Cold water swimming pretreatment reduces cognitive def i cits in a rat model of traumatic brain injury
2017-09-04ZiweiZhouYadanLiWeiweiGaoJieliChenShuyuanYueJianningZhang
Zi-wei Zhou, Ya-dan Li, Wei-wei Gao Jie-li Chen, Shu-yuan Yue Jian-ning Zhang
1 Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China
2 Tianjin Neurological Institute, Tianjin, China
3 Key Laboratory of Post-trauma Neuro-repair and Regeneration in Central Nervous System, Ministry of Education, Tianjin, China
4 Tianjin Key Laboratory of Injuries, Variations and Regeneration of Nervous System, Tianjin, China
5 Intensive Care Units, Tianjin Huanhu Hospital, Tianjin, China
6 Department of Neurology, Henry Ford Hospital, Detroit, MI, USA
Cold water swimming pretreatment reduces cognitive def i cits in a rat model of traumatic brain injury
Zi-wei Zhou1,2,3,4,*,#, Ya-dan Li5,#, Wei-wei Gao1,2,3,4, Jie-li Chen6, Shu-yuan Yue1,2,3,4, Jian-ning Zhang1,2,3,4,*
1 Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, China
2 Tianjin Neurological Institute, Tianjin, China
3 Key Laboratory of Post-trauma Neuro-repair and Regeneration in Central Nervous System, Ministry of Education, Tianjin, China
4 Tianjin Key Laboratory of Injuries, Variations and Regeneration of Nervous System, Tianjin, China
5 Intensive Care Units, Tianjin Huanhu Hospital, Tianjin, China
6 Department of Neurology, Henry Ford Hospital, Detroit, MI, USA
How to cite this article:Zhou ZW, Li YD, Gao WW, Chen JL, Yue SY, Zhang JN (2017) Cold water swimming pretreatment reduces cognitive def i cits in a rat model of traumatic brain injury. Neural Regen Res 12(8):1322-1328.

Graphical Abstract
A moderate stress such as cold water swimming can raise the tolerance of the body to potentially injurious events. However, little is known about the mechanism of benef i cial ef f ects induced by moderate stress. In this study, we used a classic rat model of traumatic brain injury to test the hypothesis that cold water swimming preconditioning improved the recovery of cognitive functions and explored the mechanisms. Results showed that aer traumatic brain injury, pre-conditioned rats (cold water swimming for 3 minutes at 4°C) spent a signif i cantly higher percent of times in the goal quadrant of cold water swim, and escape latencies were shorter than for non-pretreated rats.e number of circulating endothelial progenitor cells was signif i cantly higher in pre-conditioned rats than those without pretreatment at 0, 3, 6 and 24 hours aer traumatic brain injury. Immunohistochemical staining and Von Willebrand factor staining demonstrated that the number of CD34+stem cells and new blood vessels in the injured hippocampus tissue increased signif i cantly in pre-conditioned rats.ese data suggest that pretreatment with cold water swimming could promote the proliferation of endothelial progenitor cells and angiogenesis in the peripheral blood and hippocampus. It also ameliorated cognitive def i cits caused by experimental traumatic brain injury.
nerve regeneration; cold water swimming; cognitive def i cits; endothelial progenitor cells; angiogenesis; neural repair; stress; Morris water maze; fl uid percussion injury model; CD34; Von Willebrand factor; neural regeneration
Introduction
Chronic or severe stress on rodents can result in numerous alterations of their neuroanatomical and neurochemical properties (Buwalda et al., 2005), which have negative consequences in the central nervous system (Adlard et al., 2011). On the other hand, they could be imperative for adapting to changing circumstances. A number of studies have shown the benef i cial ef f ects to the lifespan of animals from exposure to stress or harm, including hypergravity, low exposure of toxic substances, heat shock and cold shock (Lindsay, 2005; Shevchuk, 2008; Genchi et al., 2015).ere is evidence that stress, such as inescapable shock, can facilitate subsequent learning on such tasks as conditioned eye blinks (Shors et al., 1992; Shors, 2004). It has also been reported that mild stress facilitates learning in mice (Adlard et al., 2011). A moderate amount of electric shock (electroconvulsive therapy) haslong been used to treat drug-resistant depression (Ishihara and Sasa, 1999). These stress-facilitated improvements in neurocognitive functions are believed to be mediated through activating the sympathetic nervous system, increasing blood flow, and enhancing cerebral synaptic release of noradrenaline (Jansky et al., 1996; Jedema et al., 2001; Nutt, 2002). They could also increase production of beta-endorphin (Vaswani et al., 1988), which is known to produce the sense of well-being. Synthesis of growth factors such as vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor increased extensively after cold water immersion and immobilization stress.ese growth factors promote angiogenesis and neural repair in injured tissues and organs (Kim et al., 2005; Kondo et al., 2010).
One potential mechanism for the effect of moderate stress is to mobilize stem cells so they are already available to improve a subject’s response to injury. Endothelial progenitor cells (EPCs) are the precursor cells of vascular endothelial cells, expressing CD34, CD133, VEGFR-2, that contribute to new vessel formation in postnatal angiogenesis.ey are mobilized by physiological and pathological stresses, such as exercise, trauma, tumor, and inf l ammation (Okazaki et al., 2006; Liu et al., 2007; Schlager et al., 2011).ey could increase the rate of angiogenesis and ameliorate substance metabolism to improve tissue perfusion and repair. Increases in circulating EPCs are reported to improve clinical outcomes of stroke, myocardial infarction and diabetes (Fan et al., 2010; Reinhard et al., 2010; Sorrentino et al., 2011). We have previously demonstrated that increasing EPCs, induced by progesterone or atorvastatin, promoted the functional recovery of brain trauma in a rat model (Li et al., 2012; Wang et al., 2012). In this study, we tested the hypothesis that rats pre-exposed to cold water swimming (CWS) improved cognitive defects induced by experimental TBI. We also tested whether this benef i cial ef f ect is associated with the level of mobilized EPCs in the peripheral blood.
Materials and Methods
Animals
232 adult male Wistar rats (280–320 g; Experimental Animal Laboratories of the Academy of Military Medical Sciences; Beijing, China) were housed individually in a temperature-controlled (22°C) and humidity-controlled (60%) vivarium, and maintained on a standard 12-hour light/dark cycle (7:00 a.m. to 7:00 p.m. per cycle) with free access to food and water.e study protocol was approved by the Ethics Committee of Tianjin Medical University.e experiment followed the National Guidelines for the Care and Use of Laboratory Animals, and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE). Experiments were designed to minimize the number of animals required and those used were cared for, handled and medicated as appropriate to minimize their suf f ering.
The 232 rats were randomly divided into four groups (n= 58): CWS group: rats were exposed to CWS; sham group: rats were not exposed to CWS or fluid percussion injury (FPI); TBI group: rats were exposed to FPI; CWS-TBI group: rats were subjected to CWS for 1 week before being exposed to FPI.
CWS models
Rats were placed into a tub (50 cm deep, 150 cm diameter) containing water at 4°C and allowed to swim for 3 minutes in the cold water as previously described (Commons, 2003).e rats trembled as they swam for the full 3 minutes. Subsequently, the rats were taken out from the tub and then immediately returned to home cage after drying. This was repeated each day for 1 week.is process lasted for 1 week.
FPI models
Rats with or without the pretreatment (CWS) were subjected to FPI. FPI was performed as previously described (Chen et al., 2009). Brief l y, rats were anesthetized with 10% chloride hydrate (3.0 mL/kg, intraperitoneally) and placed in a stereotaxic frame.e scalp was ref l ected with a single incision and the temporal muscles scraped from the skull. Craniotomy (4.0 mm × 4.0 mm) was performed over the right parietal skull, 2.0 mm lateral from sagittal suture and 3.0 mm caudal from coronal suture, keeping the dura intact. Subsequently, a luer-lock connector (3 mm diameter) was secured to the skull over the opening with cyanoacrylate adhesive and dental acrylic. The skull sutures were sealed with the cyanoacrylate to ensure that the fl uid bolus from the injury remained within the cranial cavity. Twenty-four hours aer surgery, the rats were subjected to experimental FPI of 2.0–2.2 atmosphere by an FPI device (model 01-B; New Sun Health Products, Cedar Bluf f, VA, USA). A rapid bolus of saline from a Plexiglas cylindrical reservoir was introduced into the closed cranial cavity, causing mechanical deformation of the brain. Rat limbs suddenly twitched, and then slowly returned to normal. Immediately aer FPI, the incision was suture-closed and the rats were placed on a heating pad until ambulatory and then returned to the home cage.
Morris water maze task
Learning abilities were assessed using a Morris water maze. Rats were trained using a Morris water maze (DMS-2, Chinese Academy of Science, China) according to the protocol by Vorhees and Williams (2006),n= 10 per group. Brief l y, a tank measuring 150 cm in diameter and 50 cm in height was fi lled with water at 20–22°C. A target platform (10 cm diameter) was hidden 2 cm below the water surface in a southeast location halfway between the center and the wall of the maze. Rats were allowed to adapt the maze without a platform for 1 minute per day for 3 days before training. Afterwards, the rats were trained to rely on visual distal cues to locate a submerged escape platform. A computerized tracking system (Etho-vision 3.0; Noldus Information Technology, Wageningen, Netherlands) was used to record latency (time to reach the platform) and swim speed. Fourtrials from four random start positions (east, north, southeast, and northwest) were tested daily (each trial lasted for 120 seconds with 15 seconds intervals) for 5 consecutive days (from 7 days through 11 days post-injury). Rats that failed to find the platform within 2 minutes were recorded for a maximum latency score of 120 seconds. Latency (seconds) and path length (cm) were recorded over time to generate a spatial learning curve. At 12 days after TBI, the platform was removed and a probe trail was performed with a novel start position, facing the tank wall.e time the rats stayed in the goal quadrant during a 30-second period was recorded.

Figure 1 Ef f ects of CWS pretreatment on learning and memory abilities in rats with brain injury.
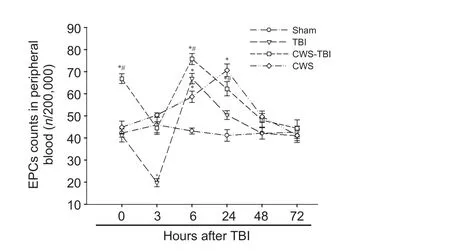
Figure 2 Flow cytometry of EPCs in peripheral blood of rats at 0, 3, 6, 24, 48 and 72 hours aer TBI.
Measurement of EPCs by fl ow cytometry
Peripheral blood samples (0.5 mL) were collected from retro-orbital venous plexus at baseline (0), 3, 6, 24, 48, and 72 hours after FPI and diluted with PBS (6 rats at each time point). Peripheral blood mononuclear cells were isolated by density-gradient centrifugation using Ficoll-Paque Plus (Chuanye, Tianjin, China). Isolated cells were washed twice with PBS and resuspended in 200 µL of PBS supplemented with 0.5% of bovine serum albumin and 2 mM of ethylenediaminetetraacetic acid. EPCs in peripheral blood were evaluated by fi rst staining with PE-conjugated CD34 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and purif i ed CD133 primary antibody (Abcam, Cambridge, UK) conjugated with FITC (Abcam), then detected by fl ow cytometry (FACScan, Becton-Dickinson, San Jose, CA, USA).e isotype-matched IgG was used as a control. CD34 and CD133 double positive cells were def i ned as EPCs. We counted the number of endothelial progenitor cells per 200,000 mononuclear cells.
Rats in each group were sacrif i ced at 1, 3, 7 and 14 days (3 rats at each time point) aer brain trauma and perfused with 0.9% NaCl through the heart to remove blood from the vasculature. Aer perfusion, brains were collected and fi xed in 4% paraformaldehyde for 24 hours and processed as 5 mm coronal paraffin-embedded tissue blocks through the TBI zone. Finally, these blocks were cut into 5–7 µm sections for immunohistochemical staining.
Immunohistochemistry
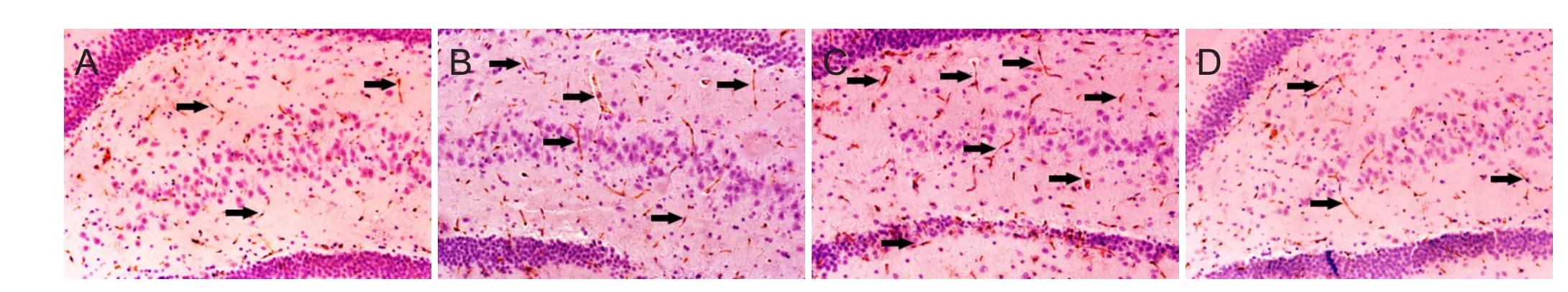
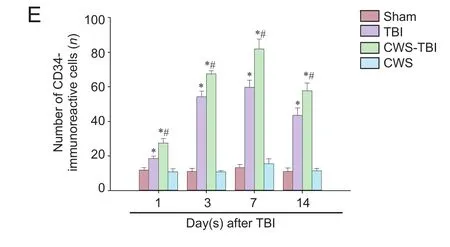
Figure 3 CD34 immunoreactivity in the injured hippocampus of brain tissue at 1, 3, 7, and 14 days aer TBI.
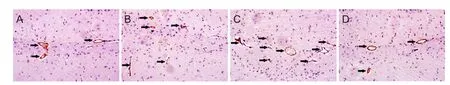
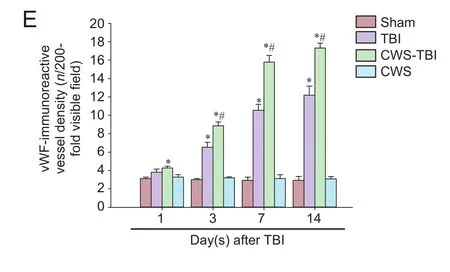
Figure 4 Detection of vascular density by vWF antibody staining in the injured hippocampus at 1, 3, 7, and 14 days aer TBI.
CD34 immunoreactivity in injured brain tissue were detected by a CD34-antibody (R&D Systems, Minneapolis, MN, USA) as recommended by the manufacturer. Briefly, after deparaffinization and rehydration, non-specific endogenous peroxidase activity was blocked by treating sections with 3% hydrogen peroxide in methanol for 30 minutes. Antigen was recovered by boiling the sections for 20 minutes in 10 mM citrate buf f er (pH 6.0). Non-specif i c binding was blocked with 3% bovine serum albumin in PBS for 30 minutes.e sections were then incubated with a goat CD34 polyclonal antibody (1:200) and Von Willebrand factor (vWF) (Abcam, Cambridge, UK) overnight at 4°C.ey were then washed with PBS, incubated with a biotinylated anti-goat IgG (1:100; Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China) for 1 hour at 37°C, washed and incubated in an avidin peroxidase conjugate solution (Beijing Zhongshan Golden Bridge Biotechnology) for 30 minutes. Finally, the sections were developed with diaminobenzidine for 3 minutes. Negative controls were similarly processed without the primary antibody. The number of endothelial-like CD34-immunoreactive cells in each section was counted (per 200×, IX2UCB; Olympus, Tokyo, Japan) in five fields by two independent observers, each blinded to the experimental conditions, to obtain an average number of CD34-immunoreactive cells per view fi eld (per 200×).
Microvasculature was quantified by counting vWF-immunoreactive vessels in a protocol similar to the CD34 staining. Two independent observers, blinded to the experimental conditions, counted vWF-immunoreactive vessels in fi ve sections under a light microscope (IX2UCB; Olympus). The brown stained vascular lumen-like structure was def i ned as vessels.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 soware (SPSS, Chicago, IL, USA). The data are presented as the mean ± SEM. One-way analysis of variance withpost hocleast significant difference test was used to analyze data. A value ofP< 0.05 was considered statistically signif i cant.
Results
Improved recovery of cognitive functions aer TBI in rats pretreated with CWS
To assess changes in cognitive function, the spatial memory of rats was tested in Morris water maze, which measures a rat’s ability to navigate from a start location in a water maze to a submerged escape platform. As expected, latency was signif i cantly shortened during the 5-day spatial acquisition test (F= 251.909,P= 0.000), suggesting that spatial memory had developed in all rats (Figure 1A). However, the escape latency of all rats was inf l uenced by grouping (F(3,36)= 8.31,P= 0.000).e rats subjected to TBI had longer latencies than those without TBI.e CWS-TBI group had a shorter mean latency than the TBI only rats (P= 0.013), which indicated that CWS improved the recovery of cognitive functions aer TBI.
On day 6 after training, the platform was removed and the ability of rats to look for the removed platform was measured as percent of time they swam in the goal quadrant (reference memory). Our study found that those rats in the TBI group had the lowest percent time (Figure 1B). Compared with the TBI group, rats in the CWS-TBI group spent signif i cantly higher percent time in goal quadrant (P= 0.025), which represented a better recovery of cognitive defect than TBI without CWS.
EPCs in peripheral blood
To test the changes of EPCs number in rats of each group, blood samples were stained for CD34 and CD133, and measured by fl ow cytometry. We found that there was no significant change in the EPCs number in the sham group at any time point (Figure 2). EPCs levels were higher in the CWS group than that in the sham group at 6 and 24 hours (P<0.05). Compared to the sham group, the number of EPCs in the TBI group decreased rapidly at 3 hours, increased to the peak level at 6 hours, and declined gradually to the normal level thereafter. The CWS-TBI group had more EPCs at 0, 6 and 24 hours compared with the sham group (P< 0.05). Although the EPCs number showed similar trends in rats of the TBI group and CWS-TBI group aer TBI, the CWS-TBI group had higher numbers of EPCs at 0, 3, 6 and 24 hours than that in TBI group (P< 0.05).
Angiogenesis in the hippocampus
CD34 is a marker for the progenitor hematopoietic cells and is expressed on new microvascular endothelial cells. CD34-immunoreactivity therefore indicates angiogenesis by the hematopoietic progenitor cells. In this study, we found that the number of CD34-immunoreactive cells increased in the hippocampus, where FPI occurred, at 1, 3, 7 and 14 days aer TBI (Figure 3). However, there was no change in the CWS group (P> 0.05).e number of CD34-immunoreactive cells in injured hippocampus was signif i cantly higher in rats from the CWS-TBI group as compared to that in the TBI group aer TBI (P< 0.05).
We also used an antibody to vWF, a vascular marker, to detect the changes in number of vascular vesicles for each group. The vWF-immunoreactivity vessel density of TBI group increased signif i cantly at 3, 7 and 14 days aer TBI as compared to the sham group (P< 0.05; Figure 4). vWF-immunoreactive vessel density increased signif i cantly at 1, 3, 7 and 14 days in CWS-TBI group (P< 0.05). As with the results of CD34 staining in the CWS group, vWF-immunoreactive vessel density was not signif i cantly dif f erent from the sham group (P> 0.05). There was a greater density of vWF-immunoreactive vessel segments in the CWS-TBI group than that in the TBI group at 3, 7 and 14 days (P< 0.05).
Discussion
Using a well-characterized model, we examined cognitive defects in rats subjected to experimentally controlled FPI and correlated such changes with EPCs in peripheral blood and CD34-immunoreactive cells and vascular density in hippocampus. We found that rats developed cognitive defects aer TBI, and those pretreated with CWS had a higher tolerance to the brain injury and improved learning ability aer TBI.
VEGF is one of the important factors of angiogenesis.ere are some reports that cold stimulation could increase VEGF expression in rat skeletal muscle cells (Sugasawa et al., 2016). Cold water immersion also augments the expression of VEGF mRNA in human skeletal muscle and the adaptive response to acute exercise (Joo et al., 2016). Aer CWS, the stress hormone glucocorticoid increased immediately (Metz et al., 2005). It has also been reported that intermediate doses of glucocorticoids for a short period of time signif i cantly increase circulating endothelial progenitor cells and promote angiogenesis in active rheumatoid arthritis (Grisar et al., 2007).ese ef f ects from CWS may explain how CWS could enhance CD34 and vWF immunoreactivity in injured hippocampus aer traumatic brain injury.
In conclusion, CWS pre-conditioned rats have better tolerance to TBI and alleviated TBI-associated cognitive defect. This benefit from CWS preconditioning is associated with improved angiogenesis in the injured brain and a high circulating level of EPCs is critical for angiogenesis.
Acknowledgments:We are very grateful to Wei-yun Cui, Lei Zhou and Ping Lei from the Department of Neurosurgery, Tianjin Medical University General Hospital in China for their excellent technical support.
Author contributions:ZWZ and JNZ designed this study. ZWZ, YDL and WWG performed experiments. JLC analyzed data. ZWZ wrote the paper. SYY revised the paper. All authors approved the fi nal version of the paper.
Conf l icts of interest:None declared.
Research ethics:
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Adlard PA, Engesser-Cesar C, Cotman CW (2011) Mild stress facilitates learning and exercise improves retention in aged mice. Exp Gerontol 46:53-59.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964-967.
Belin de Chantemele EJ, Vessieres E, Guihot AL, Toutain B, Maquignau M, Loufrani L, Henrion D (2009) Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood fl ow. Cardiovasc Res 81:788-796.
Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC (2004) Bone marrow gras restore cerebral blood fl ow and blood brain barrier in stroke rats. Brain Res 1010:108-116.
Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM (2005) Long-term ef f ects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev 29:83-97.
Chen X, Zhang KL, Yang SY, Dong JF, Zhang JN (2009) Glucocorticoids aggravate retrograde memory def i ciency associated with traumatic brain injury in rats. J Neurotrauma 26:253-260.
Commons KG (2003) Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray aer swim stress. J Comp Neurol 464:197-207.
Fadini GP, Albiero M, Boscaro E, Agostini C, Avogaro A (2009) Endothelial progenitor cells as resident accessory cells for post-ischemic angiogenesis. Atherosclerosis 204:20-22.
Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, Chen Y, Su H, Young WL, Yang GY (2010) Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol 67:488-497.
Gautier I, Geeraert V, Coppey J, Coppey-Moisan M, Durieux C (2000) A moderate but not total decrease of mitochondrial membrane potential triggers apoptosis in neuron-like cells. Neuroreport 11:2953-2956.
Genchi GG, Cialdai F, Monici M, Mazzolai B, Mattoli V, Ciofani G (2015) Hypergravity stimulation enhances PC12 neuron-like cell differentiation. Biomed Res Int 2015:748121.
Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Saemann M, Schwarzinger I, Buranyi B, Steiner G, Smolen JS (2007) Endothelial progenitor cells in active rheumatoid arthritis: effects of tumour necrosis factor and glucocorticoid therapy. Ann Rheum Dis 66:1284-1288.
Guo X, Liu L, Zhang M, Bergeron A, Cui Z, Dong JF, Zhang J (2009) Correlation of CD34+cells with tissue angiogenesis aer traumatic brain injury in a rat model. J Neurotrauma 26:1337-1344.
Ishihara K, Sasa M (1999) Mechanism underlying the therapeutic ef f ects of electroconvulsive therapy (ECT) on depression. Jpn J Pharmacol 80:185-189.
Jansky L, Sramek P, Savlikova J, Ulicny B, Janakova H, Horky K (1996) Change in sympathetic activity, cardiovascular functions and plasma hormone concentrations due to cold water immersion in men. Eur J Appl Physiol Occup Physiol 74:148-152.
Jedema HP, Finlay JM, Sved AF, Grace AA (2001) Chronic cold exposure potentiates CRH-evoked increases in electrophysiologic activity of locus coeruleus neurons. Biol Psychiatry 49:351-359.
Joo CH, Allan R, Drust B, Close GL, Jeong TS, Bartlett JD, Mawhinney C, Louhelainen J, Morton JP, Gregson W (2016) Passive and post-exercise cold-water immersion augments PGC-1alpha and VEGF expression in human skeletal muscle. Eur J Appl Physiol 116:2315-2326.
Kaneko Y, Tajiri N, Shinozuka K, Glover LE, Weinbren NL, Cortes L, Borlongan CV (2012) Cell therapy for stroke: emphasis on optimizing safety and efficacy profile of endothelial progenitor cells. Curr Pharm Des 18:3731-3734.
Kim JC, Yi HK, Hwang PH, Yoon JS, Kim HJ, Kawano F, Ohira Y, Kim CK (2005) Effects of cold-water immersion on VEGF mRNA and protein expression in heart and skeletal muscles of rats. Acta Physiol Scand 183:389-397.
Kondo Y, Saruta J, To M, Shiiki N, Sato C, Tsukinoki K (2010) Expression and role of the BDNF receptor-TrkB in rat adrenal gland under acute immobilization stress. Acta Histochem Cytochem 43:139-147.
Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, Young PP (2006) VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J 20:1495-1497.
Li Z, Wang B, Kan Z, Zhang B, Yang Z, Chen J, Wang D, Wei H, Zhang JN, Jiang R (2012) Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J Neurotrauma 29:343-353.
Lindsay DG (2005) Nutrition, hormetic stress and health. Nutr Res Rev 18:249-258.
Liu L, Liu H, Jiao J, Bergeron A, Dong JF, Zhang J (2007) Changes in circulating human endothelial progenitor cells after brain injury. J Neurotrauma 24:936-943.
Metz GA, Jadavji NM, Smith LK (2005) Modulation of motor function by stress: a novel concept of the ef f ects of stress and corticosterone on behavior. Eur J Neurosci 22:1190-1200.
Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M (2006) Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+cells in cancer animal models. Int Immunol 18:1-9.
Reinhard H, Jacobsen PK, Lajer M, Pedersen N, Billestrup N, Mandrup-Poulsen T, Parving HH, Rossing P (2010) Multifactorial treatment increases endothelial progenitor cells in patients with type 2 diabetes. Diabetologia 53:2129-2133.
Rufaihah AJ, Haider HK, Heng BC, Ye L, Tan RS, Toh WS, Tian XF, Sim EK, Cao T (2010) Therapeutic angiogenesis by transplantation of human embryonic stem cell-derived CD133+endothelial progenitor cells for cardiac repair. Regen Med 5:231-244.
Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S (2011) Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis 217:240-248.
Shevchuk NA (2008) Adapted cold shower as a potential treatment for depression. Med Hypotheses 70:995-1001.
Shin HK, Lee JH, Kim CD, Kim YK, Hong JY, Hong KW (2003) Prevention of impairment of cerebral blood fl ow autoregulation during acute stage of subarachnoid hemorrhage by gene transfer of Cu/Zn SOD-1 to cerebral vessels. J Cereb Blood Flow Metab 23:111-120.
Shors TJ (2004) Learning during stressful times. Learn Mem 11:137-144.
Sorrentino SA, Doerries C, Manes C, Speer T, Dessy C, Lobysheva I, Mohmand W, Akbar R, Bahlmann F, Besler C, Schaefer A, Hilfiker-Kleiner D, Luscher TF, Balligand JL, Drexler H, Landmesser U (2011) Nebivolol exerts benef i cial ef f ects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and leventricular dysfunction early aer myocardial infarction beyond conventional beta1-blockade. J Am Coll Cardiol 57:601-611.
Sugasawa T, Mukai N, Tamura K, Tamba T, Mori S, Miyashiro Y, Yamaguchi M, Nissato S, Ra S, Yoshida Y, Hoshino M, Ohmori H, Kawakami Y, Takekoshi K (2016) Effects of cold stimulation on mitochondrial activity and vegf expression in vitro. Int J Sports Med 37:766-778.
Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M (2008) Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells aer stroke. J Cereb Blood Flow Metab 28:764-771.
Vaswani KK, Richard CW, 3rd, Tejwani GA (1988) Cold swim stress-induced changes in the levels of opioid peptides in the rat CNS and peripheral tissues. Pharmacol Biochem Behav 29:163-168.
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848-858.
Wang B, Sun L, Tian Y, Li Z, Wei H, Wang D, Yang Z, Chen J, Zhang J, Jiang R (2012) Ef f ects of atorvastatin in the regulation of circulating EPCs and angiogenesis in traumatic brain injury in rats. J Neurol Sci 319:117-123.
Young PP, Vaughan DE, Hatzopoulos AK (2007) Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog Cardiovasc Dis 49:421-429.
Yu DC, Chen J, Sun XT, Zhuang LY, Jiang CP, Ding YT (2010) Mechanism of endothelial progenitor cell recruitment into neo-vessels in adjacent non-tumor tissues in hepatocellular carcinoma. BMC Cancer 10:435.
Zhang Y, Li Y, Wang S, Han Z, Huang X, Li S, Chen F, Niu R, Dong JF, Jiang R, Zhang J (2013) Transplantation of expanded endothelial colony-forming cells improved outcomes of traumatic brain injury in a mouse model. J Surg Res 185:441-449.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Zi-wei Zhou or Jian-ning Zhang, 1985zhouziwei@163.com or jianningzhang@hotmail.com.
Zi-wei Zhou or Jian-ning Zhang, 1985zhouziwei@163.com or jianningzhang@hotmail.com.
#
orcid: 0000-0002-5927-7835 (Zi-wei Zhou) 0000-0002-7290-0994 (Jian-ning Zhang)
10.4103/1673-5374.213553
Accepted: 2017-05-25
杂志排行
中国神经再生研究(英文版)的其它文章
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- A novel triple immunoenzyme staining enables simultaneous identif i cation of all muscle fi ber types on a single skeletal muscle cryosection from normal, denervated or reinnervated rats
- Optical coherence tomography and T cell gene expression analysis in patients with benign multiple sclerosis
- Impact of Pitx3 gene knockdown on glial cell linederived neurotrophic factor transcriptional activity in dopaminergic neurons
- Dried Rehmannia root protects against glutamateinduced cytotoxity to PC12 cells through energy metabolism-related pathways
- Dexmedetomidine mitigates isof l urane-induced neurodegeneration in fetal rats during the second trimester of pregnancy