A novel triple immunoenzyme staining enables simultaneous identif i cation of all muscle fi ber types on a single skeletal muscle cryosection from normal, denervated or reinnervated rats
2017-09-04PengWuShuyaZhangRobertSpinnerMichaelTorresLizardiYudongGuCongYuMichaelYaszemskiAnthonyWindebankHuanWang
Peng Wu, Shuya Zhang, Robert J. Spinner Michael Torres Lizardi, Yudong Gu, Cong Yu, Michael J. Yaszemski, Anthony J. Windebank Huan Wang*
1 Department of Neurological Surgery, Mayo Clinic, Rochester, MN, USA
2 Department of Hand Surgery, Huashan Hospital, Fudan University, Shanghai, China
3 Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China
4 Department of Neurology, Mayo Clinic, Rochester, MN, USA
5 Department of Medicine, New York Presbyterian Weill Cornell Medical Center, New York, NY, USA
6 Shanghai Key Laboratory of Peripheral Nerve and Microsurgery, Shanghai, China
7 Departments of Orthopedic Surgery and Biomedical Engineering, Mayo Clinic, Rochester, MN, USA
A novel triple immunoenzyme staining enables simultaneous identif i cation of all muscle fi ber types on a single skeletal muscle cryosection from normal, denervated or reinnervated rats
Peng Wu1,2,3,#, Shuya Zhang4,#, Robert J. Spinner1, Michael Torres Lizardi5, Yudong Gu2,6, Cong Yu2,6, Michael J. Yaszemski7, Anthony J. Windebank4, Huan Wang1,*
1 Department of Neurological Surgery, Mayo Clinic, Rochester, MN, USA
2 Department of Hand Surgery, Huashan Hospital, Fudan University, Shanghai, China
3 Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China
4 Department of Neurology, Mayo Clinic, Rochester, MN, USA
5 Department of Medicine, New York Presbyterian Weill Cornell Medical Center, New York, NY, USA
6 Shanghai Key Laboratory of Peripheral Nerve and Microsurgery, Shanghai, China
7 Departments of Orthopedic Surgery and Biomedical Engineering, Mayo Clinic, Rochester, MN, USA
How to cite this article:Wu P, Zhang S, Spinner RJ, Lizardi MT, Gu Y, Yu C, Yaszemski MJ, Windebank AJ, Wang H (2017) A novel triple immunoenzyme staining enables simultaneous identif i cation of all muscle fi ber types on a single skeletal muscle cryosection from normal, denervated or reinnervated rats. Neural Regen Res 12(8):1357-1364.
Triple immunofluorescence staining has recently been developed to simultaneously identify all muscle fi bers on a single cryosection which is helpful for clinical and basic research, but it has disadvantages such as fast photobleaching and unclear outlines of muscle fi bers. Triple immunoenzyme staining (TIE) is likely to avoid these disadvantages. In this study, we aimed to establish a sensitive and specif i c TIE technique to identify fi ber types in normal, denervated, and reinnervated rat muscles, and to develop a systematic sampling method for muscle fi ber quantif i cation. Tibialis anterior and soleus from normal, denervated, and reinnervated Lewis rat hind limbs were used. Five consecutive cryosections were cut from each muscle, including one for TIE and four for single immunoenzyme staining (SIE).e TIE was performed using the polymerized reporter enzyme staining system for the first two antigens (A4.74 for MyHC-IIA, BA-F8 for MyHC-I) and alkaline phosphatase staining system for the third antigen (BF-F3 for MyHC-IIB), followed by corresponding detective systems and respective chromogens.e type of muscle fi bers was quantif i ed by systematic sampling at 12.5%, 25%, 33% and 50% of all muscle fi bers, and was compared with that acquired from counting all the fi bers (100%). All muscle fi ber phenotypes, including pure and hybrid, could be simultaneously identif i ed on a single TIE cryosection with clear outlines.e fi ber types on TIE slides matched well with their respective counterpart on the consecutive SIE slides with a 95% match rate. Systematic sampling of 12.5% fi bers could represent the true fi ber type distribution of the entire muscle section. Our results suggest that novel TIE can ef f ectively visualize fi ber types in normal, denervated or reinnervated rat muscles.
nerve regeneration; muscle fiber phenotyping; immunohistochemistry; triple immunoenzyme staining; myosin heavy chain; rats; neural regeneration
Introduction
Skeletal muscles of adult rats are composed of four types of pure muscle fi bers including type I, IIA, IIB, IIX, and several types of hybrid muscle fi bers such as type I/IIA, IIA/IIX and IIB/IIX (Rivero et al., 1998; Smerdu and Soukup, 2008; Tulloch et al., 2011; McMillan and Quadrilatero, 2011). Each muscle fi ber type is characterized by a specif i c myosin heavy chain isoform (MyHC-I, IIA, IIB, IIX, I/IIA, IIA/IIX and IIB/IIX, respectively) (Lucas et al., 2000), contraction speed, metabolic and oxidative properties (Rivero et al., 1998).e fastest muscle fi bers (type IIB) mainly locate at the superf icial part while the slowest fi bers (type I) mainly locate at the deep part of tibialis anterior (Armstrong and Phelps, 1984). The distribution and proportion of muscle fibers change extensively aer exercise, disuse, denervation, and reinnervation (Michel et al., 1996; Bigard et al., 1997; Bobinac et al.,2000; Jergovic et al., 2001; Raheem et al., 2010).erefore, muscle fi ber phenotyping is of paramount importance to understand the muscle states in a wide range of fi elds including neurology, neurosurgery, orthopedics, sports medicine and aging researches (Meunier et al., 2010), and also to detect the extent of denervation or regeneration of muscle fi bers.
Myof i brillar adenosine triphosphatase (mATPase) and immunohistochemical staining are universally used to identify muscle fi ber phenotypes. mATPase staining has been wildly used for over 40 years (Guth and Samaha, 1969). It has some limitations: is sensitive to pH value and temperature change; weakens over time, cannot specifically stain capillaries; is hard to work on severely atrophic and hybrid muscle fi bers (Jergovic et al., 2001). Immunohistochemical staining can avoid the limitations of mATPase staining. Single immunostaining with specif i c antibody can recognize one pure muscle fi ber type. Monoclonal antibodies including anti-myosin BAF8, A4.74, BF-F3 and BF-35 have been described in detail in rats (Schiaf fi no et al., 1989; Rivero et al., 1998; Bobinac et al., 2000; Smerdu and Soukup, 2008; McMillan and Quadrilatero, 2011).ey are specif i cally immunoreactive with type I (Michel et al., 1996; Bobinac et al., 2000), type IIA (Schiaf fi no et al., 1989; Smerdu and Soukup, 2008; Soukup et al., 2009), type IIB (Schiaf fi no et al., 1989; Rivero et al., 1998; Smerdu and Soukup, 2008), all but type IIX fi bers (Schiaf fino et al., 1989; Rivero et al., 1998; Smerdu and Soukup, 2008), respectively. Serial consecutive sections are needed to identify all muscle fi bers by single immunostaining, which is laborious.
It is more complicated for immunohistochemical staining to identify hybrid fi bers which contain multiple MyHC isoforms, whose staining properties are intermediate and largely depend on the inner proportion of various MyHC isoforms (Tulloch et al., 2011). There are only rare hybrid fibers in normal muscles, but abundant in denervated and reinnervated muscles, due to the conversion of muscle fi bers from one type to another aer denervation and reinnervation (Gorza, 1990; Tulloch et al., 2011).erefore, identif i cation of muscle fi ber phenotypes in denervated and regenerated muscles is much more complicated than that in normal muscles.
Multiple immunohistochemical staining was first described by Nakane (1968) and has been universally used when investigators need to demonstrate multiple antigens simultaneously on a single section (Claassen et al., 1986; van der Loos et al., 1987; Gorza, 1990; Rivero et al., 1998; Smerdu and Soukup, 2008). Whereas triple immunostaining for muscle fi ber phenotyping was not established until recently because of the complexity of muscle fi ber phenotyping and coexistence of various muscle fiber types in the hybrid fibers. Triple immunof l uorescence staining (TIF) was recently introduced by which the identification and comparison of all four types of pure muscle fi bers on a single section were possible (Tulloch et al., 2011; McMillan and Quadrilatero, 2011). Triple immunoenzyme staining (TIE) has some advantages over TIF. It shows better morphologic patterns and tissue structures. The chromogen based immunoreactivity signals would not fade for a long time, making re-analysis of the staining results possible.e immunostaining results can be analyzed under the common light microscopy instead of requiring fl uorescence microscope in the dark room. It can also potentially remedy the limitation of TIF where basement membranes of muscle fibers cannot be clearly seen, particularly when there is a cluster of type IIX adjacent to each other (McMillan and Quadrilatero, 2011).
To co-localize all muscle fi bers in a single section, a novel TIE staining method was developed in this study. We chose three primary antibodies with different subtypes of immunoglobulins (anti-myosin A4.74 IgG1 for MyHC-IIA, anti-myosin BA-F8 IgGb2 for MyHC-I, anti-myosin BF-F3 IgM for MyHC-IIB) to perform the TIE in order to avoid the cross-labeling seen in previous publications (Tulloch et al., 2011; Bloemberg and Quadrilatero, 2012).e basement membranes were clearly demarcated, while little cross-labeling was seen in our novel TIE stained sections. In order to confirm the specificity of TIE, comparison with respective single immunoenzyme staining (SIE) on consecutive frozen sections was also carried out.
Materials and Methods
Muscle specimen harvest and cryopreservation
All procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC protocol# A10611). All animal studies were performed in compliance with Association for Assessment and Accreditation of Laboratory Animal Care International and under the supervision of the Institutional Animal Care and Use Committee. Eighteen female Lewis rats, weighing 200 ± 5 g, aged 3–4 months (Harlan Laboratories, Inc., Indianapolis, IN, USA) were used in this study. Tibialis anterior and soleus muscles were harvested from the left hind limb of nine normal control rats, four rats whose lesciatic nerve had been transected at mid-thigh level for various intervals (1, 4, 8, and 12 weeks, respectively), and five rats whose left sciatic nerve was repaired with 4 to 5 intermittent 10-0 monofilament sutures (Ethilon®, Ethicon US, LLC) aer dif f erent delay intervals (0 week, 1 week, 4 weeks, 6 weeks and 8 weeks, respectively). This constituted 18 normal muscles, 8 muscles at varying stages of denervation, and 10 muscles of varying degrees of reinnervation. The harvested muscle specimen (the soleus muscle and tibialis anterior muscle) was pinned at a resting length on a piece of cork and then quickly frozen in isopentane cooled with liquid nitrogen. Frozen muscle specimens were stored in a –80°C freezer before cryosectioning and immunostaining.
Immunohistochemical staining
Frozen muscle specimens were transferred to the –20°C cryostat (Leica CM3050S; Leica Microsystems, Wetzlar, Germany) on dry ice. Each specimen was cut transversely into two parts at the midpoint of the muscle belly. One of the two parts was mounted on the cryostat such that fi ve 10 µm thick consecutive crosssections were cut from the midpoint of the muscle. The cryosections were then fixed in precooled absolute acetone at 4°C for 5 minutes. Four cryosections wereused for SIE with mouse monoclonal primary antibodies anti-myosin A4.74 IgG1, anti-myosin BA-F8 IgG2b, anti-myosin BF-F3 IgM and anti-myosin BF-F35 IgG1, respectively (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA). One cryosection was used for TIE with three aforementioned primary antibodies against A4.74, BA-F8 and BF-F3.
For SIE, cryosection was incubated with the primary antibody (5 µg/mL) for 1 hour at room temperature. After washing, the slide with A4.74, BA-F8, or BF-F35 primary antibody was incubated in the polymerized peroxidase conjugated horse anti-mouse secondary antibody which was included in the ImmPRESS REAGENT Anti-Mouse IgG kit (Vector Lab, Burlingame, CA, USA) without dilution as per manufacturer’s instruction and then in respective detection kit (Vector Lab, Burlingame, CA, USA) as follows: DAB (3,3′-diaminobenzidine) (brown) for A4.74 and BFF35, SG kit (grey) for BA-F8.e slide with BF-F3 primary antibody was incubated in the goat anti-mouse secondary antibody from Alkaline Phosphatase labeled anti-mouse IgM kit (Jackson ImmunoResearch Inc, West Grove, PA, USA) diluted at 1:500 with a fi nal working concentration of 1.6 µg/mL and visualized using the Vulcan fast red chromogen kit 2 (red) (Biocare Medical, Concord, CA, USA). All staining procedures using the detection kits were performed according to manufacture instructions. TIE was performed using the ImmPRESS polymerized reporter enzyme staining system for the first two antibodies (A4.74 and BA-F8) and alkaline phosphatase staining system for the third antibody (BF-F3) as per manufacture instructions. After fixation in acetone, the sections were fi rst incubated with monoclonal anti-myosin A4.74 IgG1 primary antibody (5 µg/mL) for 1 hour.e sections were incubated with secondary antibody for 30 minutes and then treated with DAB substrate which produces a dark brown reaction. After PBS washes, the sections were incubated with the second primary antibody anti-myosin BA-F8 IgG2b (5 µg/mL) for 1 hour. Then the sections were incubated with secondary antibody for 30 minutes and treated with an SG detection kit which produces a grey reaction.e sections were then incubated with the third primary antibody, anti-myosin BF-F3 IgM (5 µg/mL) for 1 hour, alkaline phosphatase labeled anti-mouse IgM secondary antibody for 1 hour and treated with Vulcan fast red chromogen kit 2 that produces a red reaction. All the staining steps were done at room temperature. Subsequently, the sections were mounted and stored at room temperature for future imaging and analysis. The antibodies and detection reagents are summarized in Table 1.
In some SIE slides, hematoxylin staining (nuclear counterstaining) or laminin immunostaining (cell membrane counterstaining) were performed after muscle fiber immunoenzyme staining to improve visibility.
Imaging and quantif i cation
All the slides were scanned using a Nanozoomer Digital Pathology 2.0 HT scanner (Hamamatsu Inc, Houston, TX, USA) at 20× magnif i cation to acquire images of the muscle cross-sections.e distribution, relative position and morphology of various muscle fibers on the TIE section were compared with those on the four corresponding consecutive SIE sections to determine the validity of TIE.
For quantitation of the proportion of each fi ber type on a given TIE section, WebSlide Enterprise software was used.e scanned image was divided into grids.en the grid of interest was enlarged and imported to Image J soware for each fi ber type to be manually tallied.e number of fi bers stained positive for a given fiber phenotype was the sum of the tally of that fi ber type acquired from all the sampled grids.is number was then divided by the total number of fi bers tallied regardless of phenotype and determined as the proportion (percentage) of that given fiber phenotype. For each TIE stained section, muscle fibers from all the grids were tallied fi rst for the various fi ber phenotypes and their respective proportions were calculated and considered as true values. Then we systematically sampled every other, every third, every fourth and every eighth grid (50%, 33%, 25% and 12.5% sampling frequency, respectively), tallied and calculated the fi ber phenotype proportions.e proportions of each muscle fiber type acquired at 50%, 33%, 25% and 12.5% sampling frequencies were divided by the true values (acquired at 100%).e ratio was recorded as relative rate.e closer was the relative rate to 1.0, the truer was the statement that results obtained at that sampling frequency ref l ect the whole picture.
Statistical analysis
Results
TIE for rat muscle fi bers
All muscle fiber types including pure and hybrid fibers, could be identified in our novel TIE sections of normal, denervated or reinnervated tibialis anterior and soleus muscles.e TIE results of tibialis anterior are shown in Figure 1a (normal tibialis anterior), Figure 2a (1-week denervated tibialis anterior), Figure 3a (8-week denervated tibialis anterior), Figure 4a (12-week denervated tibialis anterior) and Figure 5a (reinnervated tibialis anterior).eir corresponding four consecutive SIE results are shown in panels b–e of each fi gure.
Type I, IIA, IIB, IIX fibers were distinguishable as dark grey, dark brown, dark red, unstained or very slight brown staining, respectively, in TIE sections of tibialis anterior (Figures 1a, 2a, 3a, 4a, 5a). Type I and IIA fi bers were distinguishable as dark grey and dark brown staining, respectively, in TIE sections of soleus. Hybrid type IIA/IIX and IIB/IIX fi bers were distinguishable as mild to moderate brown staining and mild to moderate red staining, respectively, in TIEsections of tibialis anterior (Figures 1a, 2a, 3a, 4a, 5a). Hybrid type IIC (I/IIA) fi bers could be distinguishable as mild to moderate brown staining, or as mild to moderate grey staining in tibialis anterior (Figure 5a).
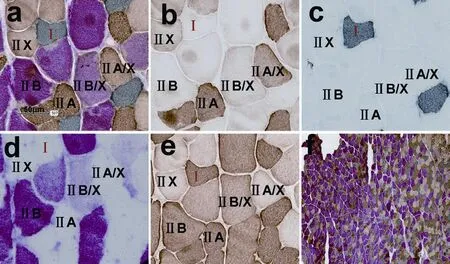
Figure 1 Triple immunoenzyme staining (TIE) and four corresponding single immunoenzyme staining (SIE) results in a normal tibialis anterior muscle.
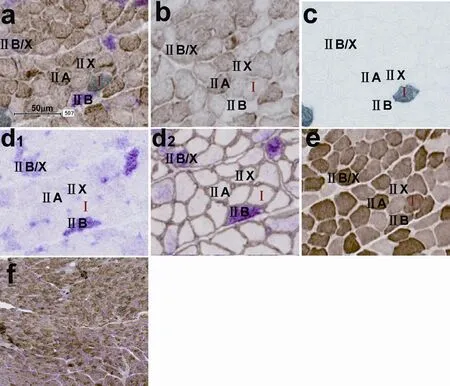
Figure 3 Triple immunoenzyme staining (TIE) and four corresponding single immunoenzyme staining (SIE) results in a tibialis anterior muscle subjected to 8-week denervation.

Figure 2 Triple immunoenzyme staining (TIE) and four corresponding single immunoenzyme staining (SIE) results in a tibialis anterior muscle subjected to 1 week denervation.
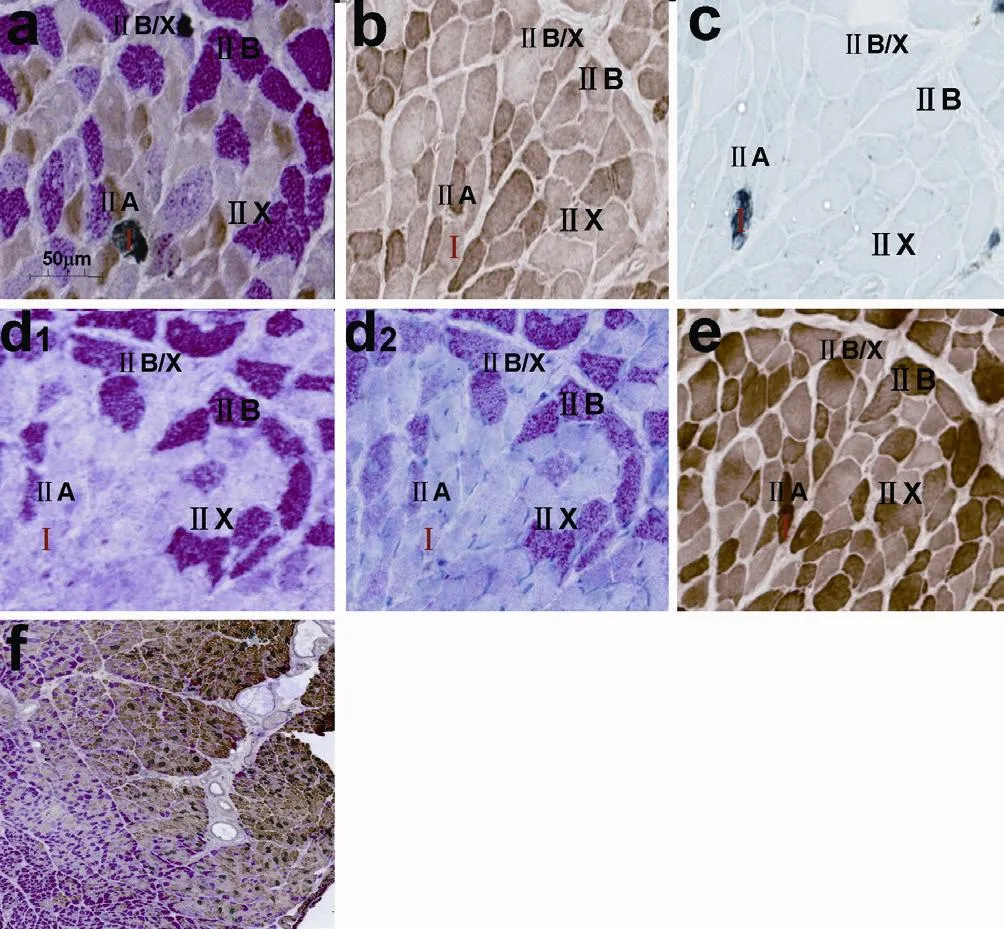
Figure 4 Triple immunoenzyme staining (TIE) and four corresponding single immunoenzyme staining (SIE) of a tibialis anterior muscle that was denervated for 12 weeks.

Table 1 Antibodies and detection systems used in the single and triple immunoenzyme staining
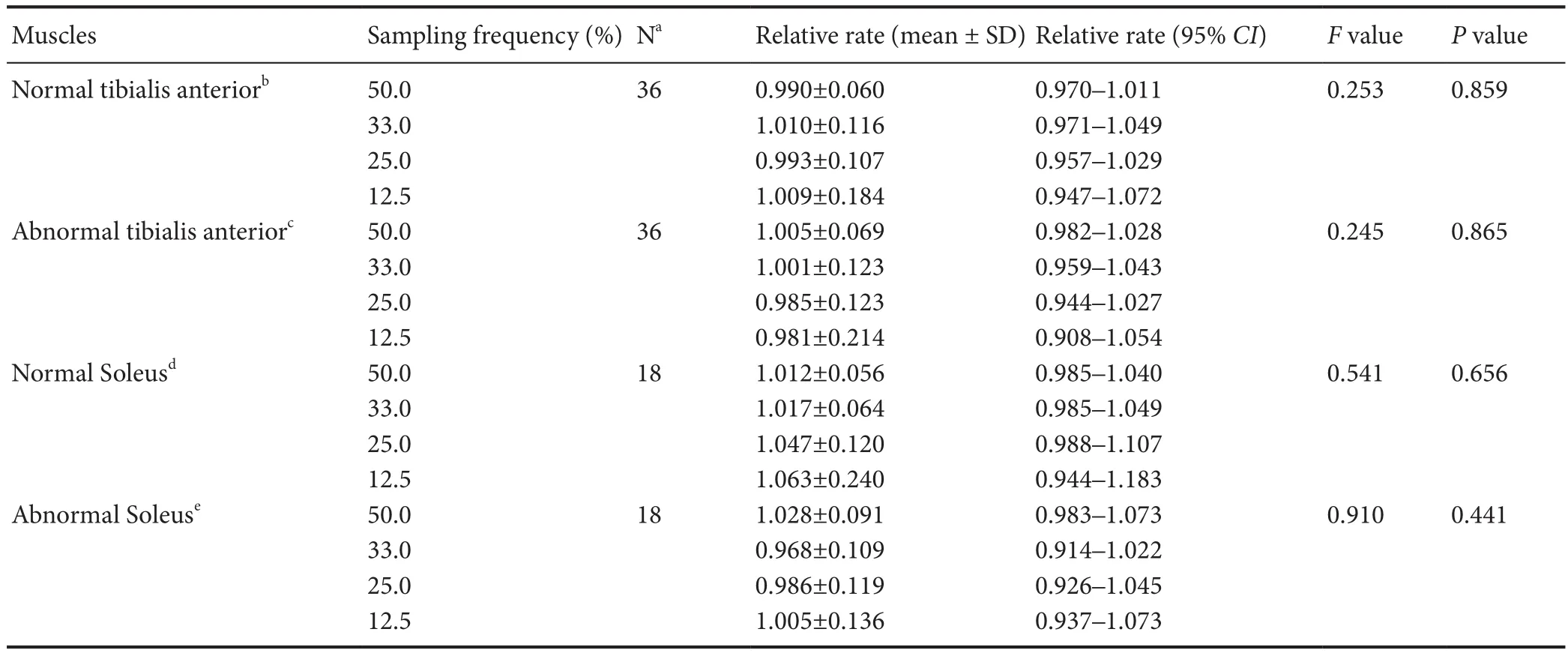
Table 2 Establishment of systematic sampling for muscle fi ber phenotyping quantif i cations

Figure 5 Triple immunoenzyme staining (TIE) and four corresponding single immunoenzyme staining (SIE) results in a reinnervated tibialis anterior muscle (16 weeks aer 4-week delayed nerve repair).
TIE specif i city
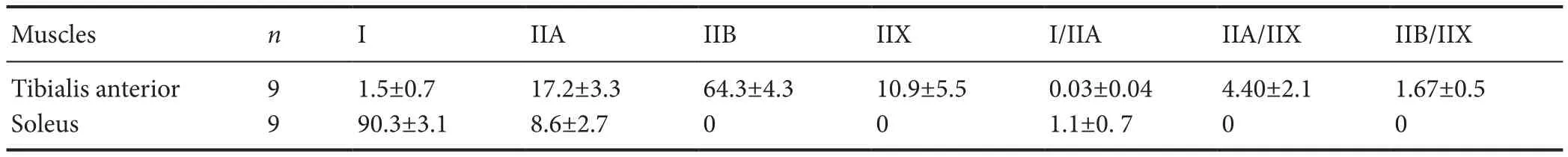
Table 3 Percentages (%) of various muscle fi ber types in normal tibialis anterior and soleus muscles in Lewis rats
Systematic sampling
We found that the relative rates at the varying sampling frequencies for both normal and abnormal tibialis anterior and soleus muscles were very close to 1.0. No statistically signif i cant dif f erences were detected for the relative rates of all muscle fi ber types among the 50%, 33%, 25% and 12.5% sampling frequencies (Table 2).
Systematic sampling of 12.5% of the entire muscle section represented the truth (100%) as well as the other three higher sampling frequencies did. A sampling frequency of 12.5% was hence determined for future studies of muscle fiber phenotyping quantif i cation, making this task less labor intensive.
Muscle fi bers in normal, denervated or reinnervated rat muscles
We found that the majority of the fastest fibers (type IIB) were located in the superficial part, while the majority of the slowest fibers (type I) were located in the deep part of the normal tibialis anterior muscle. After denervation and reinnervation of tibialis anterior muscle, the superf i cial fast fi ber area decreased and the deep slow fi ber area increased. Normal tibialis anterior muscle (fast fi ber dominant muscle) was mainly constituted of type II (type IIB, type IIX and type IIA) muscle fi ber types , a few type I muscle fi bers and rarely hybrid fibers (type I/IIA, IIA/IIX, IIB/IIX). Normal soleus (slow fi ber dominant muscle) was mostly constituted of type I muscle fi ber, a few type IIA muscle fi ber and rarely hybrid (type I/IIA) muscle fibers without type IIB and type IIX muscle fi bers (Table 3).
Discussion
A novel, reproducible, sensitive and specific TIE staining method that enables simultaneous identif i cation of all muscle fi ber phenotypes on a single cryosection of normal, denervated or reinnervated rat muscle has been successfully established. A systematic sampling method for muscle fi ber phenotype quantif i cation has also been verif i ed. Systematic sampling of 12.5% of the entire muscle cross section was found to be enough to accurately represent the true fiber phenotypes of the normal, denervated or reinnervated tibialis anterior and soleus muscles. The specimens that were analyzed comprised normal muscles, muscles of varying degrees of denervation atrophy, and muscles at varying stages of reinnervation. They represented a wide range of muscle fiber phenotyping conditions. Therefore, this systematic sampling method is suitable for analyzing not only normal rat muscles, but also muscles denervated or reinnervated at dif f erent extents.
The proportion of muscle fibers in normal, denervated or reinnervated muscles in our study is consistent with the fi nding from previous studies.e proportion of each muscle fi ber type was uniform with a small standard deviation in normal tibialis anterior and soleus muscles from nine rats (Table 3) which ref l ected the specif i city and reliability of this novel TIE staining method.e average proportion of type I and type IIA fi bers was 90.3% and 8.6% respectively, in the normal soleus muscle in our study, comparable to the data, 87–100% and 0–13%, respectively, reported in a study by Novak et al. (2010).e average proportion of type I, type IIA, type IIB and type IIX muscle fibers was 1.5%, 17.2%, 64.3% and 10.9%, respectively, in the normal tibialis anterior muscles in our study. These numbers were also consistent with those reported in other studies (Laughlin and Armstrong, 1982; Armstrong and Phelps, 1984; Gorza, 1990). In the denervated and reinnervated tibialis anterior muscles, the proportion of the fastest fi bers (type IIB) decreased dramatically, whereas that of slower fi bers (type I, type IIA, type IIX) obviously increased. With the total number of muscle fibers being unchanged, this finding suggested the conversion of faster fi bers to slower ones which is also consistent with the fi ndings from previous studies (Pette and Vrbova, 1985; Michel et al., 1996; Bigard et al., 1997; Windisch et al., 1998; Bobinac et al., 2000; Kostrominova et al., 2005).ese confirmations added credibility to this novel TIE staining technique.
3.1 由于成人脊柱侧凸存在退变、腰腿神经痛、侧凸相对僵硬等,尤其伴有其他系统性疾病患者,如心血管疾病、糖尿病、营养不良以及骨质疏松等都会加重手术难度及风险,所以术前的充分准备和手术评估尤为重要。
One of the major challenges of multiple immunostaining is the identif i cation of hybrid muscle fi bers.e immunostaining properties of hybrid fi bers are intermediate between the corresponding pure fibers, depending on the inner proportion of each MyHC isoform in hybrid fi bers (Tulloch et al., 2011). Hybrid type IIA/IIX muscle fi bers that mildly stained with A4.74 antibody and unstained with BF-35 antibody contain mostly MyHC-IIX and a low proportion of MyHC-IIA, while those contain more proportions of MyHC-IIA were moderately stained with A4.74 (Smerdu and Soukup, 2008). Hybrid type IIB/IIX muscle fi bers with more proportion of MyHC-IIB have stronger immunoreactions with BF-F3 antibody, resulting in darker red reaction on TIE sections (Figure 5a) and SIE sections stained with BF-F3 antibody (Figure 5d) and darker brown reaction on SIE sections with BF-F35 (Figure 5e). Hybrid type IIC (I/ IIA) muscle fi bers, whose staining property is intermediate between the muscle fi ber type I and type IIA, were stained mild to moderate brown on TIE sections of the soleus, or mild to moderate grey as shown on TIE and SIE sections ofreinnervated tibialis anterior (Figure 5a, c). Some publications maintained that type IIA and hybrid type IIA/X could not be distinguished by multiple immunostaining, because anti-myosin A4.74 antibody was reported to immunoreact with both type IIA and IIX which makes the dif f erentiation of hybrid type IIA/X from type IIA and IIX dif fi cult (Raheem et al., 2010). In our study, we found that type IIX fi bers were very slightly stained or unstained, type IIA fi bers were deeply stained, while hybrid type IIA/X fi bers were intermediately stained on TIE sections. It was possible to distinguish these three phenotypes with this novel staining method.
There are circumstances when hybrid fibers could not be well identif i ed. First, the immunoreaction magnitude of hybrid fi bers depends on the relative proportions of various MyHC isoforms. If the proportions of MyHC isoforms in hybrid fibers only differ very slightly from pure fibers, the difference in staining magnitude is not readily discernible by the naked eye. Second, cross reaction of secondary antibodies with primary antibodies may hinder identification of hybrid fi bers (Raheem et al., 2010).ird, the conversion of muscle fi ber phenotype from one type to another according to Gorza’s pattern (Gorza, 1990) after denervation or reinnervation occurs such that even the same type of hybrid fi ber has dif f erent constitutions of MyHC isoforms, making identif i cation of these hybrid fi bers much more complicated.
The key technical point of successfully established TIE method is to reduce the cross-reactions. Our protocol takes advantage of the fact that the antibodies against MyHC isoforms belong to dif f erent subtypes of immunoglobulins: anti-myosin A4.74 IgG1 for MyHC-IIA, anti-myosin BA-F8 IgG2b for MyHC-I, anti-myosin BF-F3 IgM for MyHC-IIB. In general, any immunohistochemical multiple-staining technique is a combination of several individual antigen visualization methods. A successful triple immunohistochemical staining protocol needs to overcome two main problems: (1) determining how to prevent cross-reactions between several individual detection methods; (2) distinguishing the color combinations that provide the best contrast between several individual colors and a mixed color at the sites of colocalization. Many investigators have tried to fi nd solutions to overcome these problems, and developed several concepts and color combinations to perform successful multiple immunostaining. Most of these concepts are based on the differences between primary antibodies including animal species, Ig isotypes or IgG subclasses, conjugates, or concentrations. Additionally, dif f erent chromogens are selected to show different colors for different antigens in tissues (van der Loos, 2008).
The advantages of our novel and effective TIE staining method include: (1) Instead of using single immunohistochemical staining on multiple muscle sections, the TIE enables simultaneous identif i cation of all muscle fi bers on a single cryosection, which saves time and tissue sections. (2) Compared to TIF staining, the TIE staining is less expensive, much more convenient for visualization and imaging, and outlines muscle fi bers better. (3)e TIE technique has been proven to work well not only on normal muscles, but also on muscles of varying degrees of denervation atrophy, and muscles at varying stages of reinnervation.is warrants its broader use in future studies.
Conclusions
A novel, reproducible, sensitive and specific TIE method has been successfully established in this study which enables simultaneous identification of all muscle fiber types on a single cryosection. It works well in normal, denervated or reinnervated rat muscles. It enables the visualization of muscle fi ber basement membranes, which facilitates the accurate identification of fiber type and morphology. Meanwhile, a systematic sampling method has been validated, making the manual counting and analysis of muscle fi ber phenotyping more accurate and less laborious.
Acknowledgments:The authors thank Jarred Nesbitt, Department of Neurology, Mayo Clinic, USA for his help with animal care and anesthesia.
Author contributions:PW, SZ and MTL conducted the experiments, analyzed and validated the experimental data. YG, CY, RJS and HW were Ph.D. candidate mentors to PW. AJW, MJY and HW were in charge of project administration and supervision. PW wrote the draft of this paper. RJS, AJW and HW edited the paper and were responsible for fundraising. HW was responsible for conceptualization of the study. All authors approved the fi nal version of this paper.
Conf l icts of interest:None declared.
Research ethics:All procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC protocol# A10611). All animal studies were performed in compliance with Association for Assessment and Accreditation of Laboratory Animal Care International and under the supervision of the Institutional Animal Care and Use Committee.
Data sharing statement:
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Armstrong RB, Phelps RO (1984) Muscle fi ber type composition of the rat hindlimb. Am J Anat 171:259-272.
Bigard AX, Serrurier B, Merino D, Lienhard F, Berthelot M, Guezennec CY (1997) Myosin heavy chain composition of regenerated soleus muscles during hindlimb suspension. Acta Physiol Scand 161:23-30.
Bloemberg D, Quadrilatero J (2012) Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunof l uorescence analysis. PLoS One 7:e35273.
Bobinac D, Malnar-Dragojevic D, Bajek S, Soic-Vranic T, Jerkovic R (2000) Muscle fi ber type composition and morphometric properties of denervated rat extensor digitorum longus muscle. Croat Med J 41:294-297.
Claassen E, Boorsma DM, Kors N, Van Rooijen N (1986) Double-enzyme conjugates, producing an intermediate color, for simultaneous and direct detection of three dif f erent intracellular immunoglobulin determinants with only two enzymes. J Histochem Cytochem 34:423-428.
Gorza L (1990) Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem 38:257-265.
Guth L, Samaha FJ (1969) Qualitative dif f erences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol 25:138-152.
Jergovic D, Stal P, Lidman D, Lindvall B, Hildebrand C (2001) Changes in a rat facial muscle aer facial nerve injury and repair. Muscle Nerve 24:1202-1212.
Kostrominova TY, Dow DE, Dennis RG, Miller RA, Faulkner JA (2005) Comparison of gene expression of 2-mo denervated, 2-mo stimulated-denervated, and control rat skeletal muscles. Physiol Genomics 22:227-243.
Laughlin MH, Armstrong RB (1982) Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol 243:H296-306.
Lucas CA, Kang LH, Hoh JF (2000) Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Commun 272:303-308.
McMillan EM, Quadrilatero J (2011) Dif f erential apoptosis-related protein expression, mitochondrial properties, proteolytic enzyme activity, and DNA fragmentation between skeletal muscles. Am J Physiol Regul Integr Comp Physiol 300:R531-543.
Meunier B, Picard B, Astruc T, Labas R (2010) Development of image analysis tool for the classif i cation of muscle fi bre type using immunohistochemical staining. Histochem Cell Biol 134:307-317.
Michel RN, Parry DJ, Dunn SE (1996) Regulation of myosin heavy chain expression in adult rat hindlimb muscles during short-term paralysis: comparison of denervation and tetrodotoxin-induced neural inactivation. FEBS Lett 391:39-44.
Nakane PK (1968) Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem 16:557-560.
Novak P, Zacharova G, Soukup T (2010) Individual, age and sex differences in fi ber type composition of slow and fast muscles of adult Lewis rats: comparison with other rat strains. Physiol Res 59:783-801.
Pette D, Vrbova G (1985) Neural control of phenotypic expression in mammalian muscle fi bers. Muscle Nerve 8:676-689.
Raheem O, Huovinen S, Suominen T, Haapasalo H, Udd B (2010) Novel myosin heavy chain immunohistochemical double staining developed for the routine diagnostic separation of I, IIA and IIX fi bers. Acta Neuropathol 119:495-500.
Rivero JL, Talmadge RJ, Edgerton VR (1998) Fibre size and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. J Muscle Res Cell Motil 19:733-742.
Schiaf fi no S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T (1989)ree myosin heavy chain isoforms in type 2 skeletal muscle fi bres. J Muscle Res Cell Motil 10:197-205.
Smerdu V, Soukup T (2008) Demonstration of myosin heavy chain isoforms in rat and humans: the specif i city of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem 52:179-190.
Soukup T, Smerdu V, Zacharova G (2009) Fiber type composition of unoperated rat soleus and extensor digitorum longus muscles aer unilateral isotransplantation of a foreign muscle in long-term experiments. Physiol Res 58:253-262.
Tulloch LK, Perkins JD, Piercy RJ (2011) Multiple immunof l uorescence labelling enables simultaneous identif i cation of all mature fi bre types in a single equine skeletal muscle cryosection. Equine Vet J 43:500-503.
van der Loos CM (2008) Multiple immunoenzyme staining: methods and visualizations for the observation with spectral imaging. J Histochem Cytochem 56:313-328.
van der Loos CM, Das PK, Houthof f HJ (1987) An immunoenzyme triple-staining method using both polyclonal and monoclonal antibodies from the same species. Application of combined direct, indirect, and avidin-biotin complex (ABC) technique. J Histochem Cytochem 35:1199-1204.
Windisch A, Gundersen K, Szabolcs MJ, Gruber H, Lomo T (1998) Fast to slow transformation of denervated and electrically stimulated rat muscle. J Physiol 510 (Pt 2):623-632.
Copyedited by Li CH, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Huan Wang, M.D., Ph.D., wang.huan@mayo.edu.
Huan Wang, M.D., Ph.D., wang.huan@mayo.edu.
#
orcid: 0000-0002-5540-0648 (Huan Wang)
10.4103/1673-5374.213560
Accepted: 2017-07-19
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- Optical coherence tomography and T cell gene expression analysis in patients with benign multiple sclerosis
- Impact of Pitx3 gene knockdown on glial cell linederived neurotrophic factor transcriptional activity in dopaminergic neurons
- Dried Rehmannia root protects against glutamateinduced cytotoxity to PC12 cells through energy metabolism-related pathways
- Dexmedetomidine mitigates isof l urane-induced neurodegeneration in fetal rats during the second trimester of pregnancy
- Cold water swimming pretreatment reduces cognitive def i cits in a rat model of traumatic brain injury