Comparisonofsubstrateandoutcomesforventriculartachycardiaablationbetweenthepatientswithischemicandnon-ischemiccardiomyopathy
2017-09-12
Comparisonofsubstrateandoutcomesforventriculartachycardiaablationbetweenthepatientswithischemicandnon-ischemiccardiomyopathy
AtulVerma
Supported by the experience and data from the Southlake in Canada and other centers, this paper compares the outcomes for ventricular tachycardia ablation between the patients with ischemic cardiomyopathy(ICM) and non-ischemic cardiomyopathy(NICM). It implies that the outcomes are better for substrate-based ablation in ICM patients compared with the outcomes in NICM patients. However, it is challenging to identify NICM substrates. Long-term outcomes for VT ablation remain worse in NICM patients than those in ICM patients. But if the reduction of VT burden is regarded as a main target rather than complete cure, the outcomes are still acceptable.
ventricular tachycardia; non-ischemic cardiomyopathy; mapping; ablation
1 Introduction
Traditionally, catheter ablation of ventricular tachycardia(VT) involved inducing the arrhythmia, mapping the circuit during VT, and using pacing manoeuvers to localize critical portions of the circuit[1-3]. Substrate-guided ablation of VT, however, involves identifying potentially arrhythmogenic regions of myocardium in sinus rhythm and thus avoids the need for mapping during VT[2-3]. This can substantially reduce the risk associated with VT ablation[4]. At present, substrate-guided ablation has been evolved as a method of choice for all VT ablations in cardiomyopathy.
Substrate-guided ablation may be less effective in patients with non-ischemic cardiomyopathy (NICM) than in those with ischemic cardiomyopathy (ICM)[5]. The reason is that scar regions in NICM may be less extensive, patchier, and more epicardially involved[6-8]. Consequently, the combined approach of activation map and substrate ablation during VT ablation in NICM patients has shown unsatisfactory results in terms of arrhythmias recurrence after ablation[9-13]. Only very limited data exist for outcomes with VT ablation in NICM and ICM patients with an exclusively substrate-guided approach[5, 14]. The purpose of this article was to compare the substrate and outcomes of a purely substrate-guided VT ablation between ICM and NICM patients, and pointed out the limitations of substrate-based mapping in NICM patients.
2 Outcomes in ischemic vs. non-ischemic cardiomyopathy
2.1 Experience from the Southlake
For the past several years, at Southlake, we have been employing a substrate-guided approach for all ICM and NICM VT ablation to reduce the difficulties introduced to the procedure with repeated inductions and maintenance of ventricular arrhythmias. Here are some data and procedures we published in 2015[15]. Scar regions were defined as areas with bipolar electrogram(EGM) voltage≤0.5 mV and scar border zone areas as those with low bipolar voltage between 0.5 mV and 1.5 mV. The normal myocardium was defined as bipolar voltage≥1.5 mV. As shown in Fig.1, we identified all late potentials in either sinus or paced rhythm within the scar, labelled as pink dots, and then all sites with good pace-match to VT(>10/12) in this scar border zone as in these yellow dots. Epicardial mapping was performed when ECG was suggestive of epicardial VT, prior endocardial ablation was unsuccessful, or no endocardial scar was seen. The definition of voltage scar in the epicardium was the same as in the endocardium, but the scar showed up in broad(>80 ms) or fractionated EGMs. We ablated all of those regions until all of the VTs became non-inducible.
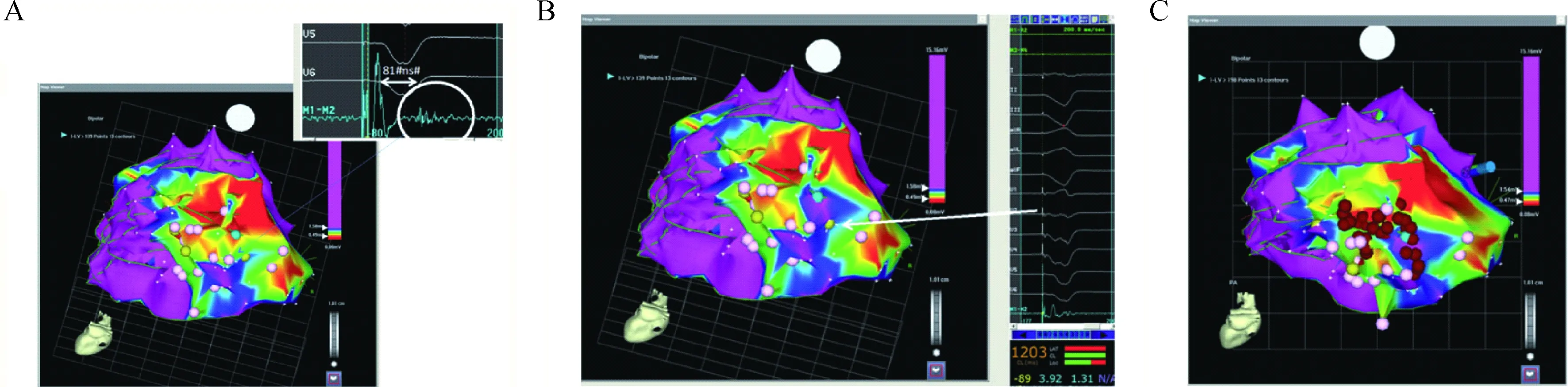
In (A) is a site showing a sample of a late potential. As can be seen, there is at least 20 ms (81 ms) from the far-field ventricular electrogram to the late potential. However , the late potential is still contained within the surface QRS (see lead V6). In (B), there is an example of a site with a 12/12 pace-match to the induced ventricular tach-ycardia. (C) shows the ablation strategy in which areas of scar or scar border zone with sites of late potentials and good pace-match sites are targeted. Red dots represent the ablation lesions
2.2 Comparison of ablation outcomes: ischemic vs. non-ischemic cardiomyopathy
Proietti et al[15]pointed out that more epicardial ablation was performed in NICM patients compared with ICM patients(25%vs. 1%), the total scar area was also substantially less (20%vs. 59%), and most importantly, there was less non-inducibility at the end of ablation(53%vs. 69%). Over a follow-up of nearly 2 years, 64% of patients were free of ventricular arrhythmias recurrence. However, both acute procedural success(complete or partial) and long-term freedom from arrhythmias were higher in ICM patients compared with NICM patients. And there was a trend of increased all-causes mortality in patients with NICM, although not statistically significant. Similar to the above results, Dinov et al[11]again showed over a mean follow-up of 1 000 days, the success rate of the NICM patients was down to about 23% whereas that of ICM patients was significantly higher.
Among many reasons why the outcomes for NICM patients are worse, first and foremost, there are less defined scar regions endocardially which impairs our ability to map for late potentials and circuits within the scar. Secondly, there are more epicardial substrates rather than endocardial substrates. Thirdly, there tends to be more inducible VTs. And finally, there also tends to be more focal VTs rather than scar re-entrant VTs.
2.3 Types of ventricular tachycardia in non-ischemic cardiomyopathy
Delacretaz et al[16]reported that in patients with NICM, about 2/3 of the VTs are in fact re-entrant, but about 27% will be due to focal automaticity in areas like the right ventricle(RV) outflow tract and mitral annulus. And about 19% will be due to bundle-branch or interfascicular re-entry. Focal VT is identified by mapping and existence of a focus with spread of activation away in all directions. Bundle-branch re-entry is identified by demonstrating His bundle potential(H) preceding each ventricular wave(V) and each H-H interval change resulting in the subsequent V-V interval change. Scar re-entry is all other VT that doesnot fall into the above categories.
3 Challenges for identifying substrate in non-ischemic cardiomyopathy
3.1 Substrates in non-ischemic cardiomyopathy
Although scar may be present in patients with endocardial NICM, it tends to be more “patchy” than patients with coronary heart disease and has predilection for the basal left ventricle. But up to 20%-60% of patients may have absolutely no endocardial scar[17]. Hsia et al[18]reported that epicardial substrates are far more common in NICM patients. Therefore, as long as we routinely go into the epicardium of NICM patients, we should be able to solve the problem of poor outcomes.
Good news comes from the meta-analysis recently published[19]that if we use a combined endo-epicardial approach, the overall outcomes will tend to be better in NICM patients than in ICM patients. However, what is interesting is that the hazard ratio of the “better outcomes” is perhaps not as pronounced as we might have thought in the combined endo-epicardial approach.
3.2 Late potentials not as frequent or reliable
Late potentials are neither as frequent nor as reliable target in NICM patients compared with ICM patients. Nakahara et al[5]found that the incidence of both mid-late potentials and very late potentials were significantly lower both in the endocardium and epicardium of NICM patients compared with ICM patients. It was concluded that (ⅰ) ICM patients had larger scar areas than NICM patients; (ⅱ) NICM patients had fewer late potentials compared with ICM patients; (ⅲ) the success rate of the exclusively late potential guided strategy was much lower in NICM patients compared with ICM patients(50%vs. 82%).
4 Limitations of substrate-based mapping in non-ischemic cardiomyopathy
4.1 Identification of true epicardial scars
Up to now, epicardial mapping still has a lot of limitations. The use of bipolar voltage in the epicardium alone may not be sufficient to identify true epicardial scar since either epicardial fat or poor epicardial contact can masquerade as scars. True epicardial scar is probably only indicated when the following two conditions[6]are satisfied at the same time: (ⅰ) low epicardial voltage areas are larger than endocardial low voltage areas; (ⅱ) EGMs are wide (>80 ms), split, or displaying late potentials. Because the EGMs satisfying condition (ⅱ) are rare in the epicardium of NICM patients, the manipulator may end up falsely ablating areas with a false epicardial scar.
The use of unipolar endocardial voltage has been proposed as a way of finding both mid-myocardial and epicardial scars. Hutchinson et al[20]suggested that the findings of confluent regions(>2 cm2) with low unipolar voltage(<8.3 mV, mean 5.5 mV) endocardially would correlate with epicardial or mid-myocardial scar regions. As shown in Fig.2, the unipolar endocardial voltage correlates with epicardial bipolar definitions of scar. And in the epicardial bipolar voltage of scar mapping, the scar regions were significantly larger than those identified by endocardial unipolar scar mapping.
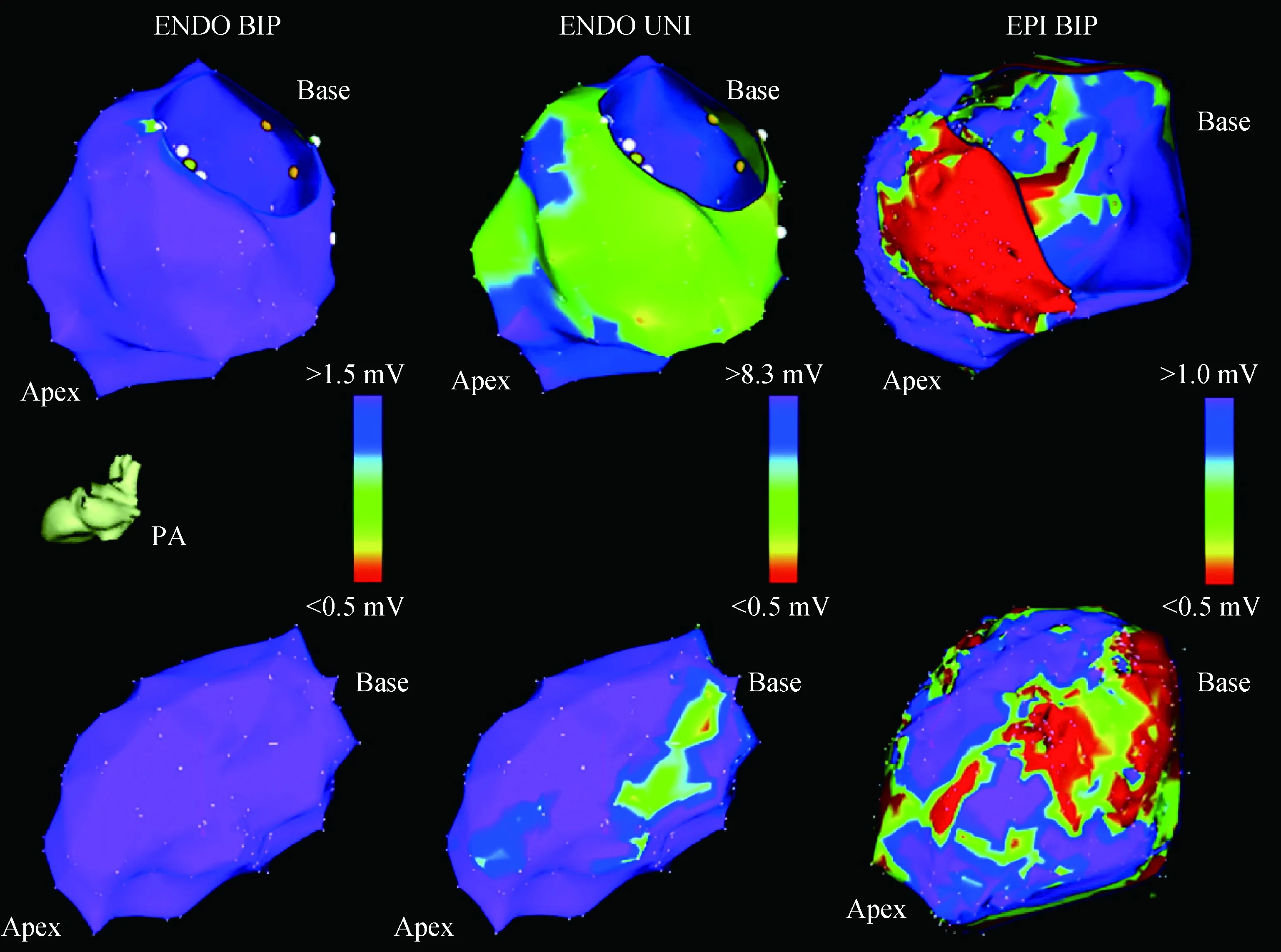
ENDO=endocardial;EPI=epicardial;BIP=bipolar;UNI=unipolar
4.2 Large size of unipolar voltage area predicts worse outcomes
The large size of the unipolar voltage abnormality predicts worse outcomes. So even if you are able to identify the unipolar voltage regions and then move to the epicardium to try to ablate all of these areas, the reality is that regardless of how well the ablation is done, the presence of large endocardial unipolar voltage would significantly worsen the outcome both in terms of cardiac mortality and cumulative probability of VT survival[21]. It suggested that these patients may be predisposed to worse outcome regardless of the ablation strategy used.
4.3 Unipolar scar progresses in non-ischemic cardiomyopathy
Recent data have also suggested that unipolar scar tends to progress in patients with NICM. Liuba et al[22]carried out a comparison between the bipolar and unipolar scar areas in 13 patients who had undergone a first or second ablation. And while the bipolar scar area did not tend to vary significantly after either the first or second ablation, the size of unipolar scar area uniformly tends to be larger in patients undergoing the second ablation compared with the ones undergoing the first procedure. It suggested that these mid-myocardial and epicardial scar regions might be more prone to progressing in NICM patients, and worsen the prognosis.
4.4 Unipolar voltage and epicardial scar regions donot always correlate
Unipolar voltage and epicardial scar regions do not always correlate with each other. Tokuda et al[23]plotted out the endocardial uniploar voltage against the epicardial bipolar voltage. Although there is a correlation, the correlation is not as strong as we might think and it tends to be a scatter plot in all of this relationship. There may be many causes for mis-match between unipolar endocardial scar voltage and epicardial bipolar scar. First of all, as we have already mentioned, epicardial fat can help us to over-estimate the occurrence of epicardial scar. Secondly, mid-myocardial scar also shows up with unipolar mapping. But there are also some situations where the unipolar voltage area is under-estimated as the epicardial scar region. Tokuda et al[23]provided an example here. As shown in Fig.3, the unipolar voltage was almost completely normal and yet there was significant voltage area when epicardial bipolar voltage mapping was performed. The exact reasons for these sorts of mis-matches cannot be explained by the above two mechanisms.
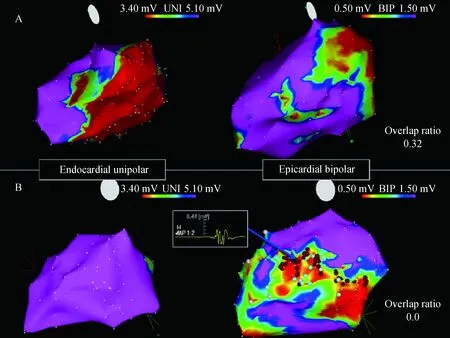
UNI=unipolar;BIP=bipolar
4.5 Long-term outcomes of ventricular tachycardia ablation in non-ischemic cardiomyopathy
There are actually very few publications on the long-term outcome of VT ablation in NICM patients. Muser et al[24]reported that the percentage of NICM patients who required either two ablation procedures, or three or more ablation procedures was significantly higher than what we might expect for ICM patients. So in the pre-therapy conversation, please make it clear that the patients may require more than one procedure in order to get a successful outcome. Furthermore, epicardial mapping was performed in nearly 40% of NICM patients and epicardial ablation was required in more than 25% of NICM patients. The incidence rate of the complications may be somewhat higher again compared to those with ICM. Particularly, complications such as pericardial effusion and tamponade mostly are related to epicardial mapping and ablation. At the end of 60 months, the overall success rate was about 70%. The result was quite reasonable but much lower than the rate of ICM patients which had been reported before. Even in the patients from whom a completely successful result failed to be obtained, the burden of VT recurrence may be less after ablation compared with that before. However, expecting a total cure in these ICM patients may not be completely realistic.
5 Conclusion
Generally, the outcomes are better for substrate-based ablation in ICM patients compared with the outcomes in those with NICM. There are many limitations in identifying NICM substrate: (ⅰ) patchy endocardial substrate, (ⅱ) multiple VT mechanisms, (ⅲ) more epicardial substrates, and (ⅳ) unipolar voltage may help to identify both epicaridal and mid-myocardial substrates. But unipolar voltage is not perfect and its size predicts worse outcomes. Long-term outcomes for VT ablation remain worse in NICM patients than those in ICM patients. However, if the reduction of VT burden is regarded as a main target rather than complete cure, the outcomes are still acceptable and we may pursue these procedures even though they may require more than one procedure.
[1] Stevenson WG, Khan H, Sager P, et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction [J]. Circulation,1993, 88(4 Pt 1):1647-1670.
[2] Di Biase L, Burkhardt JD, Mohanty P, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio[J]. Circulation, 2010, 121(23):2550-2556.
[3] Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA)[J]. Europace, 2009, 11(6):771-817.
[4] Volkmer M, Ouyang F, Deger F, et al. Substrate mapping vs. tachycardia mapping using CARTO in patients with coronary artery disease and ventricular tachycardia:impact on outcome of catheter ablation[J]. Europace, 2006, 8(11):968-976.
[5] Nakahara S, Tung R, Ramirez RJ, et al. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia[J]. J Am Coll Cardiol, 2010, 55(21):2355-2365.
[6] Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy[J]. J Am Coll Cardiol, 2009, 54(9):799-808.
[7] de Jong S, van Veen TA, van Rijen HV, et al. Fibrosis and cardiac arrhythmias[J]. J Cardiovasc Pharmacol, 2011, 57(6):630-638.
[8] Verma A, Kilicaslan F, Schweikert RA, et al. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia[J]. Circulation, 2005, 111(24):3209-3216.
[9] Kottkamp H, Hindricks G, Chen X, et al. Radiofrequency catheter ablation of sustained ventricular tachycardia in idiopathic dilated cardiomyopathy[J]. Circulation, 1995, 92(5):1159-1168.
[10] Soejima K, Stevenson WG, Sapp JL, et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy:the importance of low-voltage scars[J]. J Am Coll Cardiol, 2004, 43(10):1834-1842.
[11] Dinov B, Fiedler L, Schönbauer R, et al. Outcomes in catheter ablation of ventricular tachycardia in dilated non-ischemic cardiomyopathy in comparison to ischemic cardiomyopathy:results from the prospective HEart centre of LeiPzig VT(HELP-VT) study[J]. Circulation, 2013, 129(7):728-736.
[12] Piers SR, Leong DP, van Huls van Taxis CF, et al. Outcome of ventricular tachycardia ablation in patients with nonischemic cardiomyopathy:the impact of noninducibility[J]. Circ Arrhythm Electrophysiol, 2013, 6(3):513-521.
[13] Della Bella P, Baratto F, Tsiachris D, et al. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias:long-term outcome after ablation[J]. Circulation, 2013, 127(13):1359-1368.
[14] Tokuda M, Tedrow UB, Kojodjojo P, et al. Catheter ablation of ventricular tachycardia in nonischemic heart disease[J]. Circ Arrhythm Electrophysiol, 2012, 5(5):992-1000.
[15] Proietti R, Essebag V, Beardsall J, et al. Substrate-guided ablation of haemodynamically tolerated and untolerated ventricular tachycardia in patients with structural heart disease: effect of cardiomyopathy type and acute success on long-term outcome[J]. Europace, 2015, 17(3):461-467.
[16] Delacretaz E, Stevenson WG, Ellison KE, et al. Mapping and radiofrequency catheter ablation of the three types of sustained monomorphic ventricular tachycardia in nonischemic heart disease[J]. J Cardiovasc Electrophysiol, 2000, 11(1):11-17.
[17] Marchlinski FE, Callans DJ, Gottlieb CD, et al. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy[J]. Circulation, 2000, 101(11):1288-1296.
[18] Hsia HH, Marchlinski FE. Characterization of the electroanatomic substrate for monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy[J]. Pacing Clin Electrophysiol, 2002, 25(7):1114-1127.
[19] Hu J, Zeng S, Zhou Q, et al. Can ventricular tachycardia non-inducibility after ablation predict reduced ventricular tachycardia recurrence and mortality in patients with non-ischemic cardiomyopathy? A meta-analysis of twenty-four observational studies[J]. Int J Cardiol, 2016, 222:689-695.
[20] Hutchinson MD, Gerstenfeld EP, Desjardins B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy[J]. Circ Arrhythm Electrophysiol, 2011, 4(1):49-55.
[21] Dinov B, Schratter A, Schirripa V, et al. Procedural outcomes and survival after catheter ablation of ventricular tachycardia in relation to electroanatomical substrate in patients with nonischemic-dilated cardiomyopathy:the role of unipolar voltage mapping[J]. J Cardiovasc Electrophysiol, 2015, 26(9):985-993.
[22] Liuba I, Frankel DS, Riley MP, et al. Scar progression in patients with nonischemic cardiomyopathy and ventricular arrhythmias[J]. Heart Rhythm, 2014, 11(5):755-762.
[23] Tokuda M, Tedrow UB, Inada K, et al. Direct comparison of adjacent endocardial and epicardial electrograms: implications for substrate mapping[J]. J Am Heart Assoc, 2013, 2(5):e1-e12.
[24] Muser D, Santangeli P, Castro SA, et al. Long-term outcome after catheter ablation of ventricular tachycardia in patients with nonischemic dilated cardiomyopathy[J]. Circ Arrhythm Electrophysiol, 2016, 9(10):e1-e13.
10.13308/j.issn.2095-9354.2017.04.003
Author Unit: McGill University Health Centre, Montreal, Quebec, Canada H3A 0G4; Southlake Regional Health Centre, Newmarket, Ontario, Canada L3Y 2P6
Author Brief Introduction: Atul Verma, associate professor, director of Heart Rhythm Program in Southlake Regional Health Centre and chair of CCS AF Guidelines Committee; research interests: ventricular arrhythmia ablation, E-mail: atul.verma@utoronto.ca
