Epicardialmappingandablationforventriculartachycardia:techniquestooptimizesuccessandminimizecomplications
2017-09-12
海外论坛
Epicardialmappingandablationforventriculartachycardia:techniquestooptimizesuccessandminimizecomplications
JasonSBradfield
Epicardial mapping provides essential information to better understand the three-dimensionality of ventricular tachycardia circuits. Once epicardial access is obtained, mapping techniques such as multipolar mapping, isochronal late activation mapping and changing wavefronts can help determine essential regions for ablation, while limiting the amount of ablation required. However, successful epicardial mapping and ablation requires clear anatomic understanding to maximize success and minimize complications.
ventricular arrhythmia; epicardial; mapping; ablation
1 Introduction
In medicine, and particularly in clinical electrophysiology, we often label clinical findings with distinct terms such as “either/or”. A ventricular tachycardia(VT) circuit is often labeled as “either” endocardial “or” epicardial. However, VT circuits are three-dimensional, and often involve multiple layers of the myocardium(endocardial, mid-myocardial and/or epicardial). This distinction is based on the presumed exit site of the VT, whereas the critical isthmus of a reentrant circuit may be in a very distant location. The complex three-dimensional nature of these arrhythmias often leads to the need for epicardial mapping to better understand the critical regions of conduction that can be targeted with ablation.
In this brief review, we will discuss access and mapping of the epicardium, interpretation of epicardial electrograms(EGMs) in that setting, and related techniques to minimize collateral damage.
2 Reasons to map the epicardium
The question is not whether something is purely endocardial or purely epicardial, but rather if and when epicardial mapping can help the operator understand the VT circuit, and ultimately lead to decreased VT burden after ablation. Therefore, we must consider a number of different factors that potentially make epicardial mapping and ablation high-yield.
2.1 Non-ischemic cardiomyopathy
Epicardial mapping is commonly performed in non-ischemic cardiomyopathy(NICM) patients because of a preponderance of epicardial scar. Fig.1 shows a pathological specimen of a patient who suffered from a severe NICM with diffused fibrosis. Unlike ischemic scars, which are often endocardial, non-ischemic fibrosis can be diffuse as shown in the image. In the case of the patient from whom the pathology in Fig.1 was taken, the diffuse fibrosis made it impossible to control this patient’s VT, even with epicardial ablation. The patient ultimately underwent cardiac transplant.
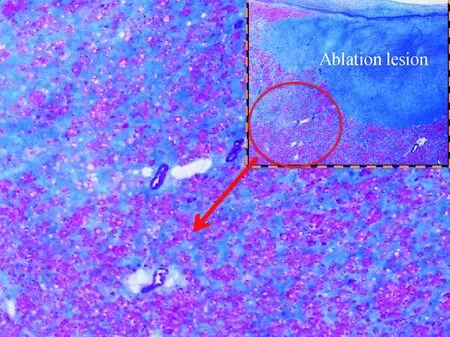
Blue tissue is diffuse fibrosis; dense blue region at top right is an ablation lesion
2.2 Imaging findings
Imaging studies can also be utilized to help determine whether epicardial mapping is of benefit. Cardiac magnetic resonance imaging(cMRI) obtained prior to ablation can demonstrate scar patterns and help determine if epicardial mapping and ablation is likely to be high-yield. The initial impetus for epicaridal mapping came from Dr Sosa and colleagues in patients with Chagas disease, and we have recently published the largest MRI series in a Chagas disease population[1]demonstrating a variety of scar patterns at various stages of the disease process. When epicardial or mid-myocardial scars are present on cMRI in patients with VT, we recommend the patient be considered of combined endocardial/epicardial mapping approach.
2.3 Elevated risk of endocardial mapping
In some cases, endocardial mapping may be high-risk and may lead us to consider the more aggressive approach of epicardial-only mapping. For example, endocardial mapping may not be feasible for VT ablation in the presence of a thrombus in the left ventricle(LV). VT ablation in the setting of LV thrombus is challenging because endocardial catheter manipulation or internal/external cardioversion of hemodynamically untolerated VT can potentially dislodge or fragment the thrombus, resulting in embolism or stroke. While we have demonstrated that in some instances(laminated chronic thrombi) endocardial VT ablation can be safely performed in the setting of LV thrombus[2]by avoiding catheter manipulation in the LV apex, this should only be considered in highly experienced centers and in very specific clinical circumstances. Generally, medical therapy, an upfront epicardial-only approach or bilateral stellate ganglionectomy should be considered prior to undertaking a higher-risk endocardial procedure in this population.
2.4 Other reasons to consider epicardial mapping
ECG criteria for epicardial exit is often utilized to determine the mapping approach. A number of ECG criteria have been published that suggest epicardial exit, most of which rely on assessment of the intial component of the QRS complex. Unfortunately, the frequent use of antiarrhythmics(in particular amiodarone) and the frequent T-wave-QRS fusion during more rapid VTs, often make assessment of this component of the QRS very inaccurate or misleading. Therefore, we have relied less on this criteria in recent years.
Previous failed ablation is a common reason for referral for epicardial ablation at experienced centers. This is often a very appropriate reason for referral. However, we have to reassess such patients on an individual basis to determine if the previous failed ablation is a technical failure, meaning endocardial ablation simply could not get to the critical region of the VT, or potentially a misinterpretation of the clinical data. For instance, if a VT ablation fails because the origin is from a structure which is difficult to reach and ablate such as a papillary muscle or certain regions of the LV summit, the endocardial ablation may have been unsuccessful, yet epicardial mapping is unlikely to provide additional benefit, and a repeat endocardial ablation is the appropriate next step.
3 Strategies to optimize mapping
3.1 Timing of epicardial access/mapping
There are really two approaches for the timing of epicardial access/mapping. In our center, we perform upfront epicardial mapping(epicardial access is obtained at the beginning of the case) for all NICM cases and consider the necessity of epicardial mapping on a case-by-case basis for ICM patients. Other centers often rely on the endocaridal findings to determine whether they will pursue epicardial mapping(access obtained later in the case after anticoagulation is reversed with protamine). Given our centers experience, we feel obtaining access early provides more data and minimizes fatigue-related risk that might be associated with epicardial access obtaining after a number of hours into a case.
3.2 Epicardial access approaches
After making the decision that epicardial access is warranted, the next question is which anatomic approach to take, anterior or posterior? In our center, we predominantly take the posterior approach, but many centers are using the anterior approach. We use the Tuouy needle shown in Fig.2, however a lot of centers have moved to use micropuncture needles in hopes of decreasing complication rates from inadvertent right ventricular punctures. The limitation of micropuncture is that the needle often does not give the tactile feedback desired to feel cardiac pulsations that the Tuouy needle provides, and the length of the needle may be an issue in larger patients. A number of new technologies are being developed to help less experienced operators perform epicardial access, but data on how these technologies will decrease complication rates when used by less experienced operators are limited at this time.

Blue arrow=posterior approach; orange arrow=anterior approach
When our trainees perform epicardial puncture for the first time, I suggest they do a right ventricular angiogram(RV gram)(Fig 3). While it may be unnecessary for an experienced operator, it can help delineate cardiac borders. Once we have a roadmap of the cardiac borders, we can have a clearer idea of when the needle is nearing the pericardium. Once the needle is

Fig.3 RAO(A) and LAO(B) views of RV gram prior to epicardial access
advanced near the cardiac border, a small amount of contrast can be injected and ultimately tenting the pericardium(Fig.4) can be verified. Once the pericardium is punctured, a J-wire is advanced and it should be clear in multiple fluoroscopic views that the wire is going around the heart boarder as apposed to within the structure of a cardiac chamber(Fig.5). Once epicardial access is obtained it is our practice to perform a pericardiogram(Fig.6) to ensure there is no adhesion that may cause increased risk of bleeding or limit catheter movement.
4 Optimization of mapping methods
4.1 Multipolar mapping
Multipolar high-density mapping is essential. An example of a duodecapolar catheter with an inter-electrode distance of 2-2-2 mm(St. Jude Medical, MN, USA) utilized for endocardial and epicardial mapping is shown in Fig.7. In this case we performed both endocardial transseptal, as well as epicardial mapping of a transmural apical scar.

Fig.4 Puncture needle tenting the pericardiumin the RAO view
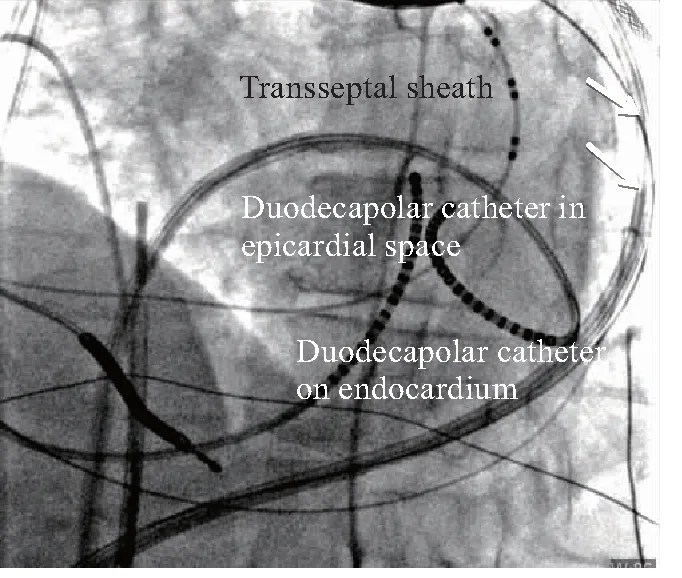
LAO=left anterior oblique; RA(RV) lead=right atrial (ventricular) lead of dual-chamber ICD; CS=coronary sinus

Fig.6 Pericardiogram performed after epicardial accessto demonstrate successful access and ensure nopericardial adhesions present
The advantages of multipolar mapping are that rapid substrate delineation and a higher density of mapping can be obtained. By utilizing this technique we can perform entrainment mapping, pace-mapping and potentially target diastolic potentials rapidly.
4.2 Limitations of scar homogenization on the epicardium
In the VT field we have moved toward the idea of “scar homogenization” as a goal for substrate-based ablation. However, as was shown by Tung et al[3]in a porcine model, mapping results are determined by the inter-electrode distance. Using a mapping catheter with closely spaced electrodes, we can see high-density local signals. However, those high-density catheters may miss distant mid-myocardium substrates due to insufficient depth of sensing. With an ablation catheter or less closely spaced electrodes, we may observe more distant signals but miss some of the local signals. True “scar homogenization” would require us to perform high-density mapping with closely spaced catheter and then perform a full re-map with the same catheter after ablation. However, most centers are generally performing high-density mapping before ablation, but cannot re-map after ablation due to time-limitations. Therefore, we have to be cognizant that we are not really preforming “scar homogenization” in the truest sense.
In addition, the risk of coronary artery damage in large regions of the epicardium makes the current concept of scar homogenization unrealistic. Therefore, we must find techniques to minimize the area of ablation required on the epicardium to have a successful result.

Fig.7 Closely spaced Duodecapolar catheter mappingboth in the epicardiun and endocardiumt
4.3 Changing the wavefront of activation
A useful technique to better understand VT substrates is changing the wavefront of activation[4]. NICM patients often have small patchy scars and limited substrate for ablation. If we change the pacing wavefront from which we are mapping, additional substrates may be evident which we did not see with original single wavefront. For example, LV pacing may demonstrate additional substrates which were not seen with RV pacing. In the study by Tung et al[4]. if a substrate was mapped in a single wavefront, 20% of the critical sites would be missed. This data suggests that when we are confronted with difficulties in finding critical substrates, it may help to change the wavefront of activation.
4.4 Isochronal late activation mapping
An additional technique we have used is “isochronal late activation mapping”(ILAM). In the study by Irie et al[5], constructing isochronal late activation maps and targeting slow conduction regions propagating into the latest zones of activation showed promise as a strategy for substrate modification with limited ablation.
Fig.8 shows an epicardial voltage map of a patient where the entire epicardium is scar(grey). If we were to attempt a substrate-based approach of ablating on all abnormal signals it would not be possible due to time limitations and the risk of coronary damage. However, when we use ILAM, the latest activation regions and slow conduction were demonstrated in a small region(Fig. 9) on the anterior apical epicardium. This site correlated with where we demonstrated clear late potentials, reasonable pacemaps and diastolic activity during VT(Fig.10). A single ablation lesion at this site terminated VT and we were able to consolidate the small region of the epicardium without having to undertake full substrate modifications of the entire epicardium.
4.5 Using the right equipment to optimize lesion delivery
When we are considering epicardial ablation, experience and lessons from previous studies must be kept in mind. First, irrigated ablation is essential. Work from Dr. André d’ Avila’s group demonstrates that in areas covered by epicardial fat standard non-irrigated radiofrequency(RF) energy did not generate any appreciable lesions, however cooled-tip RF energy did[6].

Fig.8 Epicardial voltage map demonstrating diffusescar and no clear target for ablation
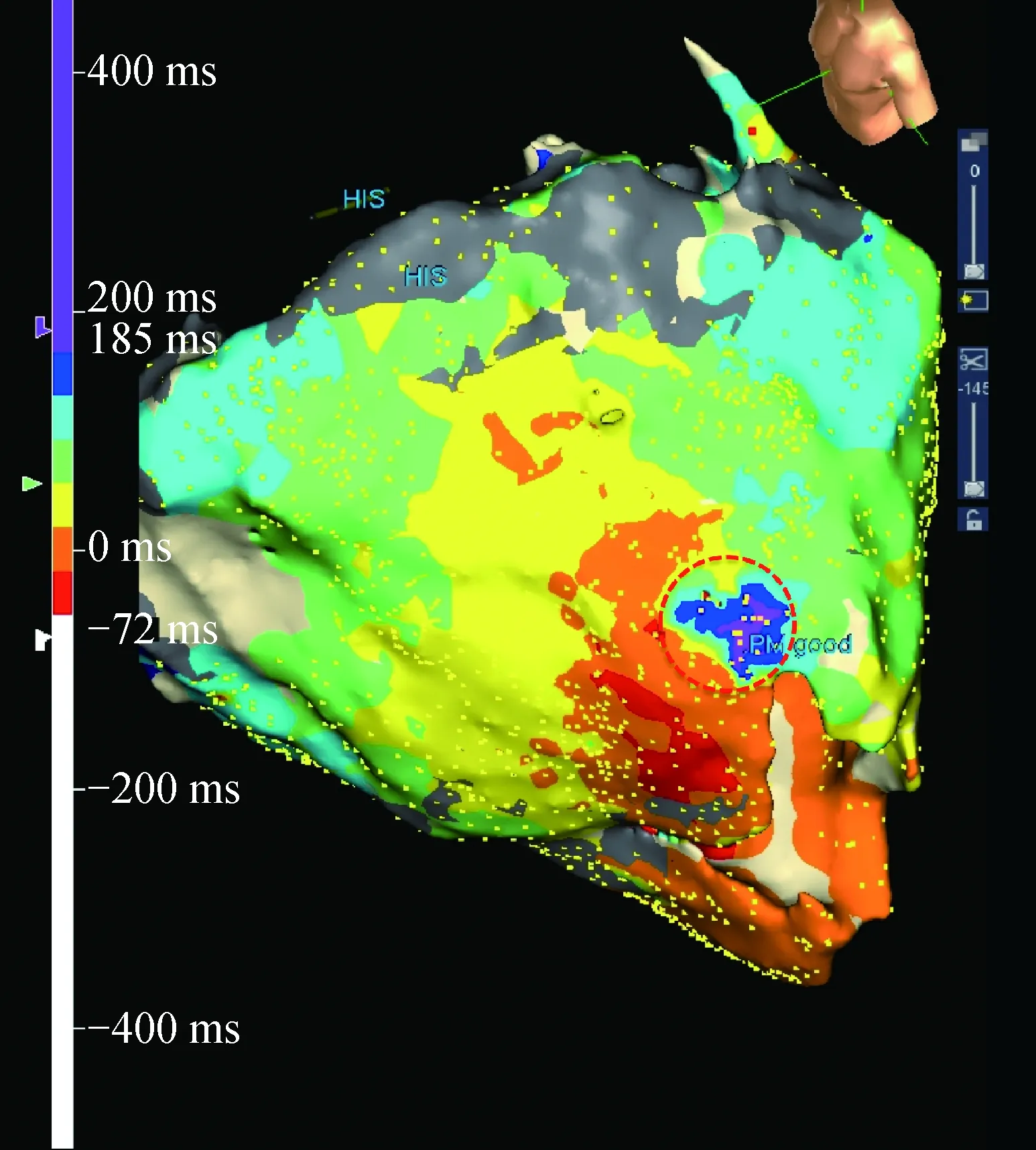
Fig.9 ILAM demonstrating a small area of slowconduction in antero-apical epicardial LV(red circle)
The second thing to remember is that too much pericardial fluid in the pericardial space from the irrigating catheter can interfere with appropriate lesion delivery. Therefore, draining the pericardium intermittently between ablation lesions sets is also highly important. Aryana et al[7]indicated that although there was no discernible difference in lesion size with irrigation flow rate(IFR), in the presence of intrapericardial fluid, the surface lesion diameter, maximal diameter, depth and volume all varied at different IFRs.

VT=ventricular tachycardia; LP= late potential; PM= pacemap
4.6 Contact force-sensing catheters
Contact force(CF) catheter are useful in the endocardium, but can be deceiving when used on the epicardium. High CF values may actually be associated with poor contact with the epicardial surface itself as the catheter may actually be pushing onto the pericardium and not the cardiac tissue. Adequate CF value may be difficult to obtain on the epicardium. Jesel et al[8]reported that high CF value epicardially is associated with poor catheter orientation. CF measurements should therefore be interpreted with caution in the pericardium.
5 How to prevent collateral damage
There are a number of potential risks of epicardial access including but not limited to: RV perforation, pericardial bleeding, liver injury, abdominal bleeding and entry into the pleural space. However, there are a number of ways to minimize/prevent these complications.
5.1 Bleeding risk of adhesions
Pericardial adhesions are infrequently seen. When they are seen they can potentially be broken up with manipulation of the mapping/ablation catheter and sheath. However, lysis of adhesions is associated with increased bleeding risk. Areas of adhesions should be avoided if possible. If lysis of adhesions is considered, it should only be done with available surgical back-up, if bleeding occurs that cannot be controlled.
5.2 Avoiding un-needed procedures
Anatomic understanding helps us know when percutaneous epicardial mapping may not be the solution for a given premature ventricular contration(PVC)/VT. For example, certain arrhythmias originating from the left ventricular summit are often not accessible from a percutaneous epicardial approach due to proximity to the coronary arteries. If the origin is identified before or early in the procedure based on data available, the operator may avoid an unnecessary epicardial puncture.
5.3 Avoiding coronary injury
Initial epicardial access should avoid the basal aspects of the RV, as puncture in this region is more likely to damage the right coronary artery or its branches. In addition, significant regions of potential ablation may be limited by coronary artery anatomy(Fig.11). Prior to any epicaridal ablation a coronary angiogram should be obtained to ensure a safe distance from the coronary. When ablating on the inferior wall, both the right and left coronary systems should be evaluated, as variable coronary dominance may be present in a given patient.
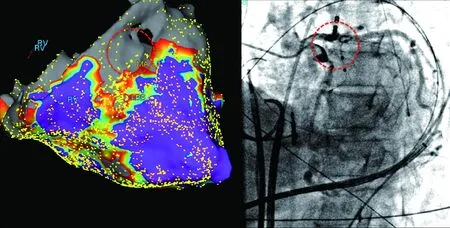
Fig.11 Epicardial voltage map and associated coronary angiography demonstrating that the site of potentialablation is high-risk for damage to the left main coronary artery
5.4 Avoiding phrenic nerve damage
The phrenic nerve runs in the region of the lateral left ventricular wall. Fig.12 shows an example of a patient with non-compaction cardiomyopathy and VT where essential lateral wall ablation lesions could not be delivered due to phrenic nerve capture with high-output pacing in that area. If this occurs a balloon can be utilized to protect the phrenic nerve.
6 Idiopathic epicardial ventricular tachycardia
Epicardial VT ablation is not always from scar-related reentry in the setting of structural heart disease. Fig.13 demonstrates a case of a patient with idiopathic VT that was incessant and only terminated with two intravenous medications. The morphology of PVC matched with that of VT at the time of ablation and therefore the PVC was mapped. The QRS pattern in the inferior leads suggests the possibility of an epicardial origin. We mapped both RV and LV endocardially without finding early signals. Epicardial mapping of the crux of the heart is shown utilizing a closely spaced duodecapolar catheter(Fig.14).

Fig.12 Phrenic nerve protection using an epicardial balloon
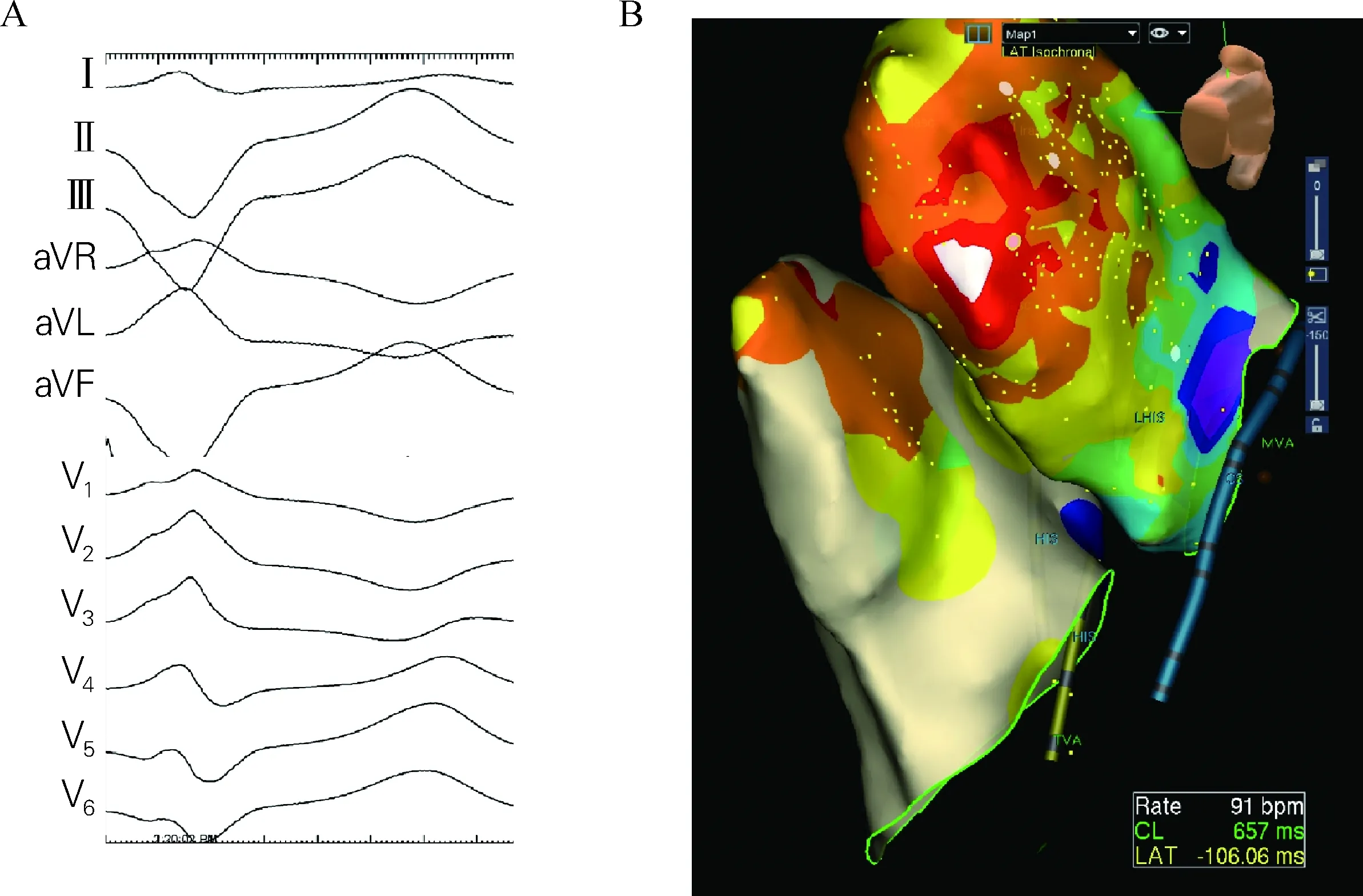
Fig.13 12-lead ECG of PVC/VT morphology(A) and endocardial map of left andright ventricle with diffusely distributed earliest activation sites(B)
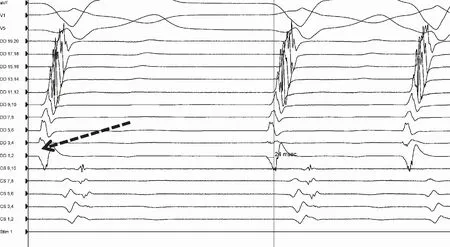
Fig.14 Epicardial mapping with closely spaced mappingmultipolar catheter in LAO view
In this case a diversion of bipolars can be observed in Fig.15, suggesting origin from that early site between the two bipoles. The local signal was significantly early relative to the QRS complex(Fig.16). With one radiofrequency lesion at 30 watt on the epicardium, the PVCs and VT were no longer inducible.
7 Conclusion
Epicardial mapping and ablation has an essential role in the management of VT. Optimizing success and minimize complications associated with this procedure requires extensive training in epicardial access and mapping techniques.
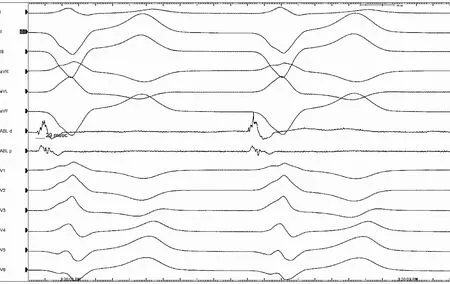
Fig.15 Multipolar mapping demonstrating reversal of polarityon the duodecapolar catheter

Fig.16 Early epicardial ablation signal
[1] Lee-Felker SA, Thomas M, Felker ER, et al. Value of cardiac MRI for evaluation of chronic Chagas disease cardiomyopathy[J]. Clin Radiol, 2016, 71(6):e1-e7.
[2] Rao HB, Yu R, Chitnis N, et al. Ventricular tachycardia ablation in the presence of left ventricular thrombus: safety and efficacy[J].J Cardiovasc Electrophysiol, 2016, 27(4):453-459.
[3] Tung R, Kim S, Yagishita D, et al. Scar voltage threshold determination using ex vivo magnetic resonance imaging integration in a porcine infarct model: influence of interelectrode distances and three-dimensional spatial effects of scar[J]. Heart Rhythm, 2016, 13(10):1993-2002.
[4] Tung R, Josephson ME, Bradfield JS, et al. Directional influences of ventricular activation on myocardial scar characterization:voltage mapping with multiple wavefronts during ventricular tachycardia ablation[J]. Circ Arrhythm Electrophysiol, 2016, 9(8):e1-e14.
[5] Irie T, Yu R, Bradfield JS, et al. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia:systematic analysis of isochronal late activation mapping[J].Circ Arrhythm Electrophysiol, 2015, 8(2):390-399.
[6] d’Avila A, Houghtaling C, Gutierrez P, et al. Catheter ablation of ventricular epicardial tissue:a comparison of standard and cooled-tip radiofrequency energy[J]. Circulation, 2004, 109(19):2363-2369.
[7] Aryana A, O’ Neill PG, Pujara DK, et al. Impact of irrigation flow rate and intrapericardial fluid on cooled-tip epicardial radiofrequency ablation[J]. Heart Rhythm, 2016, 13(8):1602-1611.
[8] Jesel L, Sacher F, Komatsu Y, et al. Characterization of contact force during endocardial and epicardial ventricular mapping[J]. Circ Arrhythm Electrophysiol, 2014, 7(6):1168-1173.
10.13308/j.issn.2095-9354.2017.04.002
Author Unit: Cardiac Arrhythmia Center, University of California at Los Angeles, Los Angeles, California, 90095
Author Brief Introduction: Jason S Bradfield, MD, FACC, FHRS, Director, Specialized Program for Ventricular Tachycardia, assistant professor of medicine; research interests: catheter ablation.
