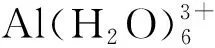水交换反应溶剂效应的量子化学团簇模型-密度泛函理论方法研究
2017-10-19董绍楠侯晓霞袁晓涵毕树平
张 婧, 董绍楠, 侯晓霞, 袁晓涵, 毕树平
(南京大学化学化工学院,江苏南京 210023)
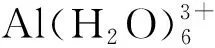
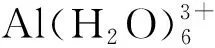
1 计算方法

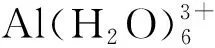
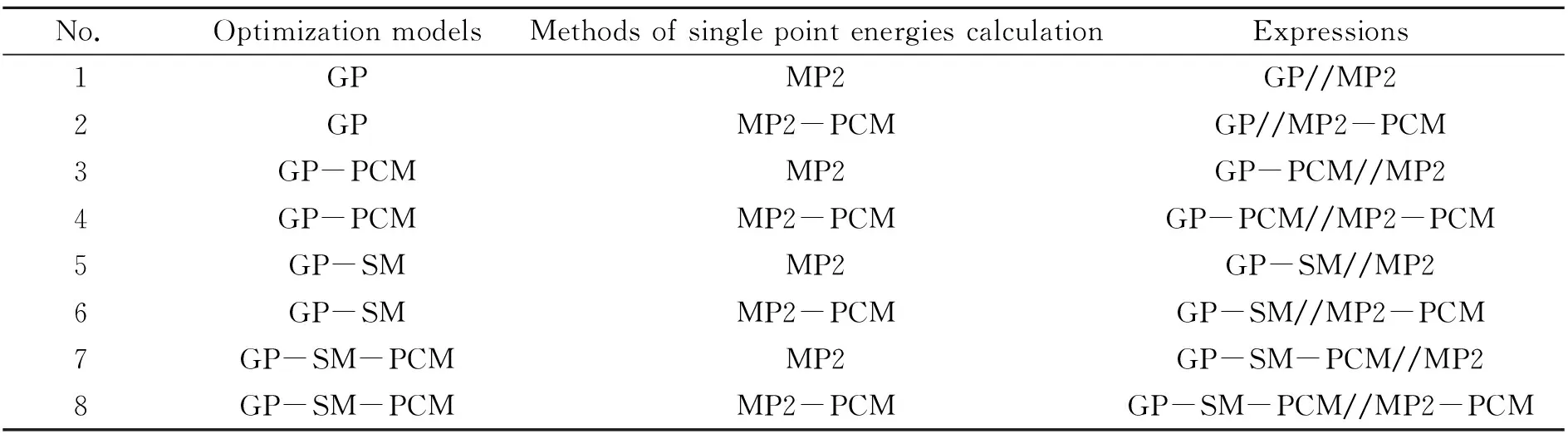
表1 不同优化模型与单点能计算方法下的能量表达方式
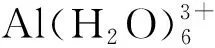
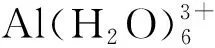
2 结果与讨论

图1 四大模型下水交换反应的反应物、过渡态以及产物的构型图(离去水以白色标记)Fig.1 The reactants,transition states and products for water-exchange reactions of complex with four models(the leaving water molecules are colored white to differ from others)

表2 四大模型下水交换反应的反应物、过渡态和产物的结构参数
Note:athe distances between Al and O of coordinated H2O;bthe average bond length between Al and O of coordinated H2O;cthe distance between Al and O of leaving H2O;dthe sum of Al-O bond lengths;eΔΣr(Al-O)I=Σr(Al-O)I,transition state-Σr(Al-O)I,reactant.

表3 四大模型下水交换反应的反应物、过渡态和产物的氢键键长(Å)
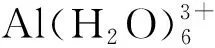


图2 不同溶剂效应下水交换反应的Gibbs自由能变化(A)和反应速率常数对数logkex(B)(图中(A)和(B)的1-8分别对应表1中的8组能量)Fig.2 The Gibbs free energy changes (A) and logkex (B) for the water-exchange reactions of with different solvent effects(1-8 in(A) and(B) are corresponding to the energies in the Table 1)
3 结论