Association and dif f erences in genetic polymorphisms in PCSK9 gene in subjects with lacunar infarction in the Han and Uygur populations of Xinjiang Uygur Autonomous Region of China
2017-09-04DengfengHanJianhuaMaChenguangHaoTuerhongTuerxunLeiDuXiaoningZhang
Deng-feng Han, Jian-hua Ma, Chen-guang Hao Tuerhong·Tuerxun Lei Du Xiao-ning Zhang
1 Department of Neurology, First Af filiated Hospital of Xinjiang Medical University, Urumqi, Xinjiang Uygur Autonomous Region, China
2 Department of Neurology, Fourth Af filiated Hospital of Xinjiang Medical University, Urumqi, Xinjiang Uygur Autonomous Region, China
Association and dif f erences in genetic polymorphisms in PCSK9 gene in subjects with lacunar infarction in the Han and Uygur populations of Xinjiang Uygur Autonomous Region of China
Deng-feng Han1,#, Jian-hua Ma1,#, Chen-guang Hao1, Tuerhong·Tuerxun1, Lei Du1, Xiao-ning Zhang2,*
1 Department of Neurology, First Af filiated Hospital of Xinjiang Medical University, Urumqi, Xinjiang Uygur Autonomous Region, China
2 Department of Neurology, Fourth Af filiated Hospital of Xinjiang Medical University, Urumqi, Xinjiang Uygur Autonomous Region, China
How to cite this article:Han DF, Ma JH, Hao CG, Tuerhong·Tuerxun, Du L, Zhang XN (2017) Association and dif f erences in genetic polymorphisms in PCSK9 gene in subjects with lacunar infarction in the Han and Uygur populations of Xinjiang Uygur Autonomous Region of China. Neural Regen Res 12(8):1315-1321.
Graphical Abstract
Polymorphisms in the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene are associated with severe hypercholesterolemia and stroke. Here, we investigated the relationship between single nucleotide polymorphisms inPCSK9and stroke in 237 patients with lacunar infarction in the Uygur and Han populations in Xinjiang Uygur Autonomous Region of China. Using the SNaPshot single-base terminal extension method, fourPCSK9gene polymorphisms were analyzed. We found a signif i cantly strong relationship between thePCSK9rs17111503 (G > A) polymorphism and increased susceptibility to lacunar infarction by variant homozygote comparison, and using the dominant and recessive models in the Han population but not in the Uygur population. Low triglyceride levels were found in AA carriers (rs17111503, G > A) in the Han population but not in the Uygur population. Association analysis revealed that the rs17111503 (G > A) polymorphism was not signif i cantly associated with smoking, alcohol drinking, history of hypertension or diabetes in the Han or Uygur lacunar infarction patients. rs11583680, rs483462 and rs505151 were not associated with risk of lacunar infarction in the Han or Uygur populations. Our fi ndings suggest that thePCSK9rs17111503 (G > A) polymorphism is associated with susceptibility to lacunar infarction in the Han population but not in the Uygur population.
nerve regeneration; genetic; proprotein convertase subtilisin/kexin type 9; lacunar infarction; polymorphisms; case control; Uygur populations; Han populations; magnetic resonance imaging; association; susceptibility; neural regeneration
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9), also known as neural apoptosis-regulated convertase 1, is the ninth member of the proprotein convertase family (Seidah et al., 2003).e humanPCSK9gene is located on chromosome 1, p32.3, and consists of 12 exons and encodes a 692 amino acid glycoprotein.PCSK9is synthesized as an inactive zymogen, pro-PCSK9(73 kDa), consisting of a signal peptide, a prodomain (residues 31–152), a catalytic domain (residues 153–451) and a C-terminal domain (residues 452–692) (Lambert et al., 2009).PCSK9acts as a molecular chaperone and serine protease that decreases low-density lipoprotein receptor levels in the hepatic and extrahepatic regions through an endosomal/lysosomal pathway and increases plasma low-density lipoprotein cholesterol (Benjannet et al., 2004; Schmidt et al., 2008).PCSK9may also regulate apolipoprotein B-containing lipoprotein synthesis and apolipoprotein B excretion (Ouguerram et al., 2004; Sun et al., 2005).
Recent studies show that a number of genetic variants ofPCSK9are associated with plasma cholesterol. There is a tight relationship between gain of function missense mutations inPCSK9and autosomal dominant hypercholesterolemia. In this form of familial hypercholesterolemia, neither the low-density lipoprotein receptor nor the ligand binding domain of apolipoprotein B100 are mutated (Leren, 2004; Allard et al., 2005).ere is also a relationship between loss of function nonsense mutations inPCSK9and low levels of plasma low-density lipoprotein and reduced morbidity from cardiovascular disease (Hallman et al., 2007; Hooper et al., 2007). Manyin vitroandin vivooverexpression and knockout/knockdown studies have shown thatPCSK9targets the low-density lipoprotein receptor for degradation (Benjannet et al., 2006; Lagace et al., 2006; Lambert et al., 2006).ese studies demonstrate that both rare mutations and common variants af f ecting the coding regions ofPCSK9impact low-density lipoprotein cholesterol levels and related diseases. However, the single nucleotide polymorphisms (SNPs) located in thePCSK9promoter, a regulatory region, have not been fully investigated. There is a strong relationship between the SNPs and the enzymatic activity ofPCSK9in the plasma. Furthermore, there is an intimate association between the SNPs and serum lipid levels and vascular disease.
In this study, we selected rs483462 and rs17111503 in the gene promoter region as well as three missense mutations–rs11583680 (p.Ala53Val), rs505151 (p.Gly377Glu) and rs149311926 (p.Gln261Glu)–to evaluate the relationship between humanPCSK9gene polymorphisms and lacunar infarction in the Uygur and Han populations.
Materials and Methods
Study protocol
All participants provided signed informed consent. DNA analyses and collection of relevant clinical data were allowed by all participants.e study protocol was approved by the Ethics Committee of the First Af filiated Hospital of Xinjiang Medical University (approval No. 20120510).
Study population
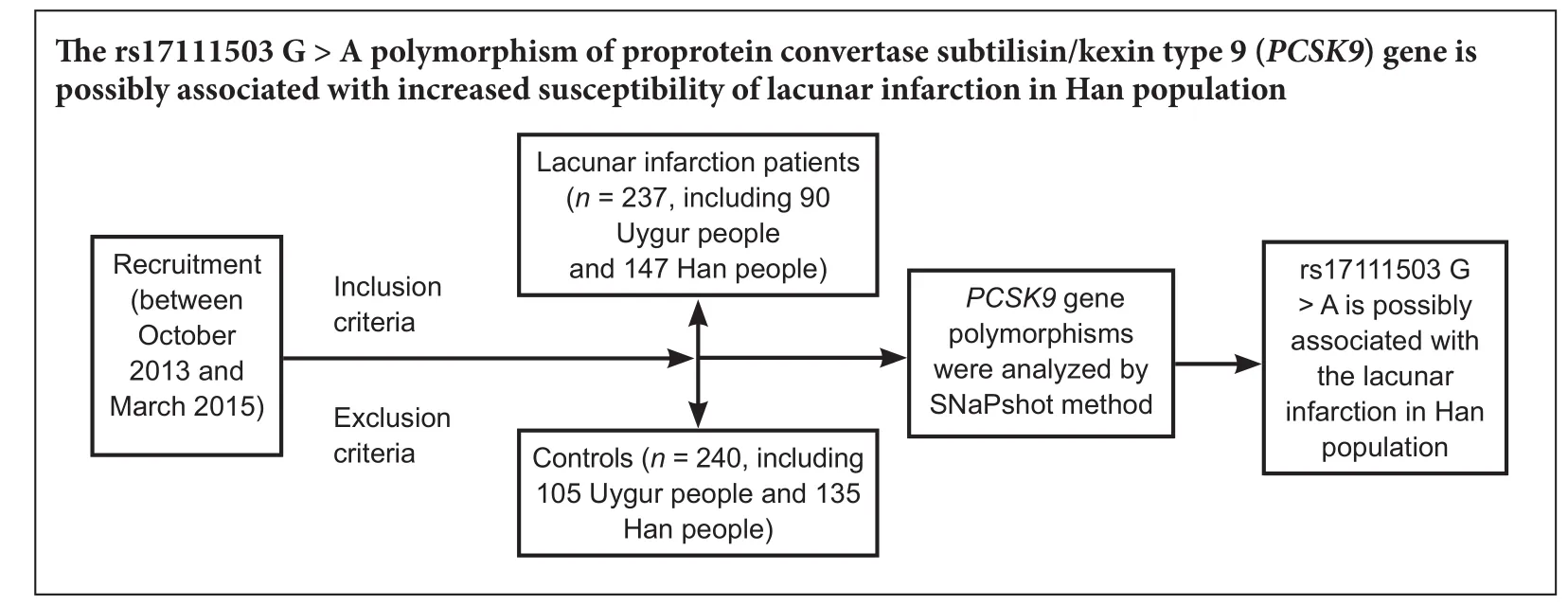
Subjects were from the Han and Uygur ethnic groups, who lived in the Urumqi region of Xinjiang Uygur Autonomous Region in China. We recruited the lacunar infarction group from the Department of Neurology of the First Af fi liated Hospital of Xinjiang Medical University of China between October 2013 and March 2015, and the control group from the same hospital in the same period.e major characteristics of study participants are shown in Table 1.e lacunar infarction group and control group were well-matched.e lacunar infarction group contained 237 lacunar infarction patients. The control group contained 240 healthy controls. Study subjects in the control group were recruited from the First Af filiated Hospital of Xinjiang Medical University by posted advertisement in the hospital newspaper asking healthy volunteers to come to the clinic for further screening.ere was no statistical difference in the severity of lacunar infarction between the Han and Uygur lacunar infarction groups by magnetic resonance imaging (MRI). Figure 1 shows representative MRIs of the brain for the Han and Uygur populations.
Inclusion criteria for the lacunar infarction group
Patients meeting all of the following criteria were included: (1) diagnosed in accordance with the standards described previously, with an infarct lesion associated with local small vasculopathy related to hypertension (Macdonell et al., 1987); (2) conf i rmed by head MRI, with an infarct lesion diameter less than 15 mm.
Exclusion criteria for the lacunar infarction group
Patients meeting any of the following criteria were excluded: (1) coronary heart disease; (2) hemorrhagic cerebrovascular disease conf i rmed by MRI; (3) refusal to participate in trials.
Inclusion criteria for the control group
Patients meeting all of the following criteria were included: (1) age > 40 years old; (2) no known family history of cerebrovascular disease; (3) no abnormality observed in cardiopulmonary physical examination and nervous system examination; (4) MRI negative for cerebrovascular disease.
Exclusion criteria for the control group
Patients meeting any of the following criteria were excluded: acute or chronic infection, malignant tumor, or autoimmune disease.
Collection of the clinical characteristics of the study participants
All data listed below were obtained from clinical medical records or questionnaires. Three trained researchers independently collected clinical medical records or questionnaires in the Department of Neurology of the First Af filiated Hospital of Xinjiang Medical University of China. Disagreement was resolved by discussion. All patients completed the standard test registration form, and disclosed the following data:
(1) General information: Age, sex and ethnic group.
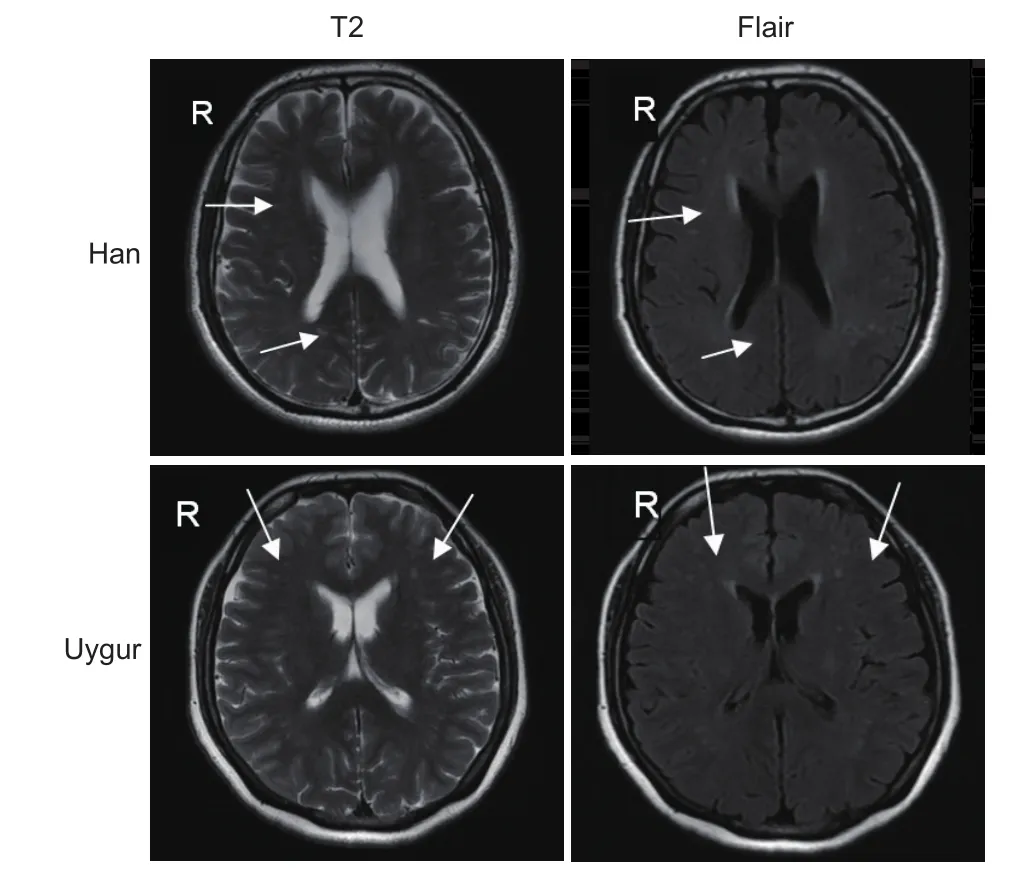
Figure 1 Magnetic resonance imaging of Uygur lacunar infarction patients.
(2) Personal history: Smoking history (daily average smoking, smoking an average of ≥ 1 cigarette/day or more, time> 1 year, defined as smoking), drinking alcohol (an aver-age of ≥ 3 times per week, more than 50 g each time over a period of > 1 year, defined as drinking), hypertension, diabetes, transient ischemic attack, atrial fibrillation, heart valve disease, heart valve replacement, and peripheral vascular disease. Hypertension: the Seventh WHO/International Society of Hypertension League Conference defined the new standard for the diagnosis of hypertension. In our study, hypertension was diagnosed if patients were treated with anti-hypertensive medication or if the average of three measurements of systolic blood pressure were > 140 mmHg (1 mmHg = 0.133 kPa) or diastolic blood pressure was >90 mmHg.e diagnosis of diabetes mellitus was based on the standard of the American Diabetes Association (1997). Individuals with daytime random blood glucose ≥ 11.1 mM or fasting glucose ≥ 7.0 mM or glucose in line 2 hours ≥ 11.1 mM or with a history of diabetes or treatment with insulin were identif i ed as diabetic.
(3) Medical history prior to admission: Treatment with antihypertensive drugs, antiplatelet drugs and anticoagulants, diabetes, lipid drug, anti-seizure medication, birth control pills, and hormones.
(4) Family history: Whether grandparents, parents, siblings or children had hypertension, diabetes, cerebral hemorrhage, cerebral infarction, myocardial infarction, coronary heart disease or arrhythmia.
(5) Physical examination: height, weight, blood pressure, pulse, and temperature.
(6) Special tests: electrocardiogram, chest X-ray, heart neck ultrasound, blood routine, blood glucose, and blood lipids.
Single nucleotide polymorphisms selection
Using the HapMap database, with the criterion of a minor allele frequency > 0.05 in Han Chinese in Beijing, we identifi ed four common SNPs (rs483462, rs630431, rs615563 and rs568052) inPCSK9, all of which are in high linkage disequilibrium in Han Chinese in Beijing.erefore, we genotyped only one SNP, rs483462. In addition, we also investigated four functional polymorphisms inPCSK9, rs17111503, rs14931192, rs11583680 and rs505151, which were selected because of their role in regulatingPCSK9gene expression.
DNA isolation and genotyping
Using standard procedures (Promega, Beijing, China), we extracted genomic DNA from peripheral blood leukocytes. Blood samples were collected under fasting conditions at 8:00 a.m. Approximately 5 mL was withdrawn from the basilic vein in the upper arm. We used the single-base terminal extension (SNaPshot) method to genotype the rs17111503 polymorphism. SNaPshot reactions were performed as described in the manufacturer’s protocol (Applied Biosystems, Warrington, UK). Brief l y, the sample was incubated with 2 U Exonuclease I and 2 U shrimp alkaline phosphatase and incubated at 37°C for 60 minutes. Aer inactivating the enzymes, 1 µL of digested reaction product was mixed with 5 µL of ready reaction premix, 1 µL of 1.0 µM primer, and 3 µL of dH2O. This mixture was placed in the thermal cycler and underwent 25 cycles of 96°C for 10 seconds, 50°C for 5 seconds, and 60°C for 30 seconds. When completed, 0.5 U of shrimp alkaline phosphatase was added, and the reaction mixture was incubated for 60 minutes. Prior to loading onto the PRISM 310, 10 µL of formamide was added to 1 µL of reaction mixture, and samples were heated to 95°C for 5 minutes.e primary data were analyzed using GeneMapper 4.0 software (Applied Biosystems). Genotypes were determined according to the nucleotide at the SNP site, which was visualized by one or two dif f erent color peaks.
Statistical analyses
The Statistical Package for Social Sciences 22.0 software (IBM, Armonk, IL, USA) was utilized for all statistical analyses. All continuous variables are expressed as the mean ± SD (such as age, body mass index, and cholesterol levels). The dif f erence between the lacunar infarction and control groups was analyzed using an independent-samplet-test and unpairedt-test with Welch’s correction, as appropriate.e chisquare test was used for analyzing the Hardy-Weinberg equilibrium and the dif f erences in general characteristics between lacunar infarction patients and matched controls, such as sex, hypertension, diabetes mellitus, smoking, drinking and genotype.e latent relationship of genotypic frequencies of the PCSK9 polymorphisms with the risk of lacunar infarction was assessed by the odds ratios (ORs) with their 95% conf i dence intervals (CIs) from logistic regression models. A value ofP<0.05 was considered statistically signif i cant.
Results
Comparison of clinical data between the lacunar infarction and control groups
A total of 477 subjects were registered, including 237 lacunar infarction patients and 240 healthy controls, in this case-control study.e clinical characteristics of the lacunar infarction patients and matched participants are shown in Table 1. For all Han and Uygur subjects, there were no signif i cant dif f erences in sex or age between the lacunar infarction patients and matched subjects. We observed a few dif f erences between the lacunar infarction group and the control group. Between these two groups, several common risk factors for lacunar infarction were significantly different: fasting plasma glucose, systolic blood pressure, diastolic blood pressure (except Uygur subjects), total cholesterol (except Uygur subjects), and low-density lipoprotein cholesterol (except Han subjects) (P< 0.05). Other lacunar infarction risk factors, such as apolipoprotein A1, apolipoprotein B, triglyceride levels and body mass index, were not signif i cantly dif f erent.
Hardy–Weinberg equilibrium test
The Hardy–Weinberg equilibrium test of the observed and expected genotype values suggested the lacunar infarction and control groups were in Hardy–Weinberg equilibrium at rs17111503, rs11583680, rs483462 and rs505151.e characteristics of the populations in these groups are shown in Table 2. Only the CC genotype was found for rs149311926 (data not shown).
Association analysis
For rs17111503, the dominant model (GGvs. GA + AA:OR= 1.70, 95%CI= 1.06–2.73,P= 0.03) and the recessivemodel (GG + GAvs. AA:OR= 2.31, 95%CI= 1.09–4.90,P= 0.02) suggested a signif i cant dif f erence between the lacunar infarction and matched controls in the total and Han groups but not in the Uygur group. In addition, the AA genotype of rs17111503 was signif i cantly higher in frequency in the lacunar infarction patients than in matched controls in the Han group (Han: 17.01%vs. 8.15%, AAvs. GG:OR= 2.81, 95%CI= 1.27–6.21,P= 0.02) but not in the Uygur group. When the analysis was adjusted for gender, age and body mass index, similar results were obtained (Table 3). However, logistic regression analyses showed that the relationships between the three polymorphisms (rs11583680, rs483462, and rs505151) and the risk of lacunar infarction were weak (Table 3).
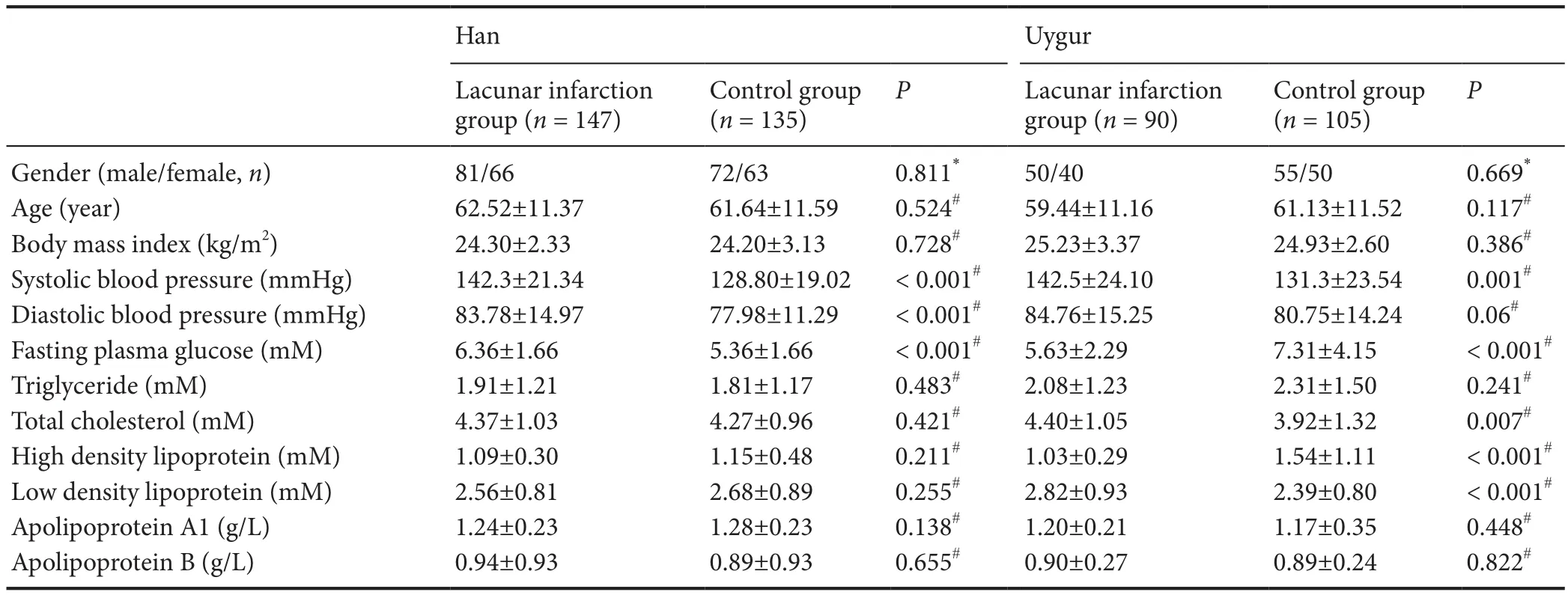
Table 1 Major clinical characteristics of study participants
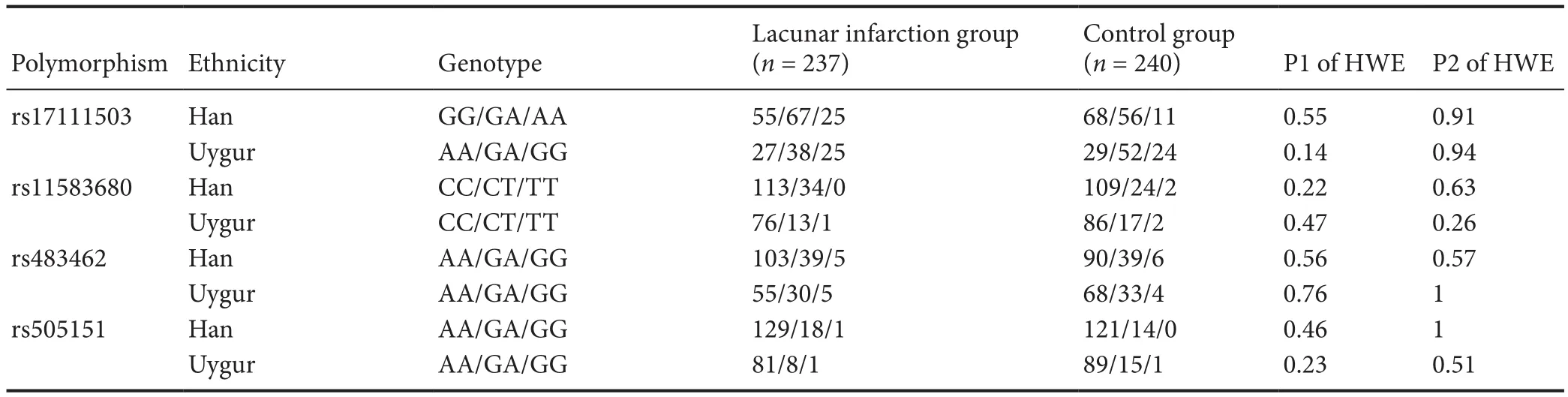
Table 2 Genotype frequencies (n) of the four polymorphisms in the studied subjects
Genotype of rs17111503 and the clinical characteristics of patients
In addition, from the chi-square analysis for smoking, alcohol drinking, history of hypertension and diabetes, the results were not signif i cant in the Han and Uygur populations, as theP-values were greater than 0.05 (Table 5).
Discussion
Cerebral ischemic stroke is a primary cause of morbidity and mortality, and is expected to remain so until at least 2030 (Mathers and Loncar, 2006). Cerebral ischemic stroke and coronary heart disease are major manifestations of atherosclerotic processes. Lacunar infarction is the most common type of ischemic stroke, and is associated with sustained hypertension and cerebrovascular atherosclerosis.ere is a strong relationship between improved living conditions and increased morbidity from lacunar infarction (Wang et al., 2014). High levels of low-density lipoprotein cholesterol are a risk factor for atherosclerosis (Hobbs et al., 1990). Activi-ty of the low-density lipoprotein receptor in the liver is the primary factor that determines the serum concentrations of low-density lipoprotein cholesterol.PCSK9was recently discovered to play a key role in cholesterol homeostasis through the enhanced degradation of low-density lipoprotein receptor (Benjannet et al., 2004; Park et al., 2004; Peterson et al., 2008; Horton et al., 2009), and possibly in neural development. In addition, both rare mutations and common variants in the coding regions ofPCSK9can af f ect low-density lipoprotein cholesterol levels and stroke risk. Recent studies have identified severalPCSK9variants influencing circulating low-density lipoprotein cholesterol levels (Chen et al., 2005; Yue et al., 2006; Horton et al., 2007; Miyake et al., 2008; Folsom et al., 2009). However, polymorphisms in the promoter region have not been well investigated. Our previous study found that the rs17111503 G > A polymorphism is associated with cerebral ischemic stroke in the Han population of China (Han et al., 2014). However, the study further revealed the relationship between thePCSK9rs17111503 G > A polymorphism and lacunar infarction in the Uygur and Han populations. Therefore, we investigated the association between the five polymorphisms and the risk of lacunar infarction in Chinese Han and Uygur populations in this case-control study of lacunar infarction. Finally, these preliminary fi ndings show that thePCSK9rs17111503 G > A polymorphism is possibly associated with the susceptibility to lacunar infarction in Han population, but not in Uygur population.
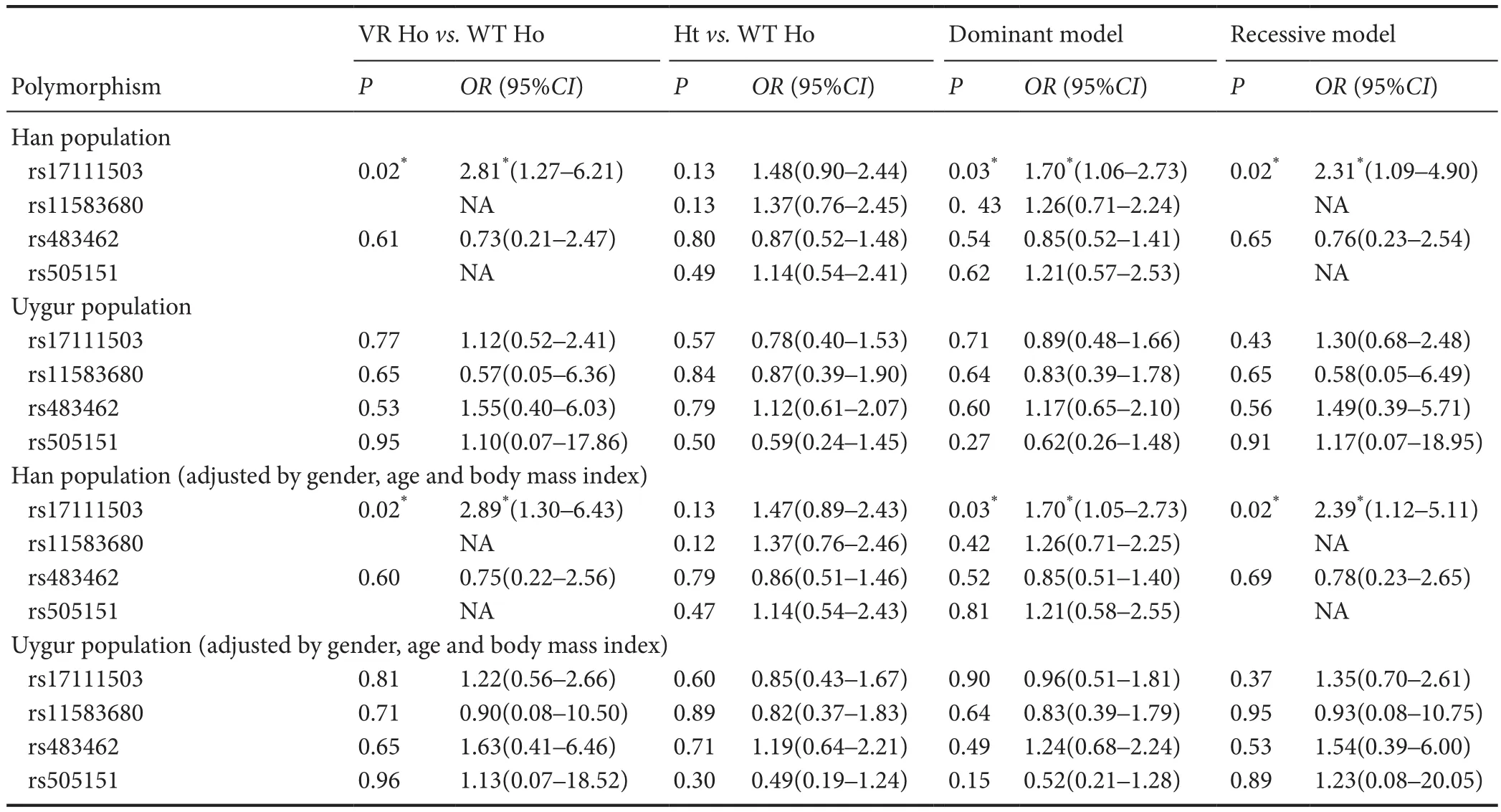
Table 3 Association between the risk of lacunar infarction and the four polymorphisms
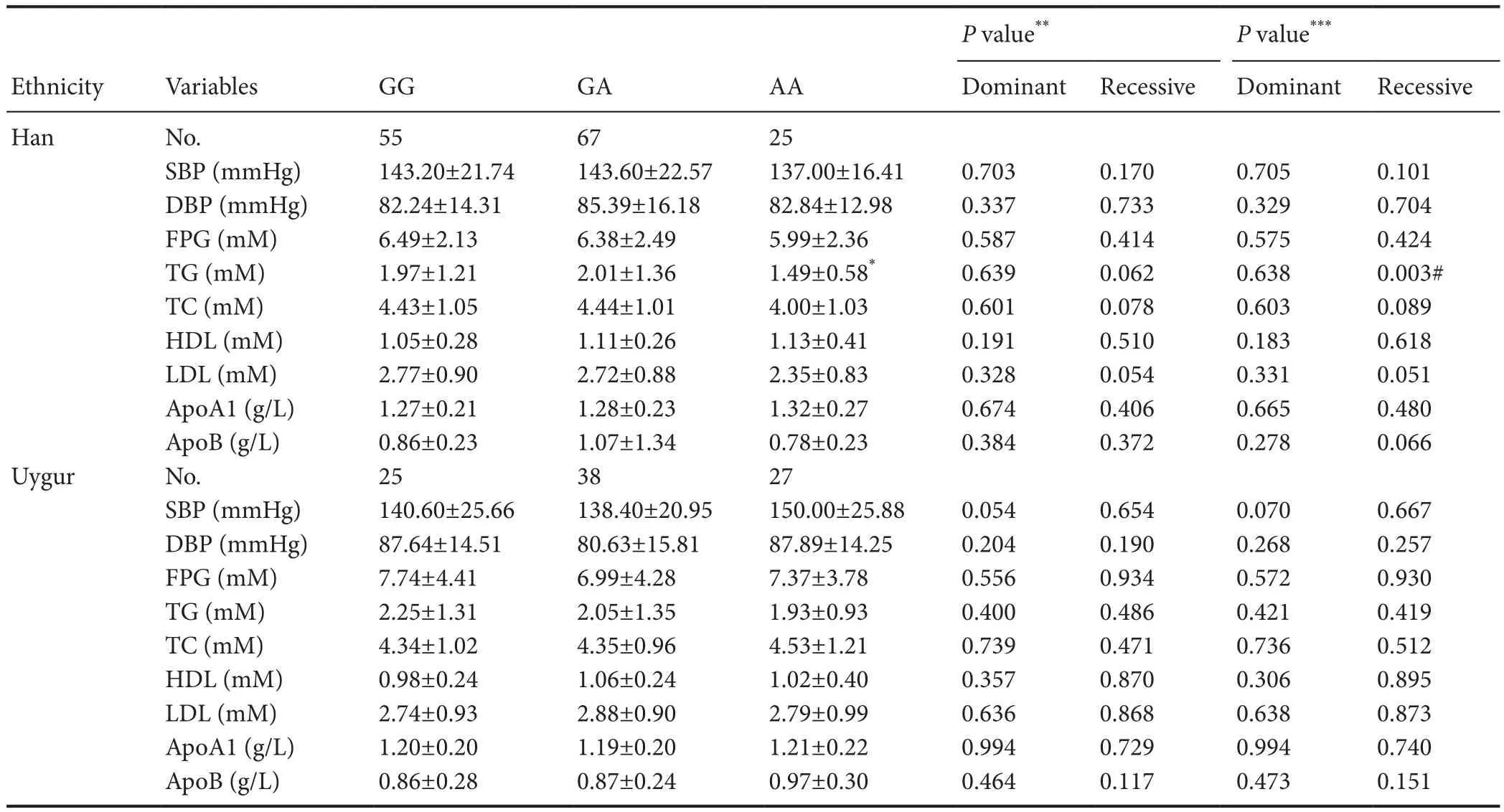
Table 4 Genotype of rs17111503 and the clinical characteristics of the patients

Table 5 Impact of the rs17111503 polymorphism on hypertension, diabetes, smoking and alcohol drinking in patients
To our knowledge, the present study is the fi rst to demonstrate an association between the polymorphisms and the susceptibility to lacunar infarction.
We found that thePCSK9rs17111503 G > A polymorphism has a statistically signif i cant impact on the susceptibility to lacunar infarction in the Han population but not in the Uygur population.PCSK9was synthesized by liver, kidney and small intestine of vertebrates, primarily (Gupta et al., 2010). It was primarily expressed in liver and small intestine of vertebrates. To the best of our knowledge, little is reported regarding whether the rs17111503 polymorphism affectedPCSK9enzyme activity (does it increase or decrease?) and the activity of rs17111503 in blood was ambiguous. This needs further investigation.
ThePCSK9rs11583680, rs483462, rs505151 and rs149311926 polymorphisms were not associated with the risk of lacunar infarction in the Han or Uygur population in China. However, we found a strong relationship between the rs17111503 G > A SNP in thePCSK9gene promoter and the morbidity of lacunar infarction in the Han population but not in the Uygur population, suggesting that ethnicity contributes to the differences. In particular, there was a significantly strong relationship between the AA genotype and morbidity from lacunar infarction, suggesting that the AA genotype may have an important pathogenetic role in lacunar infarction. In the Han population, there was a strong relationship between the polymorphism at this locus and abnormal lipid metabolism, as the AA genotype was associated with lowered levels of triglycerides. Low-density lipoprotein cholesterol levels displayed borderline causative properties in the recessive model (P= 0.051, calculated by unpairedt-test with Welch’s correction). However, no signif i cant association was observed for the other clinical characteristics in the Han and Uygur populations. In addition, subgroup analysis of the Han and Uygur lacunar infarction patients by hypertension, diabetes mellitus, smoking and drinking showed no signif icant association with the rs17111503 G > A polymorphism.
Taken together, our fi ndings suggest that the A allele at this locus is causative for lacunar infarction by increasing the risk for ischemic stroke by af f ecting low-density lipoprotein cholesterol levels and other serum components in the Chinese Han population. However, there are a number of limitations to this study.e patients and healthy controls were registered from the First Af fi liated Hospital of Xinjiang Medical University of China and may not adequately represent the general population. Furthermore, a comprehensive assessment of the impact of genetic variability inPCSK9may not have been given by the polymorphisms investigated in our study. Further fine mapping needs to be performed to better understand the impact of susceptibility regions on stroke. In addition, in this study, the moderate sample size also limited the statistical power of the analyses. Further studies are required to validate our fi ndings, and gene-environment interaction studies should also help elucidate the genetic mechanisms of lacunar infarction.
Despite the limitations of this study, it is the fi rst to investigate the effect ofPCSK9polymorphisms on the susceptibility to lacunar infarction in the Chinese Han and Uygur ethnic groups.e major fi nding is that the rs17111503 G >A polymorphism has a statistically signif i cant impact on the susceptibility to lacunar infarction in the Han population but not the Uygur population.
Acknowledgments:We are very grateful to director of Li-xin Ying and Tuerxun·Shabier from the Department of Neurology of First Affiliated Hospital of Xinjiang Medical University of China for their good proposals.Meanwhile, we are very grateful to head nurse of Jian Jiang and nurses in the Department of Neurology of First Af fi liated Hospital of Xinjiang Medical University for their help in blood sample collection.
Author contributions:XNZ designed this study, performed experiments and analyzed data. DFH performed experiments, analyzed data and wrote the paper. JHM performed experiments and analyzed data. CGH, Tuerhong•Tuerxun and LD performed data collection. All authors approved the fi nal version of the paper.
Conf l icts of interest:None declared.
Research ethics:
Declaration of patient consent:
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Allard D, Amsellem S, Abifadel M, Trillard M, Devillers M, Luc G, Krempf M, Reznik Y, Girardet JP, Fredenrich A, Junien C, Varret M, Boileau C, Benlian P, Rabes JP (2005) Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum Mutat 26:497.
American Diabetes Association: clinical practice recommendations 1997 (1997) Diabetes Care 20 Suppl 1:S1-70.
Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, Asselin M C, Hamelin J, Varret M, Allard D, Trillard M, Abifadel M, Tebon A, Attie AD, Rader DJ, Boileau C, Brissette L, Chretien M, Prat A, Seidah NG (2004) NARC-1/PCSK9 and its natural mutants: zymogen cleavage and ef f ects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem 279:48865-48875.
Benjannet S, Rhainds D, Hamelin J, Nassoury N, Seidah N G (2006)e proprotein convertase (PC) PCSK9 is inactivated by furin and/ or PC5/6A: functional consequences of natural mutations and post-translational modif i cations. J Biol Chem 281:30561-30572.
Chen SN, Ballantyne CM, Gotto AM, Jr., Tan Y, Willerson JT, Marian AJ (2005) A common PCSK9 haplotype, encompassing the E670G coding single nucleotide polymorphism, is a novel genetic marker for plasma low-density lipoprotein cholesterol levels and severity of coronary atherosclerosis. J Am Coll Cardiol 45:1611-1619.
Folsom A R, Peacock J M, Boerwinkle E, Atherosclerosis Risk in Communities Study I (2009) Variation in PCSK9, low LDL cholesterol, and risk of peripheral arterial disease. Atherosclerosis 202:211-215.
Gupta N, Fisker N, Asselin MC, Lindholm M, Rosenbohm C, Orum H, Elmen J, Seidah NG, Straarup EM (2010) A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo. PLoS One 5:e10682.
Hallman DM, Srinivasan SR, Chen W, Boerwinkle E, Berenson GS (2007) Relation of PCSK9 mutations to serum low-density lipoprotein cholesterol in childhood and adulthood (from The Bogalusa Heart Study). Am J Cardiol 100:69-72.
Han D, Ma J, Zhang X, Cai J, Li J, Tuerxun T, Hao C, Du L, Lei J (2014) Correlation of PCSK9 gene polymorphism with cerebral ischemic stroke in Xinjiang Han and Uygur populations. Med Sci Monit 20:1758-1767.
Hobbs HH, Russell DW, Brown MS, Goldstein JL (1990)e LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet 24:133-170.
Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR (2007)e C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 193:445-448.
Horton JD, Cohen J C, Hobbs HH (2007) Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 32:71-77.
Horton JD, Cohen JC, Hobbs HH (2009) PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 50 Suppl:S172-177.
Lagace TA, Curtis DE, Garuti R, Mcnutt MC, Park SW, Prather HB, Anderson NN, Ho YK, Hammer RE, Horton JD (2006) Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest 116:2995-3005.
Lambert G, Charlton F, Rye KA, Piper DE (2009) Molecular basis of PCSK9 function. Atherosclerosis 203:1-7.
Lambert G, Jarnoux A L, Pineau T, Pape O, Chetiveaux M, Laboisse C, Krempf M, Costet P (2006) Fasting induces hyperlipidemia in mice overexpressing proprotein convertase subtilisin kexin type 9: lack of modulation of very-low-density lipoprotein hepatic output by the low-density lipoprotein receptor. Endocrinology 147:4985-4995.
Leren TP (2004) Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin Genet 65:419-422.
Macdonell RA, Kalnins RM, Donnan GA (1987) Cerebellar infarction: natural history, prognosis, and pathology. Stroke 18:849-855.
Mathers C D, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3:e442.
Miyake Y, Kimura R, Kokubo Y, Okayama A, Tomoike H, Yamamura T, Miyata T (2008) Genetic variants in PCSK9 in the Japanese population: rare genetic variants in PCSK9 might collectively contribute to plasma LDL cholesterol levels in the general population. Atherosclerosis 196:29-36.
Ouguerram K, Chetiveaux M, Zair Y, Costet P, Abifadel M, Varret M, Boileau C, Magot T, Krempf M (2004) Apolipoprotein B100 metabolism in autosomal-dominant hypercholesterolemia related to mutations in PCSK9. Arteriosclerromb Vasc Biol 24:1448-1453.
Park SW, Moon YA, Horton JD (2004) Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem 279:50630-50638.
Peterson AS, Fong LG, Young SG (2008) PCSK9 function and physiology. J Lipid Res 49:1595-1599.
Schmidt RJ, Beyer TP, Bensch WR, Qian YW, Lin A, Kowala M, Alborn WE, Konrad R J, Cao G (2008) Secreted proprotein convertase subtilisin/kexin type 9 reduces both hepatic and extrahepatic low-density lipoprotein receptors in vivo. Biochem Biophys Res Commun 370:634-640.
Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M (2003)e secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal dif f erentiation. Proc Natl Acad Sci U S A 100:928-933.
Sun XM, Eden ER, Tosi I, Neuwirth CK, Wile D, Naoumova RP, Soutar AK (2005) Evidence for ef f ect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum Mol Genet 14:1161-1169.
Wang J, Zheng B, Wang QS, Wang J, Cheng SY, Li J (2014) Association of endothelial lipase genetic polymorphism with lacunar infarction in a Chinese population. Int J Clin Exp Med 7:4427-4433.
Yue P, Averna M, Lin X, Schonfeld G (2006)e c.43_44insCTG variation in PCSK9 is associated with low plasma LDL-cholesterol in a Caucasian population. Hum Mutat 27:460-466.
Copyedited by Patel B, Norman C, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Xiao-ning Zhang, M.D., 32193860@qq.com.
Xiao-ning Zhang, M.D., 32193860@qq.com.
orcid: 0000-0002-6763-7634 (Xiao-ning Zhang)
10.4103/1673-5374.213552
Accepted: 2017-05-13
杂志排行
中国神经再生研究(英文版)的其它文章
- Transcriptional inhibition in Schwann cell development and nerve regeneration
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- Effects of estrogen receptor modulators on cytoskeletal proteins in the central nervous system
- Optogenetics and its application in neural degeneration and regeneration
- Live-cell imaging: new avenues to investigate retinal regeneration
- Neurotrophic factors and corneal nerve regeneration
