冠状动脉疾病的代谢组学特点及其诊断价值
2017-10-18赵娟滕丽新毛梅
赵娟,滕丽新,毛梅
冠状动脉疾病的代谢组学特点及其诊断价值
赵娟1,滕丽新2,毛梅1
目的分析代谢产物变化对不同类型冠状动脉疾病(CAD)的诊断价值。方法选自重庆市急救中心、重庆市肿瘤医院、解放军第三二四医院及重庆市第三人民医院等4所医院老年病科于2003年1月~2016年1月住院患者1086例,男性712例,女性374例。依据症状和检查结果分为:对照组(NCA组,116例,无冠状动脉狭窄)、非阻塞性冠状动脉粥样硬化组(NOCA组,276例,冠状动脉狭窄<50%)、急性心肌梗死组(AMI组,324例)、不稳定型心绞痛组(UA组,307例)和稳定型心绞痛组(SA组,63例)。液相色谱-质谱联用(LC-MS)检测不同血样中代谢产物的质谱峰,从而确定其中所有的代谢产物。结果针对CAD代谢紊乱做了12个交叉比较,对89种不同的代谢产物进行鉴定。代谢途径的改变包括磷脂代谢增加,氨基酸代谢降低,短链酰基肉碱增多,三羧酸循环减少,原发性胆汁酸合成减少。受试者工作特征曲线(ROC)评估各组对比有差异的代谢产物诊断价值,有差异的代谢产物诊断NOCA与NCA(n=392)的曲线下面积(AUC)、敏感性和特异性分别为0.952、94.2%和80.7%;SA与NOCA(n=339)分别为0.993、96.4%和95.6%;UA与SA(n=370)分别为0.990、97.4%和91.1%;AMI与UA(n=631)分别为0.992、94.5%和95.3%。结论不同类型冠状动脉疾病患者发生代谢紊乱,小分子代谢产物的变化对冠状动脉疾病的鉴别诊断具有潜在价值。
冠状动脉疾病;代谢组学;代谢产物;诊断
冠状动脉疾病(CAD)是世界范围内构成死亡的主要原因,根据2013年全球疾病负担研究,每年有814万人因冠心病死亡(16.8%)[1]。根据临床症状、动脉阻塞程度和心肌损伤,CAD分为不同的类别:非梗阻性冠状动脉粥样硬化(NOCA),稳定型心绞痛(SA)、不稳定型心绞痛(UA)、急性心肌梗死(AMI)[2]。UA和AMI也被称为急性冠脉综合征(ACS)。
动脉粥样硬化是心绞痛和急性心肌梗死的常见原因,是一个缓慢而复杂的过程[3]。CAD的早期筛查和鉴别诊断,可使患者尽早获得干预。目前临床区分CAD主要基于临床症状、心电图(ECG)、心肌损伤标志物和冠状动脉造影[4-7]。其中,冠状动脉造影是诊断的“金标准”[8],但其存在专业技术和高成本的限制[9-12]。代谢组学是系统生物学中一个快速发展的领域,代谢改变在疾病的进展中具有重要意义[12]。心脏代谢改变也会导致体液代谢组学变化[13],多种小分子代谢产物的组合为疾病诊断提供参考[14,15]。本研究探讨了冠状动脉疾病中代谢的改变和意义。
1 资料与方法
1.1 研究对象选自重庆市急救中心、重庆市肿瘤医院、解放军第三二四医院及重庆市第三人民医院等4所医院老年病科于2003年1月~2016年1月住院患者1086例,其中男性712例,女性374例。冠状动脉疾病患者纳入标准:胸痛症状;合并心血管危险因素;心电图缺血性改变(ST段抬高或压低、T波低平或倒置、出现U波等),或心肌酶升高(乳酸脱氢酶及其同工酶:>380 U/L、肌酸激酶及其同工酶>12 U/L);冠状动脉造影检查明确诊断。排除主动脉夹层、肺栓塞、恶性肿瘤、自身免疫性疾病、严重传染病、外伤、近期手术史、重度心力衰竭伴左室射血分数<20%、肝功能异常(谷丙转氨酶>135 U/L)、肾功能不全(肌酐>3 mg/dl);心肌炎、心包炎、Takotsubo心肌病。所有患者均知情同意。本研究经我院伦理学委员会通过。
1.2 代谢组学检测行冠状动脉造影术前采集血浆标本,立刻放置于-80℃保存,以便进行代谢组学分析。与甲醇、乙醇、甲醇/乙醇(1:1),和甲醇/乙腈/丙酮(1:1:1)比较,选用乙腈为最佳提取溶剂。为确保代谢分析的数据质量,纳入正常冠状动脉患者(82例)和冠心病患者(125例),通过混合等量的血浆(10 ml),制备质量控制样品。液相色谱-质谱联用(LC-MS)检测不同血样中代谢产物的质谱峰,从而确定其中所有的代谢产物。
1.3 研究分组依据症状和检查结果分为:对照组(NCA 组,116例,无冠状动脉狭窄)、非阻塞性冠状动脉粥样硬化组(NOCA组,276例,冠状动脉狭窄<50%)、急性心肌梗死组(AMI组,324例)、不稳定型心绞痛组(UA组,307例)和稳定型心绞痛组(SA组,63例)。
1.4 统计分析所有数据均采用SPSS 19.0统计学软件分析。计量资料采用均数±标准差(±s)表示,两组比较采用t检验,多组间均数的比较采用方差分析,计数资料采用例数(构成比)表示,组间比较采用χ2检验。潜在的结构判别分析(OPLS-DA)采用SIMCA 14.0.1的正交投影模型。每个代谢产物的交叉比较P值均采用Bonferroni校正。聚类分析使用MeV 4.6.0进行。用Cytoscape 3.2.0绘制相关网络。受试者工作特征曲线(ROC)分析代谢产物差异的诊断价值。P<0.05为差异有统计学意义。
2 结果
2.1 一般情况比较四组在年龄、左室射血分数、收缩压、用药情况、血脂和血糖等方面比较,差异有统计学意义(P均<0.01),表1。
2.2 与冠心病患者的比较及交叉比较在峰值校对和去除缺失值后,共检测到2032个正模式特征,共有1130种离子发生了显著变化。使用OPLS-DA模型描述代谢紊乱。VIP值>1的离子被认为是潜在差异代谢产物。比较不同组代谢产物和代谢途径差异,在斑块形成期(NOCA组 vs. NCA组),斑块发展期(SA组 vs. NOCA组),斑块稳定到不稳定期(UA组 vs. SA组),斑块脱落期(AMI组vs. UA组)。结果如下:NOCA相对正常动脉,累计R2Y=0.655,Q2=0.503(图1A);SA相对NOCA,R2Y=0.626,Q2=0.518(图1B);UA相对SA,R2Y=0.645,Q2=0.548(图1C);AMI和UA,R2Y=0.641,Q2=0.595(图1D)。图1E~1H表示代谢途径变化。NOCA相对NCA,甘油、嘌呤和鞘脂代谢改变;SA相对NOCA,甘油、缬氨酸、亮氨酸、异亮氨酸、原发性胆汁酸、精氨酸和脯氨酸的生物合成改变;UA相对SA,甘油、天冬氨酸、谷氨酸、精氨酸、丙氨酸、脯氨酸的合成改变;AMI相对UA,甘油磷脂、鞘磷脂、精氨酸和脯氨酸的代谢均发生改变。甘油磷脂代谢在代谢中占有重要地位。
2.3 CAD血浆中代谢产物的网络分析除上述4组外,还进行了8个交叉比较的OPLS-DA图,包括SA与NCA,UA与NCA,AMI与NCA,UA与NOCA;AMI与NOCA,AMI与SA,严重CAD(SA/UA/AMI)与相对不严重CAD(NCA/NOCA)以及ACS(UA/AMI)与SA。通过这12个不同的CAD组的综合交叉比较,确定了89种不同的代谢产物,其中44个被证实可作为参考。图2A是正常动脉和CAD组中89种不同代谢产物的平均归一化量的热图,以正常动脉组为基准,共27种代谢产物升高,62种降低。CAD组在三羧酸循环和磷脂代谢产物下调有高度相关系数,相关氨基酸水平上调,短链酰基肉碱在网络中心,是桥梁的改变代谢物,胆汁酸代谢产物减少(图2B)。
2.4 代谢生物标志物的鉴别诊断鉴别诊断的生物标志物VIP>1.5,具有参考价值。NOCA相对NCA有10种特定的代谢生物标志物被识别,SA相对NOCA有8种,UA相对SA有10种,AMI相对UA有9种(表2)。制作ROC曲线,NOCA与NCA(n=392)曲线下面积(AUC)、敏感性和特异性分别为0.952、94.2%和80.7%(图3A);SA与NOCA组(n=339)分别为0.993、96.4%和95.6%(图3B);UA与SA组(n=370)分别为0.990、97.4%和91.1%(图3C);AMI与UA组(n=631)分别为0.992、94.5%和95.3%(图3D)。在观察阶段,ROC最高预测的敏感性和特异性,最佳界值分别为NOCA对NCA为0.635,SA对NOCA为0.205,UA对SA为0.692,AMI对UA为0.475。临界值用来预测CAD在测试阶段和外部采集的不同阶段。NOCA对NCA预测值在采集阶段是95%,观察阶段是91.5%(图3E);SA对NOCA是94.5%和89.7%(图3F);UA对SA是91.8%和96.4%(图3G);AMI对UA组是96%和85.3%(图3H)。

表1 入组患者的基线资料比较
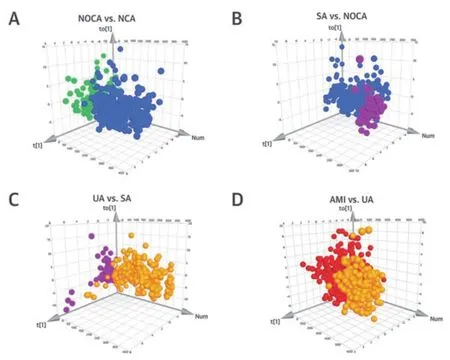
图1 OPLS-DA评分图和干扰代谢途径

图2 CAD类型和代谢相关网络中的产物差异
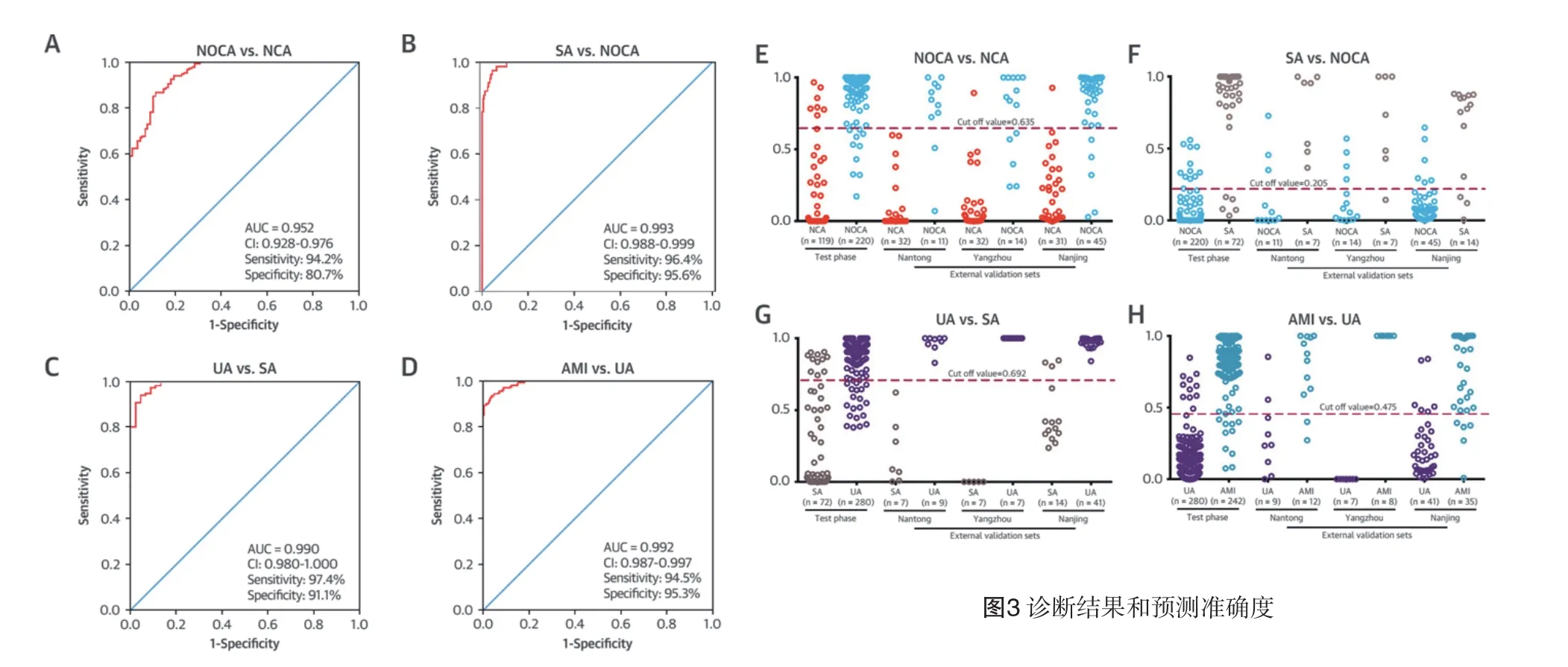
图3 诊断结果和预测准确度
2.5 各类型CAD的代谢特征和鉴别诊断对怀疑CAD患者进行冠状动脉造影,通过液相色谱-质谱法(LC-MS)测定代谢产物变化,将显著改变的代谢物用于鉴别诊断。潜在结构判别分析(OPLS-DA)评分图的正交投影显示了显著与非显著CAD之间的明显差异。受试者工作特征曲线(ROC)分析提供曲线下面积(AUC)为0.938,灵敏度为83.3%,特异度为发现阶段的91.6%,在试验阶段提供了93.0%的预测值,100%的以3为中心的外部验证集。
3 讨论
本研究分析了冠心病进展各个阶段患者代谢组学的变化,血浆样品中89种代谢产物发生变化。在CAD的进展中存在磷脂代谢和三羧酸循环下降,氨基酸代谢和短链酰基肉碱增高与原发性胆汁酸合成下降。CAD患者卵磷脂胆固醇酰基转移酶活性降低[16,17]。鞘脂水平升高是肥胖和心血管疾病的风险特征[18]。
动脉粥样硬化斑块的生长导致冠状动脉狭窄。与NOCA组相比,SA组磷酸胆碱、磷脂酰乙醇胺、磷脂酰胆碱、溶血磷脂酰胆碱降低,植物鞘氨醇和磷脂酰肌醇升高。磷脂酰胆碱的减少对冠心病有一定的影响[19]。它和CAD之间的负相关关系已被报道[20]。SA组患者磷脂酰肌醇增加。磷脂酰肌醇的增加会导致患者严重的血管钙化[21]。与SA相比,UA患者肌酸、2-羟基月桂酸、色氨酸和乙酰肉毒碱、天冬氨酸、卵磷脂、胆碱磷酸和胆碱水平下降。肌酸激酶系统可保护心血管系统免受缺血和收缩力增加带来的影响[22]。2-羟基月桂酸与斑块破裂、脂肪酸代谢紊乱相关[23]。色氨酸与免疫系统激活和炎症密切相关[24]。短链酰基肉碱水平升高提示激活脂肪酸代谢尿酸[25]。UA天冬氨酸降低提示心肌损害的高风险[26]。
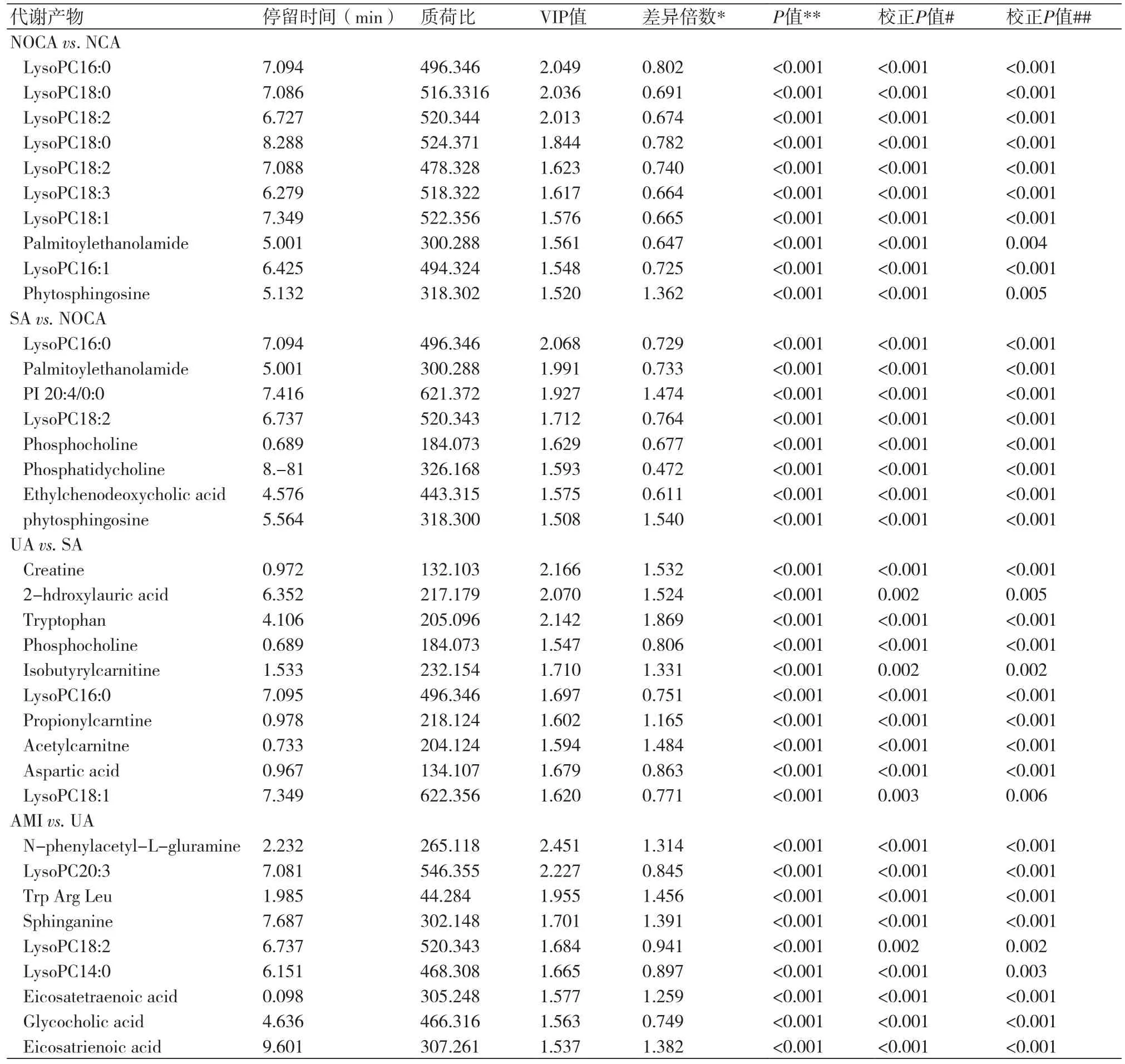
表2 各组患者的代谢生物标志物的统计分析

图4 各类型CAD的代谢特征和鉴别诊断
与UA相比,AMI患者鞘氨醇、二十碳酸和色氨酸水平增加,磷酸胆酸、溶血卵磷脂、胆碱水平降低[27]。鞘氨醇是鞘氨醇碱的合成中间体[28],表明AMI患者神经鞘脂类代谢受阻。二十碳三烯酸存在反映了AMI患者存在炎症[29]。甘氨胆酸是合成胆汁酸和胆固醇的关键[30],AMI患者降低是因为胆固醇与磷脂代谢有明显的抑制[31]。激活的氨基酸生物合成是急性心肌梗死的重要指标[32]。
在未来的研究中,建议使用双分析平台,如气相色谱-质谱联用液相色谱-质谱法,并进一步扩大人群。
[1] Naghavi M,Wang H,Lozano R,et al. Global, regional, and national agesex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013[J]. Lancet,2015,385:117-71.
[2] Epstein SE,Zhou YF,Zhu J,et al. Infection and atherosclerosis emerging mechanistic paradigms[J]. Circulation,1999,100(4):e20-8.
[3] De Backer GG. The global burden of coronary heart disease[J]. Medicogr aphia,2009,31:343-8.
[4] Libby P,Ridker PM,Hansson GK,et al. Progress and challenges in translating the biology of atherosclerosis[J]. Nature,2011,473:317-25.
[5] Bassand JP,Hamm CW,Ardissino D,et al. Guidelines for the diagnosis and treatment of nonST-segment elevation acute coronary syndromes[J].Eur Heart J,2007,2(8):1598-660.
[6] Lindahl B,Toss H,Siegbahn A,et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease[J]. N Engl J Med,2000,343(16):1139-47.
[7] Hambrecht R,Wolf A,Gielen S,et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease[J]. N Engl J Med,2000,342(7):454-60.
[8] Achenbach S,Daniel WG. Noninvasive coronary angiography—an acceptable alternative[J]. N Engl J Med,2001,345(26):1909-10.
[9] Patel MR,Peterson ED,Dai D,et al. Low diagnostic yield of elective coronary angiography[J]. N Engl J Med,2010,362(10):886-95.
[10] Amsterdam EA,Wenger NK,Brindis RG,et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines[J]. J Am Coll Cardiol, 2014,64:e139-228.
[11] Sabatine MS,Liu E,Morrow DA,et al. Metabolomic identification of novel biomarkers of myocardial ischemia[J]. Circulation,2005,112(25):3868-75.
[12] Cheng ML,Wang CH,Shiao MS,et al. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure:diagnostic and prognostic value of metabolomics[J]. J Am Coll Cardiol,2015,65(15):1509-20.
[13] Sreekumar A,Poisson LM,Rajendiran TM,et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression[J].Nature,2009,457(7231):910-4.
[14] Huang Q,Tan Y,Yin P,et al. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics[J]. Cancer Res,2013,73(16):4992-5002.
[15] Gika HG,Theodoridis GA,Wingate JE,et al. Within-day reproducibility of an HPLCMS-based method for metabonomic analysis: application to human urine[J]. J Proteome Res,2007, 6(8):3291-303.
[16] Ganna A,Salihovic S,Sundström J,et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease[J]. PLoS Genet,2014,10(12):e1004801.
[17] Stegemann C,Pechlaner R,Willeit P,et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study[J]. Circulation,2014,129(18):1821-31.
[18] Wymann MP,Schneiter R. Lipid signalling in disease[J]. Nat Rev Mol Cell Bio,2008, 9(2):162-76.
[19] Tang WHW,Wang Z,Levison BS,et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk[J]. N Engl J Med,2013,368(17):1575-84.
[20] Lin Z,Pan X,Wu F,et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory elementbinding protein-2 and induction of adiponectin in mice[J]. Circulation,2015,131(21):1861-71.
[21] Masuda M,Miyazaki-Anzai S,Keenan AL,et al. Saturated phosphatidic acids mediate saturated fatty acid-induced vascular calcification and lipotoxicity[J]. J Clin Invest,2015,125(12):4544-58.
[22] Bottomley PA,Panjrath GS,Lai S,et al. Metabolic rates of ATP transfer through creatine kinase (CK Flux) predict clinical heart failure events and death[J]. Sci Transl Med,2013,5(215):215re3.
[23] Rizos EC,Ntzani EE,Bika E,et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis[J]. J Am Med Assoc,2012,308(10):1024-33.
[24] Mellor AL,Sivakumar J,Chandler P,et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy[J]. Nat Immunol,2001,2(1):64-8.
[25] Koves TR,Ussher JR,Noland RC,et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance[J]. Cell Metab,2008,7(1):45-56.
[26] Lazo M,Rubin J,Clark JM,et al. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease[J]. J Hepatol,2015,(62):841-7.
[27] Shah SH,Sun JL,Stevens RD,et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease[J].Am Heart J,2012,163(5):844-50.
[28] Knapp M,Baranowski M,Lisowska A,et al. Decreased free sphingoid base concentration in the plasma of patients with chronic systolic heart failure[J]. Adv Med Sci,2012,57(1):100-5.
[29] Akasaka H,Ruan KH. IDENTIFICATION OF THE TWO-PHASE MECHANISM OF ENDOGENOUS OMEGA-6 FATTY ACID,ARACHIDONIC ACID, REGULATING VASCULAR INFLAMMATION BY TARGETING CYCLOOXYGENASE-2 AND MICROSOMAL PROSTAGLANDIN E2 SYNTHASE-1[J]. J Am College Cardiol,2016,67(13):2318.
[30] Sayin SI,Wahlström A,Felin J,et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist[J]. Cell Metab,2013,17(2):225-35.
[31] Chapman MJ,Ginsberg HN,Amarenco P,et al. Triglyceride-rich lipoproteins and highdensity lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management[J].Eur Heart J,2011,32(11):1345-61.
[32] Lewis GD,Wei RU,Liu E,et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury[J]. J Clin Invest, 2008,118(10):3503-12.
本文编辑:姚雪莉
Metabolomic characteristics and their value in diagnosis of coronary artery disease
Zhao Juan*, Teng Lixin, Mao Mei.
*Ward of Geriatrics, Chinese PLA 324 Hospital, Chongqing 400020, China.
ObjectiveTo analyze the diagnostic value of metabolomic changes to different types of coronary artery disease (CAD).MethodsThe hospitalized patients (n=1086, male 712 and female 374) were chosen from the departments of geriatrics in the Emergency Center of Chongqing City, Tumor Hospital of Chongqing City,Chinese PLA 324 Hospital and the Third People’s Hospital of Chongqing City from Jan. 2003 to Jan. 2016. All patients were divided, according to symptoms and examination results, into normal coronary artery group (NCA group, n=116, without coronary stenosis), non-occlusion coronary atherosclerosis group (NOCA group, n=276,coronary stenosis<50%), acute myocardial infarction group (AMI group, n=324), unstable angina group (UA group,n=307) and stable angina group (SA group, n=63). The mass spectra peaks of metabolites in different blood samples were detected by using liquid chromatography-tandem mass spectrometry (LC-MS) for determining all metabolites.ResultsThere were 12 cross comparisons conducted aiming at metabolic disorders of CAD, and 89 different metabolites identified. The changes of metabolic pathways included increase of phospholipid metabolism, decrease of amino acid metabolism, increase of short chain acyl carnitine, decrease of three-carboxylic acid cycle, and decrease of primary bile acid synthesis. The results of receiver operating characteristic curve (ROC) in reviewing diagnostic value of varying metabolites in all groups showed that area under curve (AUC) was 0.952, sensitivity was 94.2% and specificity was 80.7% in NOCA group and NCA group (n=392), 0.993, 96.4% and 95.6% in SA group and NOCA group (n=339), 0.990, 97.4% and 91.1% in UA group and SA group (n=370), and 0.992, 94.5% and 95.3% in AMI group and UA group (n=631).ConclusionThe patients with different types of CAD will suffer from metabolic disorders. The changes of small molecular metabolites have potential value in the antidiastole of CAD.
Coronary artery disease; Metabolomics; Metabolites; Diagnosis
Zhao Juan, E-mail: zhaojuanqc78@163.com
R543.3 【文献标志码】 A 【文献标志码】1674-4055(2017)09-1112-06
1400020 重庆,解放军第三二四医院干部病房;2518000 深圳,香港大学深圳医院心血管内科
赵娟,E-mail:zhaojuanqc78@163.com
10.3969/j.issn.1674-4055.2017.09.26
