miR-21对TGF-β1诱导的肾小管上皮细胞间质转分化的影响*
2017-10-14蔡月琴褚燕青朱科燕王德军
蔡月琴, 褚燕青, 朱科燕, 陈 诚, 王德军
(浙江中医药大学 动物实验研究中心/比较医学研究所, 杭州 315700)
miR-21对TGF-β1诱导的肾小管上皮细胞间质转分化的影响*
蔡月琴, 褚燕青, 朱科燕, 陈 诚, 王德军1△
(浙江中医药大学 动物实验研究中心/比较医学研究所, 杭州 315700)
目的观察miR-21在转化生长因子β1(TGF-β1)诱导的人肾小管上皮细胞(HK-2细胞)上皮间质转分化(EMT)中的作用,并探讨miR-21参与调控HK-2细胞EMT的可能靶点。方法体外培养的HK-2细胞分为6组:正常对照组、转化生长因子β1(TGF-β1)模型组、miR-21 mimic阴性组、miR-21 mimic组、miR-21 inhibitor阴性组和miR-21 inhibitor组。细胞经4 ng/ml TGF-β1处理建立EMT模型,检测miR-21和EMT相关指标的表达变化,利用基因转染技术,将miR-21 mimic质粒或miR-21 inhibitor质粒转染经TGF-β1处理的HK-2细胞,使细胞过表达或抑制表达miR-21,在此基础上观察细胞EMT相关指标的变化以及磷酸酯酶(PTEN)基因的影响。结果①与正常组相比,模型组的miR-21含量显著升高(P<0.05),上皮表型标志物E-cadherin的mRNA和蛋白表达水平均显著降低(P<0.01),间质表型标志物α平滑肌肌动蛋白(α-SMA) mRNA和蛋白水平也显著升高(P<0.05,P<0.01);②转染miR-21 mimic后,与miR-21 mimic阴性对照组相比,miR-21含量显著升高(P<0.01),PTEN、E-cadherin的mRNA和蛋白水平显著降低(P<0.05,P<0.01),α-SMA mRNA和蛋白水平显著升高(P<0.05,P<0.01);转染miR-21 inhibitor后,与miR-21 inhibitor阴性对照组相比,miR-21含量显著降低(P<0.01),PTEN、E-cadherin的mRNA和蛋白含量显著升高(P<0.05,P<0.01),α-SMA mRNA、蛋白水平显著降低(P<0.05,P<0.01)。结论miR-21在TGF-β1诱导的HK-2细胞EMT发生中具有重要作用,并且可能通过靶基因PTEN参与EMT相关分子的表达调控。
微小RNA-21;HK-2细胞;转化生长因子-β1;上皮间质转分化
肾间质纤维化(renal interstitial fibrosis,RIF)是各种不同病因的慢性肾脏病进展到终末期肾衰竭的最终共同病变过程,RIF轻重程度决定各种肾脏疾病进行性肾功能的恶化程度,已经成为威胁世界公共健康的主要疾病之一[1-4]。RIF的关键发病机制是肾小管上皮细胞向间充质细胞的转分化(epithelial-mesenchymal transition,EMT),EMT 是RIF发生的中心环节,表现为上皮细胞失去粘附能力、α-SMA 表达和肌动蛋白的重组、基底膜破坏和细胞迁移和侵袭能力增强[5]。EMT受许多生长因子、细胞因子、激素和细胞外基质的调节[6]。在众多的调节因子中,转化生长因子-β1(transformation growth factor-β1,TGF-β1)被公认为是最主要的致纤维化因子,始动并调节肾小管上皮细胞EMT的全过程[7]。但目前肾小管细胞EMT的具体分子机制仍不明确,这也是目前研究的热点之一。
近年来研究发现,microRNA(miRNA)对EMT和肿瘤的侵袭转移具有调控作用。miR-10 b可以通过上调HOXD10 基因的翻译、下调RhoC的表达,从而促进肿瘤的侵袭和转移,其表达水平与乳腺癌的进展密切相关[8]。miR-200 不仅可以通过抑制TGF-β对EMT的诱导作用,而且可以直接作用于ZEB1 及SIP1 的mRNA,上调肿瘤细胞中E-cadherin的表达,抑制EMT的发生,降低肿瘤细胞的侵袭性[9, 10]。研究发现,miR-21在肾纤维化小鼠模型和肾移植病人中都显著上调,并且miR-21敲除小鼠在肾脏损伤后很少会发生纤维化[11, 12]。另外,有研究发现TGF-β1能够上调miR-21表达[13]。这些结果提示,miR-21可能参与TGF-β1诱导的EMT,但至今miR-21在EMT中发挥的功能仍不十分清楚。因此,本研究通过在人肾小管上皮细胞(HK-2细胞)中过表达或抑制表达miR-21,观察miR-21在TGF-β1诱导的HK2细胞EMT转分化中的作用,探讨miR-21参与调控HK2细胞EMT的可能的靶点。
1 材料与方法
1.1 细胞株
人肾小管上皮细胞(HK-2细胞)购自武汉大学中国典型培养物保藏中心,用含10%胎牛血清的DMEM/F12培养基,于37℃细胞培养箱培养。
1.2 药物与试剂
TGF-β1购自R&D公司,DMEM/F12培养基、胰蛋白酶、胎牛血清均购自Gibco公司,Lipofectamine 3000购自invitrogen公司, RNA提取试剂盒、RT-PCR反转录试剂盒、SYBR Green荧光定量试剂均购自TaKaRa,PTEN、α-SMA、E-cadherin抗体购自Abcam公司,内参β-actin抗体购自CST公司,Odyssey荧光羊抗鼠二抗购自LI-COR公司,蛋白提取试剂盒、蛋白定量试剂盒购自凯基公司。
1.3 EMT细胞模型制备
参照Wang等操作方法,将HK-2细胞用含10%胎牛血清的DMEM/F12培养液,置37℃、5% CO2细胞培养箱内培养并传代。按5×105cells/well密度分别将上述细胞均匀种于6孔培养板中,80%融合后,换无血清DMEM/F12培养液,继续培养16 h同步化细胞,培养液中加入终浓度为4 ng/ml的TGF-β1,用于转染miR-21类似物、抑制剂。
1.4 转染miR-21类似物mimic、抑制剂inhibitor
将不同浓度的miR-21 mimic或inhibitor溶于250 μl无血清的Opti-MEMⅠ Reduced Serum Medium中,混合均匀。将适量 Lipofectamine 3000 溶于 250 μl 无血清的Opti-MEMⅠ中,混合均匀,在室温下孵育5 min。孵育5 min后,将上述两种混合物混合(总体积500 μl)。轻轻混合均匀在室温下孵育20 min(可能出现雾状沉淀)。在6孔板中每孔加入500 μl 的复合物,十字法混合均匀。细胞在37℃ 5% CO2培养箱中培养18~48 h后备用。
细胞分组:(1)正常对照组:加入新鲜无血清的DMEM/F12培养液;(2)TGF-β1诱导组:培养液中加入终浓度为4 ng/ml的TGF-β1;(3)miR-21 mimic阴性组:培养液中加入TGF-β1后,脂质体转染FAM标记的miR-21 mimic阴性对照;(4)miR-21 mimic组:培养液中加入TGF-β1后,转染miR-21 mimic;(5)miR-21 inhibitor阴性组:培养液中加入TGF-β1后,脂质体转染FAM标记miR-21 inhibitor阴性对照;(6)miR-21 inhibitor组:培养液中加入TGF-β1后,转染miR-21 inhibitor。每组3个复孔,继续培养48 h,收集细胞。
1.5 HK-2细胞miR-21含量检测
总RNA提取:各组6孔细胞培养板中的HK-2细胞,用0.01 mol/L PBS(pH 7.4)洗涤两次,用RNA提取试剂盒(TaKaRa)提取总RNA,操作按照说明书进行。提取的总RNA浓度和纯度用Nanodrop 2000微量核酸测定仪测定后,备用。
miR-21逆转录RT:将上述已提取的总RNA分别用has-miR-21 RT Primer、has-U6 snRNA RT Primer引物逆转录为miR-21和U6 cDNA。加入总RNA 250 ng,RT primer (2 μmol/L) 0.5 μl,并补足ddH2O至总体积3.0 μl,RT反应条件:65℃ 10 min,冰上放置3 min。在上述反应液中依次加入Denatured total RNA and RT primer、dNTP、5x RT缓冲液、Rnase inhibitor、M-MLV,在42℃下孵育60 min,然后再70℃孵育15 min,进行逆转录反应。
miR-21荧光定量PCR检测:将反转录的miR-21和U6 cDNA用实时荧光定量PCR仪进行扩增。PCR反应体系:SYBR Green Mix 10 μl,10 μmol/L Forward Primer 0.4 μl,10 μmol/L Reverse Primer 0.4 μl,加入0.5 μl miR-21或U6 cDNA,ddH2O补足至20 μl。PCR扩增程序如下:95℃预变性5 min,然后95℃变性10 s,60℃复性30 s,40个循环后进入熔解曲线程序。每个样品PCR反应重复3次,根据熔解曲线判断产物特异性,miR-21表达量通过公式2-△△CT计算。has-miR-21 RT Primer、has-miR-21荧光定量PCR引物和内参照has-U6 snRNA RT Primer、has-U6 snRNA荧光定量PCR引物序列由invitrogen设计并合成,各引物序列见表1。
Tab.1RT and quantitative PCR primer sequence of has-miR-21, has-U6 snRNA

miRNAprimernameSequencesofprimershas⁃miR⁃21RTPrimerGTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAAChas⁃miR⁃21forwardF5’⁃GCCGCGTAGCTTATCAGACT⁃3’has⁃miR⁃21reverseR5’⁃CAGTGCAGGGTCCGAGGTATT⁃3’has⁃U6snRNARTPrimerCGAATTTGCGTGTCATCCThas⁃U6snRNAfor⁃wardF5’⁃CTCGCTTCGGCAGCACATA⁃3’has⁃U6snRNAre⁃verseR5’⁃CGAATTTGCGTGTCATCCT⁃3’
1.6HK-2细胞PTEN、α-SMA、E-cadherinmRNA检测
总RNA逆转录为cDNA:将上述已提取的总RNA用逆转录试剂盒逆转录为cDNA,按照逆转录试剂盒PrimeScript TM RT reagent(TaKaRa)说明书配置RT反应液,将反转录反应体系加到无RNase的PCR管内,逆转录反应条件:37°C 15 min,85°C 5 s。反应结束后,将cDNA保存于-20°C冰箱备用。Real time PCR:将cDNA用实时荧光定量PCR仪进行扩增,PCR反应体系:SYBR Green Mix 10 μl,10 μmol/L Forward Primer 1 μl,10 μmol/L Reverse Primer 1 μl,加入2 μl cDNA,ddH2O补足至20 μl。PCR扩增反应条件:95℃预变性30 s;95℃ 变性10 s,60℃ 复性30 s,40个循环;熔解曲线:从55℃开始,每10 s升高0.5℃,直到95℃,循环1次。所有反应信息资料由Bio-Rad iQ5 PCR仪收集,目的基因mRNA水平通过公式2-△△CT计算。PTEN、α-SMA、E-cadherin和管家基因β-actin荧光定量PCR引物序列由invitrogen设计并合成,各引物序列见表2。
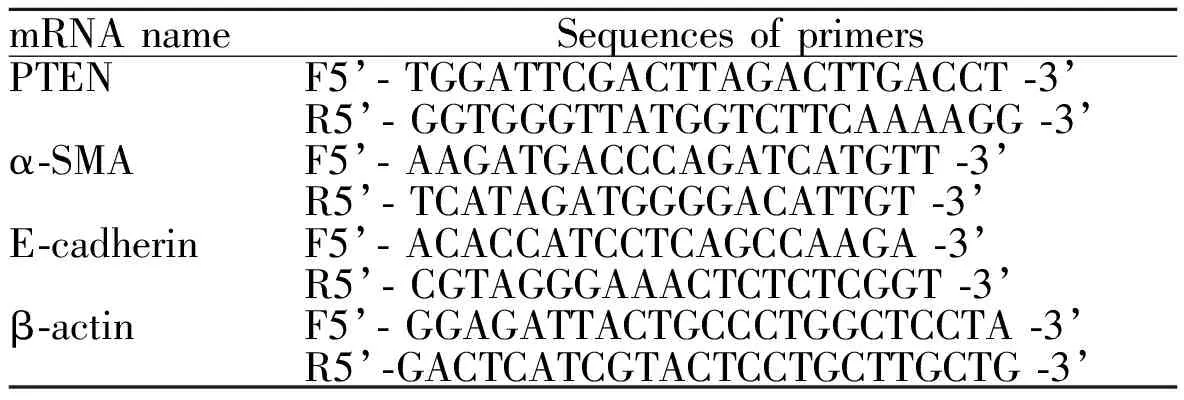
Tab. 2 Primer sequences for quantitative PCR
1.7Westernblot检测HK-2细胞PTEN、α-SMA、E-cadherin蛋白
各组6孔细胞培养板中的HK-2细胞质粒转染48 h后,用0.01 mol/L PBS(pH 7.4)清洗两次,用细胞刮将细胞从6孔培养板中分离,用蛋白提取试剂盒提取总蛋白,提取的总蛋白经BCA蛋白质定量试剂盒测定其蛋白浓度,取部分样品加蛋白上样缓冲液进行煮沸5 min变性。取50 μg总蛋白样品经10% SDS-PAGE电泳分离蛋白质,凝胶蛋白质转膜于硝酸纤维膜,经5% BSA 37℃封阻2 h后,每张膜分别加一抗PTEN、α-SMA、E-cadherin或内参β-actin抗体,一抗用5% BSA 1∶1 000稀释,4℃摇动孵育过夜,加odyssey荧光抗兔二抗,室温下摇动1.5 h,含0.1% Tween 20的TBST缓冲液洗5次,每次5 min,将膜置于odyssey近红外激光成像系统上扫描目的条带和内参条带,经odyssey图像软件系统分析PTEN、α-SMA、E-cadherin蛋白表达量。
1.8 统计学方法
2 结果
2.1TGF-β1处理后HK-2细胞miR-21水平以及EMT相关指标α-SMA、E-cadherin的变化
与正常组相比,TGF-β1诱导的模型组细胞的miR-21含量显著升高(P<0.05),间质表型标志物α-SMA的 mRNA含量和蛋白水平均显著升高(P<0.05,P<0.01),上皮表型标志物E-cadherin的mRNA含量和蛋白表达水平均显著降低(P<0.01,表3,图1),表明已成功建立EMT模型。
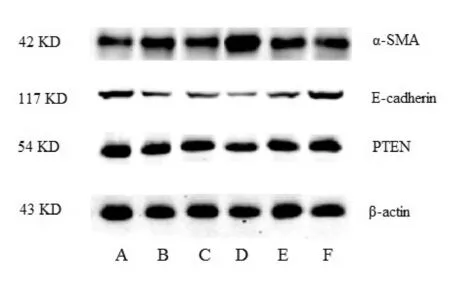
Fig.1Changes of protein expressions of α-SMA, E-cadherin and PTEN by Western blot analysis in all groups A: Control group; B: Model group; C: MiR-21 mimic negative control group; D: MiR-21 mimic group; E: MiR-21 inhibitor negative control group; F: MiR-21 inhibitor group

Tab. 3 Changes of miR-21 level, mRNA and protein expressions of α-SMA, E-cadherin in HK-2 cells after treating with TGF-β1(±s, n=3)
α-SMA: α-smooth muscle actin; TGF-β1: Transformation growth factor-β1
*P<0.05,**P<0.01vscontrol group
2.2HK-2细胞过表达或抑制表达miR-21对PTENmRNA和蛋白水平的影响
从表4、图1可以看出,HK-2细胞转染miR-21 mimic后,miR-21含量较miR-21 mimic阴性对照组显著升高(P<0.01),PTEN的mRNA和蛋白水平显著下降(P<0.05,P<0.01);HK-2细胞转染miR-21 inhibitor后,miR-21含量较miR-21 inhibitor阴性对照组显著降低(P<0.01),PTEN的mRNA和蛋白水平显著升高(P<0.05,P<0.01)。
2.3HK-2细胞过表达或抑制表达miR-21对α-SMA、E-cadherinmRNA和蛋白水平的影响
从表5、图1可以看出,HK-2细胞转染miR-21 mimic后,与miR-21 mimic阴性组相比,α-SMA mRNA和蛋白水平显著升高(P<0.05,P<0.01),E-cadherin的mRNA和蛋白水平显著降低(P<0.05,P<0.01);HK-2细胞转染miR-21 inhibitor后,与miR-21 inhibitor阴性组相比,α-SMA mRNA和蛋白水平显著降低(P<0.05,P<0.01),E-cadherin的mRNA和蛋白水平显著升高(P<0.05,P<0.01)。

Tab. 4 Changes of miR-21 level on the mRNA and protein expression of PTEN in HK-2 cells after transfecting with miR-21 mimic or inhibitor plasmid(±s, n=3)
PTEN: Phosphatase and tensin homolog
*P<0.05,**P<0.01vsmiR-21 mimic negative control group;#P<0.05,##P<0.01vsmiR-21 inhibitor negative control group

Tab. 5 Changes of mRNA and protein expression of α-SMA and E-cadherin in HK-2 cells after transfecting with miR-21 mimic or inhibitor plasmid(±s, n=3)
*P<0.05,**P<0.01vsmiR-21 mimic negative control group;#P<0.05,##P<0.01vsmiR-21 inhibitor negative control group
3 讨论
TGF-β1 是目前公认的最主要致纤维化细胞因子,是诱导EMT过程的关键因素,EMT是指上皮细胞在特定的情况下向间质细胞转分化的现象,其主要特征为上皮细胞粘附分子(E-cadherin)表达的丧失,并获得间质细胞特征性表型蛋白(α-SMA)的高表达[14]。E-cadherin的作用是维持上皮细胞间连接的稳定性,其表达水平与EMT的发生及肿瘤的侵袭和转移能力呈负相关,是EMT的关键分子,α-SMA是间质细胞的特征性表型蛋白,也是EMT的主要分子标志之一。本实验中,HK-2细胞经TGF-β1诱导后,模型组的上皮细胞表型标志物E-cadherin的 mRNA含量和蛋白表达水平均显著降低,而间质表型标志物α-SMA的mRNA含量和蛋白水平显著升高,表明已成功建立EMT模型。
miRNA是一类长约19~23 nt的单链非编码RNA,其与靶mRNA的3’-UTR(3’非编码区)特异性结合,引起靶 mRNA 的翻译抑制或切割降解,调控基因的表达。miRNA可通过靶基因的抑制和降解在EMT调控中起重要作用。miR-21在EMT中发挥调控作用的靶mRNA可能是与张力蛋白同源的磷酸酯酶基因(phosphatase and tensin homolog,PTEN)[15, 16]。miR-21通过降解PTEN的表达和结合于靶mRNA 的3’UTR端抑制其翻译两种作用方式调控基因的表达,使肾小管上皮细胞发生EMT。本实验发现,TGF-β1诱导的EMT模型细胞中miR-21表达显著升高,提示miR-21可能在肾纤维化过程中具有重要调控作用。这和前期Wang等报道的在肾纤维化小鼠模型中miR-21显著上调的结论相一致[11]。为验证这一结论,我们通过转染试验,将EMT模型组细胞分别转染miR-21 mimic或miR-21 inhibitor,转染后miR-21含量进一步升高或者显著降低,表明质粒转染成功。转染miR-21 mimic后,miR-21过表达下调了PTEN的mRNA和蛋白水平,上调了间质表型标志物α-SMA的mRNA和蛋白表达、下调了上皮表型标志物E-cadherin的mRNA和蛋白表达,即miR-21过表达加剧了HK-2细胞的EMT;正好相反,转染miR-21 inhibitor后,miR-21抑制表达,上调了PTEN的mRNA和蛋白水平,下调了间质表型标志物α-SMA的mRNA和蛋白表达,上调了上皮表型标志物E-cadherin的mRNA和蛋白表达,逆转了HK-2细胞的EMT。这些结果表明miR-21作为体内调控基因表达的重要分子,参与了EMT相关分子的表达调控。
以上实验结果表明,miR-21能够加剧HK2细胞TGF-β1诱导的EMT,并且可能通过靶基因PTEN参与了EMT相关分子的表达调控。
[1] Woo KT, Choong HL, Wong KS,etal. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases [J].KidneyInt, 2012, 81(10): 1044-1045.
[2] Nugent RA, Fathima SF, Feigl AB,etal. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health [J].NephronClinPract, 2011, 118(3): c269-277.
[3] Farris AB, Colvin RB. Renal interstitial fibrosis: mechanisms and evaluation [J].CurrOpinNephrolHypertens, 2012, 21(3): 289-300.
[4] Mahdavi-Mazdeh M, Hatmi ZN, Shahpari-Niri S. Does a medical management program for CKD patients postpone renal replacement therapy and mortality? A 5-year-cohort study [J].BMCNephrol, 2012, 13: 138.
[5] 林成成, 陆 红, 梁 勇, 等. 肾小管上皮细胞表型转化在大鼠输尿管梗阻及再通中的意义[J]. 中国应用生理学杂志, 2013, 29(5): 454-456.
[6] Wang QL, Tao YY, Yuan JL,etal. Salvianolic acid B prevents epithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro [J].BMCCellBiol, 2010, 11(31): 1668-1681.
[7] Fan DM, Qi PW, Gao SG,etal. TGF-β1 mediates estrogen receptor-induced epithelial-to-mesenchymal transition in some tumor lines [J].TumourBiol, 2014, 35(11): 11277-11282.
[8] Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer [J].Nature, 2007, 449(7163): 682-688.
[9] Korpal M, Lee ES, Hu G,etal. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2[J].JBiolChem, 2008, 283(22): 14910-14914.
[10]Li Y, Vanden Boom TG 2nd, Kong D,etal. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells[J].CancerRes, 2009, 69(16): 6704-6712.
[11]Wang JY, Gao YB, Zhang N,etal. miR-21 overexpression enhances TGF-β1-induced epithelial-to- mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy [J].MolCellEndocrinol, 2014, 392(1-2): 163-172.
[12]Wilflingseder J, Sunzenauer J, Toronyi E,etal. Molecular pathogenesis of post-transplant acute kidney injury: assessment of whole-genome mRNA and miRNA profiles [J].PLoSOne, 2014, 9(8): e104164.
[13]Liu Z, Wang J, Guo C,etal. microRNA-21 mediates epithelial-mesenchymal transition of human hepatocytes via PTEN/Akt pathway [J].BiomedPharmacother, 2015, 69: 24-28.
[14]涂容芳, 张秀峰, 何振华. 5-HTR2B、E-cad、α-SMA在博莱霉素致大鼠肺纤维化中的表达变化[J]. 中国应用生理学杂志, 2016, 32(4): 365-369.
[15] Haghikia A, Hilfiker-Kleiner D. MiRNA-21: a key to controlling the cardiac fibroblast compartment [J].CardiovascRes, 2009, 82(1): 1-3.
[16]Zhang Z, Peng H, Chen J,etal. MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice [J].FEBSLett, 2009, 583(12): 2009-2014.
TheinfluenceofmiR-21onHK-2EMTcellsinducedbyTGF-beta1
CAI Yue-qin, CHU Yan-qing, ZHU Ke-yan, CHEN Cheng, WANG De-jun△
(Zhejiang Chinese Medical University, Laboratory Animal Research Center/Comparative Medicine Institude, Hangzhou 310053, China)
Objective: To investigate the effect of the miR-21 and its target mRNA in renal tubular epithelial mesenchymal transformation (EMT) model induced by transformation growth factor-β1(TGF-β1) in human renal tubular epithelial (HK-2) cells.MethodsHK-2 cells were divided into 6 groups: normal control group, TGF-β1 group, miR-21 mimic negative group, miR-21 mimic group, miR-21 inhibitor negative group and miR-21 inhibitor group. EMT model was established in HK-2 cells induced by 4 ng/ml TGF-β1. The level of miR-21, the mRNA and protein expression of EMT related factors were detected. MiR-21 mimic plasmid and miR-21 inhibitor plasmid were transfected into HK-2 cells that treated with TGF-β1 respectively using liposome transfection technique. Observe the impact of overexpression or inhibition expression of miR-21 on the mRNA and protein expression of EMT related factors and PTEN.Results①Compared with the normal group, the level of miR-21 was significantly increased in model group (P<0.05), the mRNA and protein expression levels of epithelial cells marker E-cadherin was significantly decreased (P<0.01), while the mRNA and protein levels of mesenchymal cells marker α-SMA was significantly increased (P<0.05,P<0.01). ②Compared with the miR-21 mimic negative group, the level of miR-21 in miR-21 mimic group increased significantly (P<0.01), the mRNA and protein expression levels of PTEN and E-cadherin decreased significantly (P<0.05,P<0.01), the mRNA and protein levels of α-SMA increased significantly (P<0.05,P<0.01). Compared with the miR-21 inhibitor negative control group, the level of miR-21 in miR-21 inhibitor group decreased significantly (P<0.01), the mRNA and protein expression levels of PTEN and E-cadherin increased significantly (P<0.05,P<0.01), the mRNA and protein levels of α-SMA decreased significantly (P<0.05,P<0.01).ConclusionMiR-21 may play an important role in EMT induced by TGF-β1 in HK-2 cells and regulate the expression of EMT related factors its target gene PTEN.
microRNA-21; HK-2 cells; transformation growth factor-β1; epithelial mesenchymal transformation
R392.11
A
1000-6834(2017)04-346-05
浙江省自然科学基金资助项目(LQ14H290004);浙江中医药大学比较医学创新团队资助项目(XTD201301)
2016-07-21
2017-01-30
△
Tel: 0571-86613662; E-mail: wdj0369@126.com
10.12047.j.cjap.5477.2017.084
