Transcriptional inhibition in Schwann cell development and nerve regeneration
2017-09-04SusanneQuintesBastianBrinkmann
Susanne Quintes, Bastian G. Brinkmann
1 Max-Planck-Institute of Experimental Medicine, Department of Neurogenetics, Göttingen, Germany
2 Department of Clinical Neurophysiology, University Medical Center Göttingen (UMG), Göttingen, Germany
Transcriptional inhibition in Schwann cell development and nerve regeneration
Susanne Quintes1,2, Bastian G. Brinkmann1,*
1 Max-Planck-Institute of Experimental Medicine, Department of Neurogenetics, Göttingen, Germany
2 Department of Clinical Neurophysiology, University Medical Center Göttingen (UMG), Göttingen, Germany
How to cite this article:Quintes S, Brinkmann BG (2017) Transcriptional inhibition in Schwann cell development and nerve regeneration. Neural Regen Res 12(8):1241-1246.
Schwann cells, the myelinating glial cells of the peripheral nervous system are remarkably plastic after nerve trauma.eir transdif f erentiation into specialized repair cells aer injury shares some features with their development from the neural crest. Both processes are governed by a tightly regulated balance between activators and inhibitors to ensure timely lineage progression and allow re-maturation aer nerve injury. Functional recovery aer injury is very successful in rodents, however, in humans, lack of regeneration aer nerve trauma and loss of function as the result of peripheral neuropathies represents a signif i cant problem. Our understanding of the basic molecular machinery underlying Schwann cell maturation and plasticity has made significant progress in recent years and novel players have been discovered. While the transcriptional activators of Schwann cell development and nerve repair have been well def i ned, the mechanisms counteracting negative regulation of (re-)myelination are less well understood. Recently, transcriptional inhibition has emerged as a new regulatory mechanism in Schwann cell development and nerve repair.is mini-review summarizes some of the regulatory mechanisms controlling both processes and the novel concept of “inhibiting the inhibitors” in the context of Schwann cell plasticity.
Schwann cell; Zeb2; myelin; transcription factors; regeneration; remyelination; neuropathy
Schwann Cell Development and Plasticity
Schwann cells form the lipid-rich myelin sheath enwrapping peripheral nerve axons thereby facilitating saltatory impulse conduction and providing trophic support. Aer nerve injury and, to some extent, in peripheral neuropathies, Schwann cells show a remarkable plasticity, which is a prerequisite for successful nerve regeneration. Schwann cells develop from the neural crest, a transient highly motile cell population giving rise to a multitude of derivatives, including enteric ganglion cells and melanocytes. Their differentiation from Schwann cell precursors to immature Schwann cells and fi nally mature myelinating and non-myelinating Schwann cells is governed by a multitude of factors, some Schwann cell-intrinsic, others stemming from the axon or the extracellular matrix (Jessen and Mirsky, 2005). After peripheral nerve injury, Schwann cells form repair cells, a process which partially recapitulates early Schwann cell development. These highly specialized cells gain unique features, such as the ability to phagocytose myelin and form regenerative tracts (Bands of Büngner) for axons to reinervate their targets. Repair Schwann cells share some properties with the immature Schwann cells in development and have therefore oen been considered to be de-dif f erentiated. However, in addition to the re-expression of immature genes, repair Schwann cells activate injury-related genes, such as those coding for neurotrophins, recruitment of macrophages and phagocytosis, thereby actively supporting nerve repair (Jessen and Mirsky, 2016). Some transcripts, such as oligodendrocyte transcription factor 1 (Olig1), glial cell-derived neurotrophic factor (Gdnf), sonic hedgehog (shh) or artemin are exclusively expressed in the repair cell and absent or only detectable at very low levels in immature cells (Arthur-Farraj et al., 2012; Fontana et al., 2012).is cell type conversion should therefore rather be considered as a transdifferentiation, similar to that observed in other cell types (Jessen et al., 2015).
Positive Regulation of Peripheral Nerve Myelination and Repair
In addition to transcriptional control, epigenetic mechanisms also play an important role in the regulation of the peripheral myelination and remyelination program. Sox10 binding to Oct6 during Schwann cell development leads to the recruitment of Brahma-related gene 1 (Brg1) containing Brg1/Brm-associated factor (BAF) chromatin-remodeling complexes and the histone deacetylases 1 (HDAC1) and HDAC2 (Jacob et al., 2011; Weider et al., 2012). While HDAC2 primarily promotes myelination by transcriptional regulation of Sox10, Oct6 and myelin protein zero, HDAC1 is involved in the control of Schwann cell survival (Jacob et al., 2011). There is also evidence for the involvement of histone modification in the activation of genes after nerve injury (Ma et al., 2015).
Negative Regulators of Peripheral Nerve Myelination and Repair
The positive signals driving peripheral myelination described above have been well defined. The function of negative regulators of myelination and the mechanisms suppressing them to allow Schwann cell maturation are less well understood.ese factors are especially interesting as they are expressed in immature Schwann cells, inactivated in myelinating Schwann cells, and, in many cases, re-expressed aer nerve injury and in peripheral neuropathies.is implies that some of them may play important roles in the establishment and maintenance of the repair Schwann cell or in creating a favourable environment for nerve regeneration. However, just as important as their activation in pathological conditions is the need to silence them again to allow re-di ff erentiation and remyelination. A multitude of pathways and second messenger systems have to be precisely balanced during peripheral nerve myelination (Boerboom et al., 2017). The PI3 kinase/Akt/mTOR pathway is counteracted by the phosphatase and tensin homolog (PTEN), which dephosphorylates PtdIns (3,4,5)P3, thereby ensuring the correct myelin sheath thickness. Inactivation of PTEN in Schwann cells leads to focal hypermyelination of PNS axons and causes a tomaculous neuropathy (Goebbels et al., 2010). PTEN activity is potentiated by the scaffolding protein discs large homolog 1 (Dlg1) and mice lacking Dlg1 speci fi cally from Schwann cells display transient hypermyelination (Noseda et al., 2013). Furthermore, DNA damage-inducible transcript 4 protein (Ddit4) has been identifi ed as a sustained negative regulator of PNS myelination, as loss of expression results in increased PI3kinase/Akt/ mTOR activity and persistent hypermyelination (Noseda et al., 2013).e p38 mitogen-activated protein (Map) kinase also functions as an inihibitor of PNS myelination. P38 MAPK inactivation in myelinating co-cultures promotes myelination and lack of the major isoform p38alpha in conditional knockout mice accelerates developmental myelination (Roberts et al., 2017). An imbalance of PI3 kinase and Map kinase signalling has been demonstrated in a rat model for Charcot-Marie-Tooth disease 1A (CMT1A) and may have implications for other peripheral neuropathies (Fledrich et al., 2014).
Secreted molecules have also been implicated in negative regulation of myelination. Collagen triple-helix containing 1 (Cthrc1) led to prolonged proliferation and delayed myelination when transgenically overexpressed in mice (Apra et al., 2012). Endothelin 1 binding to the endothelin receptor B has been shown to delay lineage progression of Schwann cells and Ednrb-def i cient rats display premature expression of S100 beta protein, a marker for immature Schwann cells (Brennan et al., 2000).
In addition to these pathways, several transcription factors function as inhibitors of peripheral myelination.e pluripotency factor Sox2 has been shown to repress myelin gene expressionin vitro (Le et al., 2005) and, together with Sox10, interacts with positive transcription elongation factor b in Schwann cells (Arter and Wegner, 2015). During regeneration of peripheral nerves, Sox2 acts downstream of EphrinB2 to mediate sorting through relocalization of N-cadherin (Parrinello et al., 2010). More recently, the role of Sox2 as a negative regulator of myelination wasconf i rmedin vivo. Maintained Sox2 expression in a transgenic mouse line led to increased proliferation and suppression of myelination during development and reduced functional recovery aer injury (D.B. Parkinson personal communication).
The transmembrane receptor Notch is cleaved upon ligand binding and its intracellular domain (NICD) enters the nucleus to function as a transcriptional regulator.is signalling pathway is active in Schwann cell precursors, needs to be inactivated to allow final maturation and is then again re-activated in the repair Schwann cell to drive demyelination after injury (Woodhoo et al., 2009). Notch promotes the generation of Schwann cells from precursors and inhibits myelination by opposing Krox20. Furthermore, activation of Notch signalling in adult mice led to myelin breakdown (Woodhoo et al., 2009).
Negative regulators of myelination are absent or expressed at very low levels in mature Schwann cells and are re-expressed in the repair Schwann cell to promote regeneration (Jessen and Mirsky, 2016).is requires mechanisms actively suppressing differentiation inhibitors in a timely manner during development and in order to allow re-maturation and remyelination. It has so far remained elusive how this is accomplished, while the positive feedback loops in Schwann cell development and peripheral nerve regeneration have been well established. Direct transcriptional repression is one possibility to silence genes stalling Schwann cell maturation in development and re-dif f erentiation aer nerve injury.
Zeb2-Mediated Transcriptional Inhibition as a Novel Mechanism Promoting Schwann Cell Dif f erentiation
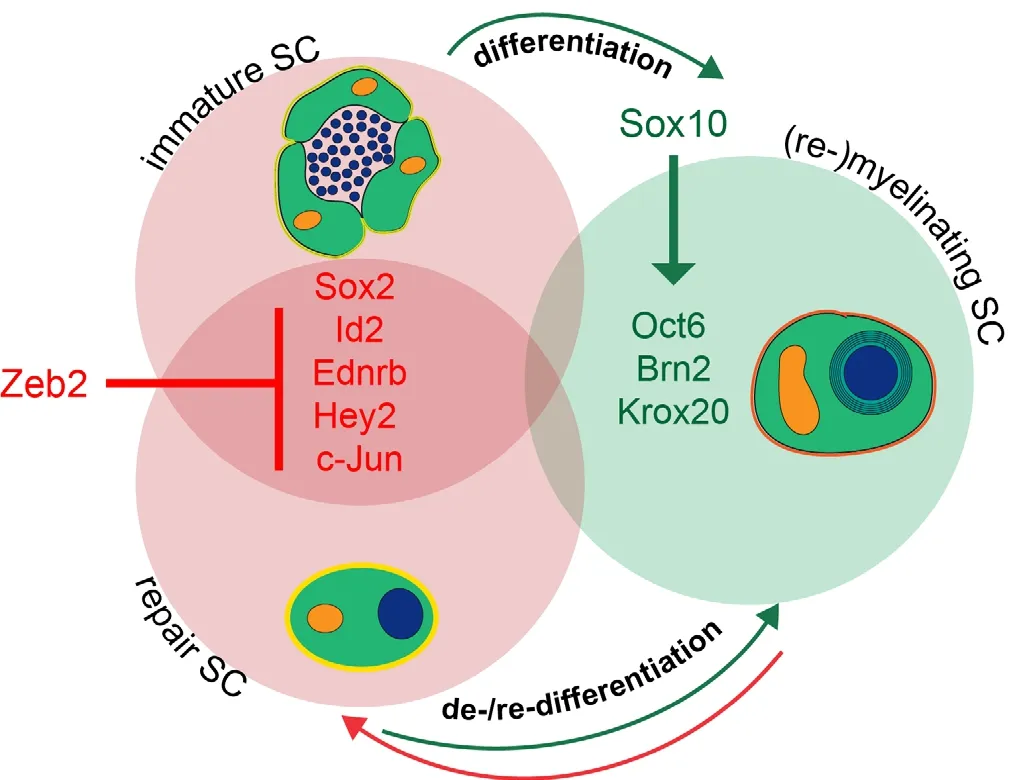
Figure 1 Schematic depiction of transcriptional regulation during SC development and nerve repair.
In accordance with the effects observed in Schwann cell development, re-maturation and regeneration after nerve injury dramatically failed in mice with Zeb2-deficient Schwann cells (Quintes et al., 2016; Wu et al., 2016). Functional recovery of hind limb movement as well as remyelination was severely impaired. The absence of Zeb2 did not a ff ect the formation of the repair cell or removal of myelin debris. Instead, the re-differentiation into remyelinating Schwann cells was hampered. Expression levels of Sox2 and Ednrb remained high in distal stumps of sciatic nerve of conditional Zeb2 mutant mice and Krox20 mRNA expression was low (Quintes et al., 2016). Aberrantly high Hey2 expression levels were not observed aer nerve injury, which led us to hypothesize that this is a unique feature of Zeb2-defi cient Schwann cells during development. We conclude, that Zeb2 acts as a potent transcriptional inhibitor and is crucial for the silencing of factors inhibiting Schwann cell maturation and re-di ff erentiation.is “inhibition of inhibitors” is a novel concept in Schwann cell development and peripheral nerve regeneration.
While the degenerative and regenerative events occurring aer an acute peripheral nerve injury (Wallerian degeneration) have been known since the nineteenth century, major progress has been made recently in understanding the pivotal role of Schwann cell plasticity during peripheral nerve regeneration. Sciatic nerve crush or transection has been used to study axon regrowth and remyelination in genetically altered mice, which has led to the identif i cation of a multitude of factors controlling these processes. In the future it will be crucial to fully understand the function of each individual pathway and how they cooperate to enable nerve regeneration.
It has become clear, that the generation of the repair Schwann cell is not merely a passive de-differentiation or“going backwards” in development, but an active process, which is dependent on the AP1 transcription factor c-Jun (Arthur-Farraj et al., 2012; Jessen and Mirsky, 2016). Just as important as the generation of a proliferating, motile repair cell upon injury is the re-dif f erentiation of this cell into a mature (or Remak) Schwann cell once it comes to remyelination. In contrast to Schwann cells lacking c-Jun, Zeb2-deficient Schwann cells are still able to form repair cells, however, in accordance with our data in development, they constantly show high mRNA expression levels of maturation inhibitors and their re-differentiation to mature myelinating cells is impaired.e resulting failure of nerve regeneration substantiates the importance of the silencing of transcription factors, which are needed to successfully activate the repair process, but at the same time, prevent remyelination (e.g., c-Jun, Sox2, Pax3 and Id2).
Induction of the repair mechanisms in Schwann cells leads to an adaptive cellular reprogramming (Jessen et al., 2015) of mature cells upon nerve injury, which is extremely interesting in regenerative medicine and peripheral neuropathy research. Understanding common molecular control mechanisms involved in both, developmental differentiation and regeneration aer trauma or in peripheral neuropathies, will be valuable to develop new therapeutic strategies. However, one major challenge is the complexity of the regulatory network.
In addition, more research is needed in the analysis of transcription factors expressed in the neural crest and in developing Schwann cells and their possible role in Schwann cell plasticity aer injury. As direct repression of maturation inhibitors is evolving as a novel concept in Schwann cell development and nerve regeneration, transcriptional inhibitors are moving into the focus. One way of identifying possible candidates of interest is to look at genes causative of human neurocristopathies, diseases characterized by malformations of neural crest derivatives. Patients carrying mutations in one of the two alleles of Zeb2 suf f er from one of these rare disorders, Mowat-Wilson-Syndrome (Mowat et al., 1998). Notably, mutations in the Sox10 gene also lead to a neurocristopathy, Waardenburg syndrome (Bondurand et al., 2007). Recent data suggest that transcriptional repression by Zeb2 poses a counterpart to the transcriptional activator Sox10 during Schwann cell maturation (Quintes et al., 2016; Wu et al., 2016).
Another possibility is looking at physiological or patho-logical processes known to require cellular reprogramming, such as the epithelial-to-mesenchymal transition (EMT), a process in which mature, stationary cells regain properties of immature cells such as the ability to migrate and loss of cell-cell contacts (Baum et al., 2008; Conidi et al., 2011). These massive phenotypical changes can be compared to the processes characterizing Schwann cell plasticity and adaptive cellular reprogramming aer injury (Jessen et al., 2015). Zeb2 plays a pivotal role in EMT during tumor metastasis by repressing E-cadherin expression (Comijn et al., 2001).e analysis of Zeb2 function in Schwann cells thereby examplifies that by analyzing genes involved in rare diseases or cell type transitions new insights can be obtained for Schwann cell development and adaptive changes occurring during somatic healing. Moreover, specific inactivation of Zeb2-mediated transcriptional repression in Schwann cells also allowed the identification of novel inhibitors of myelination, such as the downstream ef f ector of Notch signalling Hey2 (Quintes et al., 2016; Wu et al., 2016).
Transcriptional regulators are highly interesting as potential therapeutic targets, however, one enormous challenge for any future treatment trials will be to reach a fi nely tuned balance between proliferation and dif f erentiation. It may prove too dif ficult to pharmacologically regulate the transcription factors themselves, because of their actions as activators or repressors depending on the regulated gene. In addition, unknown downstream targets may cause unwanted side ef f ects. In the case of Zeb2, simultaneous activation of genes related to maturation and myelination cannot be excluded at the moment and additional direct and epigenetic mechanisms of gene silencing in the Schwann cell lineage remain to be elucidated. For therapeutic purposes, it may thus be more promising to focus on single downstream targets. Endothelin receptor antagonists have been used for the treatment of pulmonary hypertension and could be tested in models of nerve injury or peripheral neuropathies.
Some genes re-expressed in Schwann cells after acute nerve injury may also be involved in long-term maintenance of the repair capacity, which holds implications for nerve trauma, where regeneration of severed axons may take years, and peripheral neuropathies where Schwann cells are in a chronically de-differentiated state. Recently, the transcription factor Stat3 was identif i ed to play a major role in longterm denervation by not only supporting the survival of long term denervated Schwann cells, but also maintaining their repair capacity (Benito et al., 2017).
Finally, any future therapeutical approach will have to be carefully timed in order to potentiate regenerative capacity of Schwann cells and at the same time prevent overproliferation and tumor development.
Author contributions:
Conf l icts of interest:None declared.
Plagiarism check: Checked twice by ienticate.
Peer review: Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Open peer reviewer:Patrick Küry, Heinrich-Heine-Universitat Duesseldorf, Germany.
Additional fi le:Open peer review report 1.
Apra C, Richard L, Coulpier F, Blugeon C, Gilardi-Hebenstreit P, Vallat JM, Lindner V, Charnay P, Decker L (2012) Cthrc1 is a negative regulator of myelination in Schwann cells. Glia 60:393-403.
Arter J, Wegner M (2015) Transcription factors Sox10 and Sox2 functionally interact with positive transcription elongation factor b in Schwann cells. J Neurochem 132:384-393.
Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633-647.
Baum B, Settleman J, Quinlan MP (2008) Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 19:294-308.
Benito C, Davis CM, Gomez-Sanchez JA, Turmaine M, Meijer D, Poli V, Mirsky R, Jessen KR (2017) STAT3 Controls the long-term survival and phenotype of repair Schwann cells during nerve regeneration. J Neurosci 37:4255-4269.
Boerboom A, Dion V, Chariot A, Franzen R (2017) Molecular mechanisms involved in Schwann cell plasticity. Front Mol Neurosci 10:45.
Bondurand N, Dastot-Le Moal F, Stanchina L, Collot N, Baral V, Marlin S, Attie-Bitach T, Giurgea I, Skopinski L, Reardon W, Toutain A, Sarda P, Echaieb A, Lackmy-Port-Lis M, Touraine R, Amiel J, Goossens M, Pingault V (2007) Deletions at the SOX10 gene locus cause Waardenburg syndrome types 2 and 4. Am J Hum Genet 81:1169-1185.
Brennan A, Dean CH, Zhang AL, Cass DT, Mirsky R, Jessen KR (2000) Endothelins control the timing of Schwann cell generation in vitro and in vivo. Dev Biol 227:545-557.
Colognato H, Tzvetanova ID (2011) Glia unglued: How signals from the extracellular matrix regulate the development of myelinating glia. Dev Neurobiol 71:924-955.
Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F (2001)e two-handed E box binding zinc fi nger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7:1267-1278.
Conidi A, Cazzola S, Beets K, Coddens K, Collart C, Cornelis F, Cox L, Joke D, Dobreva MP, Dries R, Esguerra C, Francis A, Ibrahimi A, Kroes R, Lesage F, Maas E, Moya I, Pereira PN, Stappers E, Stryjewska A, et al. (2011) Few Smad proteins and many Smad-interacting proteins yield multiple functions and action modes in TGFβ/BMP signaling in vivo. Cytokine Growth Factor Rev 22:287-300.
Doddrell RD, Dun XP, Moate RM, Jessen KR, Mirsky R, Parkinson DB (2012) Regulation of Schwann cell dif f erentiation and proliferation by the Pax-3 transcription factor. Glia 60:1269-1278.
Domènech-Estévez E, Baloui H, Meng X, Zhang Y, Deinhardt K, Dupree JL, Einheber S, Chrast R, Salzer JL (2016) Akt regulates axon wrapping and myelin sheath thickness in the PNS. J Neurosci 36:4506-4521.
Fledrich R, Stassart RM, Klink A, Rasch LM, Prukop T, Haag L, Czesnik D, Kungl T, Abdelaal TAM, Keric N, Stadelmann C, Brück W, Nave K-A, Sereda MW (2014) Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med 20:1055-1061.
Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB (2008) Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci 28:7174-7183.
Fontana X, Hristova M, Da Costa C, Patodia S,ei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A (2012) c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol 198:127-141.
Ghislain J, Charnay P (2006) Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep 7:52-58.
Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Mobius W, Liu X, Lappe-Siee C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA (2010) Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci 30:8953-8964.
Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Grif fi th M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jef f eries HB, Aspichueta P, Elortza F, Aransay AM, Martínez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, et al. (2015) Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol 210:153-168.
Hantke J, Carty L, Wagstaf f LJ, Turmaine M, Wilton DK, Quintes S, Koltzenburg M, Baas F, Mirsky R, Jessen KR (2014) c-Jun activation in Schwann cells protects against loss of sensory axons in inherited neuropathy. Brain 137:2922-2937.
Heinen A, Kremer D, Gottle P, Kruse F, Hasse B, Lehmann H, Hartung HP, Kury P (2008)e cyclin-dependent kinase inhibitor p57kip2 is a negative regulator of Schwann cell dif f erentiation and in vitro myelination. Proc Natl Acad Sci U S A 105:8748-8753.
Heinen A, Tzekova N, Graf f mann N, Torres KJ, Uhrberg M, Hartung HP, Küry P (2012) Histone methyltransferase enhancer of zeste homolog 2 regulates Schwann cell dif f erentiation. Glia 60:1696-1708.
Jacob C, Christen CN, Pereira JA, Somandin C, Baggiolini A, Lötscher P, Ozçelik M, Tricaud N, Meijer D, Yamaguchi T, Matthias P, Suter U (2011) HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat Neurosci 14:429-436.
Jessen KR, Mirsky R (2005)e origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6:671-682.
Jessen KR, Mirsky R (2016)e repair Schwann cell and its function in regenerating nerves. J Physiol 594:3521-3531.
Jessen KR, Mirsky R, Arthur-Farraj P (2015) The role of cell plasticity in tissue repair: adaptive cellular reprogramming. Dev Cell 34:613- 620.
Le N, Nagarajan R, Wang JYT, Araki T, Schmidt RE, Milbrandt J (2005) Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identif i es Sox2 as an inhibitor of Schwann cell dif f erentiation and myelination. Proc Natl Acad Sci U S A 102:2596-2601.
Ma KH, Hung HA, Srinivasan R, Xie H, Orkin SH, Svaren J (2015) Regulation of peripheral nerve myelin maintenance by gene repression through polycomb repressive complex 2. J Neurosci 35:8640-8652.
Makoukji J, Shackleford G, Meffre D, Grenier J, Liere P, Lobaccaro JMA, Schumacher M, Massaad C (2011) Interplay between LXR and Wnt/ β-catenin signaling in the negative regulation of peripheral myelin genes by oxysterols. J Neurosci 31:9620-9629.
Maurel P, Salzer JL (2000) Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci 20:4635-4645.
Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA (2004) Axonal neuregulin-1 regulates myelin sheath thickness. Science 304:700-703.
Mindos T, Dun XP, North K, Doddrell RDS, Schulz A, Edwards P, Russell J, Gray B, Roberts SL, Shivane A, Mortimer G, Pirie M, Zhang N, Pan D, Morrison H, Parkinson DB (2017) Merlin controls the repair capacity of Schwann cells aer injury by regulating Hippo/YAP activity. J Cell Biol 216:495-510.
Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR (2013) Gpr126 functions in Schwann cells to control dif f erentiation and myelination via G-protein activation. J Neurosci 33:17976-17985.
Mogha A, Harty BL, Carlin D, Joseph J, Sanchez NE, Suter U, Piao X, Cavalli V, Monk KR (2016) Gpr126/Adgrg6 has Schwann cell autonomous and nonautonomous functions in peripheral nerve injury and repair. J Neurosci 36:12351-12367.
Morton PD, Johnstone JT, Ramos AY, Liebl DJ, Bunge MB, Bethea JR (2012) N Nuclear factor-κB activation in Schwann cells regulates regeneration and remyelination. Glia 60:639-650.
Mowat DR, Croaker GD, Cass DT, Kerr BA, Chaitow J, Adès LC, Chia NL, Wilson MJ (1998) Hirschsprung disease, microcephaly, mental retardation, and characteristic facial features: delineation of a new syndrome and identif i cation of a locus at chromosome 2q22-q23. J Med Genet 35:617-623.
Nodari A, Previtali SC, Dati G, Occhi S, Court FA, Colombelli C, Zambroni D, Dina G, Del Carro U, Campbell KP, Quattrini A, Wrabetz L, Feltri ML (2008) Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J Neurosci 28:6714-6719.
Nodari A, Zambroni D, Quattrini A, Court FA, D’Urso A, Recchia A, Tybulewicz VLJ, Wrabetz L, Feltri ML (2007) Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol 177:1063-1075.
Noseda R, Belin S, Piguet F, Vaccari I, Scarlino S, Brambilla P, Boneschi FM, Feltri ML, Wrabetz L, Quattrini A, Feinstein E, Huganir RL, Bolino A (2013) DDIT4/REDD1/RTP801 is a novel negative regulator of Schwann cell myelination. J Neurosci 33:15295-15305.
Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D’Antonio M, Mirsky R, Jessen KR (2004) Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol 164:385-394.
Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR (2008) Switching myelination on and of f. J Cell Biol 181:625-637.
Parrinello S, Napoli I, Ribeiro S, Digby PW, Fedorova M, Parkinson DB, Doddrell RDS, Nakayama M, Adams RH, Lloyd AC (2010) EphB signaling directs peripheral nerve regeneration through Sox2-dependent schwann cell sorting. Cell 143:145-155.
Poitelon Y, Lopez-Anido C, Catignas K, Berti C, Palmisano M, Williamson C, Ameroso D, Abiko K, Hwang Y, Gregorief f A, Wrana JL, Asmani M, Zhao R, Sim FJ, Wrabetz L, Svaren J, Feltri ML (2016) YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat Neurosci 19:879-887.
Quintes S, Brinkmann BG, Ebert M, Fröb F, Kungl T, Arlt FA, Tarabykin V, Huylebroeck D, Meijer D, Suter U, Wegner M, Sereda MW, Nave KA (2016) Zeb2 is essential for Schwann cell dif f erentiation, myelination and nerve repair. Nat Neurosci 19:1050-1059.
Roberts SL, Dun XP, Dee G, Gray B, Mindos T, Parkinson DB (2017)e role of p38alpha in Schwann cells in regulating peripheral nerve myelination and repair. J Neurochem 141:37-47.
Sherman DL, Krols M, Wu LMN, Grove M, Nave KA, Ganglof f YG, Brophy PJ (2012) Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J Neurosci 32:1817-1825.
Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL (2005) Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47:681-694.
Van de Putte T, Francis A, Nelles L, van Grunsven LA, Huylebroeck D (2007) Neural crest-specif i c removal of Zx1b in mouse leads to a wide range of neurocristopathies reminiscent of Mowat-Wilson syndrome. Human Mol Genet 16:1423-1436.
Verschueren K, Remacle JE, Collart C, KraH, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D (1999) SIP1, a novel zinc fi nger/homeodomain repressor, interacts with Smad proteins and binds to 5’-CACCT sequences in candidate target genes. J Biol Chem 274:20489-20498.
Weider M, Küspert M, Bischof M, Vogl MR, Hornig J, Loy K, Kosian T, Müller J, Hillgärtner S, Tamm ER, Metzger D, Wegner M (2012) Chromatin-remodeling factor Brg1 is required for Schwann cell dif f erentiation and myelination. Short Article. Dev Cell 23:193-201.
Woodhoo A, Alonso MBD, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR (2009) Notch controls embryonic Schwann cell dif f erentiation, postnatal myelination and adult plasticity. Nat Neurosci 12:839-847.
Wu LM, Wang J, Conidi A, Zhao C, Wang H, Ford Z, Zhang L, Zweier C, Ayee BG, Maurel P, Zwijsen A, Chan JR, Jankowski MP, Huylebroeck D, Lu QR (2016) Zeb2 recruits HDAC-NuRD to inhibit Notch and controls Schwann cell dif f erentiation and remyelination. Nat Neurosci 19:1060-1072.
*< class="emphasis_italic">Correspondence to: Bastian G. Brinkmann, M.D., brinkmann@em.mpg.de.
Bastian G. Brinkmann, M.D., brinkmann@em.mpg.de.
orcid: 0000-0002-7177-5749 (Susanne Quintes) 0000-0003-1482-2788 (Bastian G. Brinkmann)
10.4103/1673-5374.213537
Accepted: 2017-06-28
杂志排行
中国神经再生研究(英文版)的其它文章
- LINGO-1 and AMIGO3, potential therapeutic targets for neurological and dysmyelinating disorders?
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- Effects of estrogen receptor modulators on cytoskeletal proteins in the central nervous system
- Optogenetics and its application in neural degeneration and regeneration
- Live-cell imaging: new avenues to investigate retinal regeneration
- Neurotrophic factors and corneal nerve regeneration
