Extract of Ginkgo biloba promotes neuronal regeneration in the hippocampus after exposure to acrylamide
2017-09-04WeilingHuangYuxinMaYubaoFanShengminLaiHongqingLiuJingLiuLiLuoGuoyingLiSuminTian
Wei-ling Huang, Yu-xin Ma, Yu-bao Fan Sheng-min Lai Hong-qing Liu Jing Liu Li Luo Guo-ying Li Su-min Tian
1 Department of Anatomy, School of Basic Medicine, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
2 Department of Physiology, School of Basic Medicine, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
Extract of Ginkgo biloba promotes neuronal regeneration in the hippocampus after exposure to acrylamide
Wei-ling Huang1,#, Yu-xin Ma1,#, Yu-bao Fan1, Sheng-min Lai1, Hong-qing Liu1, Jing Liu1, Li Luo1, Guo-ying Li1, Su-min Tian2,*
1 Department of Anatomy, School of Basic Medicine, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
2 Department of Physiology, School of Basic Medicine, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China
How to cite this article:Huang WL, Ma YX, Fan YB, Lai SM, Liu HQ, Liu J, Luo L, Li GY, Tian SM (2017) Extract of Ginkgo biloba promotes neuronal regeneration in the hippocampus aer exposure to acrylamide. Neural Regen Res 12(8):1287-1293.
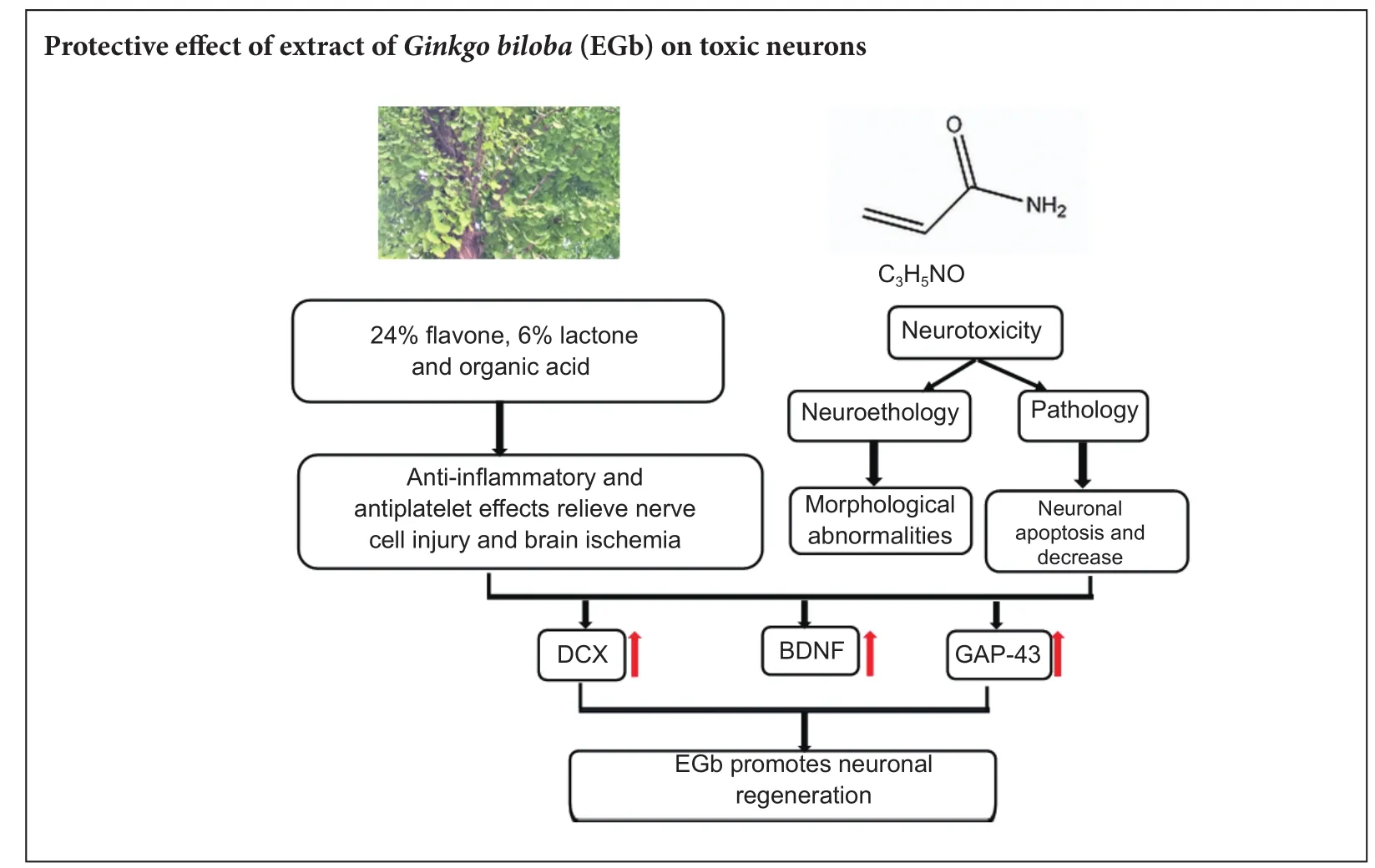
Graphical Abstract
Previous studies have demonstrated a neuroprotective ef f ect of extract ofGinkgo bilobaagainst neuronal damage, but have mainly focused on antioxidation of extract ofGinkgo biloba. To date, limited studies have determined whether extrasct ofGinkgo bilobahas a protective ef f ect on neuronal damage. In the present study, acrylamide and 30, 60, and 120 mg/kg extract ofGinkgo bilobawere administered for 4 weeks by gavage to establish mouse models. Our results showed that 30, 60, and 120 mg/kg extract ofGinkgo bilobaef f ectively alleviated the abnormal gait of poisoned mice, and up-regulated protein expression levels of doublecortin (DCX), brain-derived neurotrophic factor, and growth associated protein-43 (GAP-43) in the hippocampus. Simultaneously, DCX- and GAP-43-immunoreactive cells increased.ese fi ndings suggest that extract ofGinkgo bilobacan mitigate neurotoxicity induced by acrylamide, and thereby promote neuronal regeneration in the hippocampus of acrylamide-treated mice.
nerve regeneration; brain injury; extrat of Ginkgo biloba; acrylamide; doublecortin; brain-derived neurotrophic factor; growth associated protein-43; neurons; damage; hippocampus; mice; neural regeneration
Introduction
Acrylamide (ACR) is a white crystal chemical that is a common raw material of polyacrylamide product. In many industries around the world, ACR is used for water purification, the inner coating of pipelines, and pulp processing (Rosen et al., 2002; Dybing et al., 2003). Moreover, foods rich in starch can produce ACR after high temperature cooking (above 120°C) (Ma et al., 2011; Krishna et al., 2015; Sen et al., 2015). ACR produces defective neurological hallmarks such as skeletal muscle weakness and ataxia. Quantitative morphometric and electrophysiological analyses show that nerve terminals are the primary sites of ACR action(LoPachin et al., 2005). However, current studies are not standardized to evaluate the neurotoxicity of ACR, making it difficult to define a toxic level in the nervous system (Friedman et al., 1999; Santhanasabapathy et al., 2015). At present, most laboratories study neurotoxicity by analyzing morphometric, molecular, and biochemical changes. For behavior, dif f erent functional tests have been used to assess development of hindlimb skeletal muscle weakness and ataxia (LoPachin et al., 2002).us, we used gait score and the open-f i eld test in the present study to investigate features of mouse behavior. Previous studies have revealed that ACR shows neurotoxicity, reproductive toxicity, and carcinogenic properties (especially in neurotoxicity). Further, many studies have reached a consensus on the strong relationship between ACR neurotoxicity and doublecortin (DCX) expression (LoPachin et al., 2004; Ogawa et al., 2012). In addition, brain-derived neurotrophic factor (BDNF) and growth associated protein-43 (GAP-43) increase DCX expression (Song et al., 2013).erefore, we investigated the ef f ects of extract ofGinkgo biloba(EGb) on neuronal regeneration in the hippocampus of mice treated with ACR. Accordingly, we demonstrate that expression levels of DCX, BDNF, and GAP-43 are strongly interconnected.
In recent years, numerous herbal medicines have attracted the attention of many researchers for the treatment of neurological diseases. For example,Radix PuerariaeandRhizoma Acori Tatarinowiiexert neuroprotective effects (Zhu et al., 2016). EGb shows protective ef f ects against senile dementia (Stackman et al., 2003; Tan et al., 2015) and cardiovascular disease (Schneider et al., 2010). Likewise, an effect of EGb has been demonstrated on anxiety-like behavior and locomotor activity (Ribeiro et al., 2016), modulation of inf l ammatory mediators and the cholinergic system (Kim et al., 2016), and in dementia treatment (Hashiguchi et al., 2015). In particular, EGb enhances regeneration of injured peripheral nerves. Nonetheless, previous studies on the ef f ect of EGb on brain damage have mainly focused on oxidative damage (Aydin et al., 2016; Sener et al., 2017) and neuronal damage (Massieu et al., 2004; Eckert et al., 2005), with neuronal regeneration insuf ficiently researched. Newborn neurons are labeled by DCX in the brain, and there is a strong relationship between neuronal regeneration and BDNF, GAP-43, and DCX (Song et al., 2013). Therefore, in this study, we investigated the protective effect of EGb on neuronal regeneration in the hippocampus of mice treated with ACR. Our study may provide a scientif i c foundation for the use of EGb in preventing and treating ACR neurotoxicity.
Materials and Methods
Animal model preparation
Forty male Kunming mice weighing 22–26 g (at the start of the experiment) were purchased from the Animal Experimental Center of Guangdong Province of China (certif i cation No. SYXK (Yue) 2013-0002). All animals were housed in an animal room on 12-hour dark/light cycles, and allowed free access to food and water. EGb was purchased from Ruilin Biotechnology Co., Ltd., (Xi’an, China). Its content was: 24% fl avone, 6% esters, and organic acids.
The study protocol was approved by the Animal Ethics Committee of Guangdong Pharmaceutical University of China (approval No. gdpu2016022). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Mice were randomly divided into fi ve groups, with eight mice per group. In the control group, mice did not undergo any procedure. In the ACR group, mice were administrated saline in the morning and 20 mg/kg/d ACR (Yongda, Inc., Tianjin, China) in the afternoon by gavage for 4 weeks. In the 30, 60, and 120 mg/kg EGb groups, mice were administrated 30, 60, and 120 mg/kg/d EGb, respectively, in the morning and 20 mg/kg/d ACR in the afternoon by gavage for 4 weeks.
Assessment of locomotor function
Gait score
Standards of grading were as follows (LoPachin et al., 2002): score 1, normal gait; score 2, a slightly abnormal gait (slightly inharmonious and increased foot distance); score 3, moderately abnormal gait (foot weakness, obvious movement abnormalities characterized by abduction of legs); score 4, severely abnormal gait (hind limbs paralyzed and unable to support the body, and foot splays).
Open fi eld test
Immunohistochemistry
After behavioral testing, four mice from each group were deeply anesthetized with 4% chloral hydrate by peritoneal injection and perfused with 0.9% saline followed by 4% paraformaldehyde in 0.01 M phosphate buf f er solution (pH 7.4). Brains were immediately removed, post-fixed overnight in paraformaldehyde, washed with running tap water overnight, dehydrated, embedded in paraffin, and sliced into 4 µm-thick coronal sections with a microtome. Sections were mounted onto glass slides, hydrated in graded ethanol, immersed in 0.01 M citrate buf f er (0.01 M; pH 6.0), and heated for 20 minutes in a microwave oven at 90°C for antigen retrieval. After cooling to room temperature, sec-tions were treated with 3% hydrogen peroxide at 37°C for 15 minutes to inactivate endogenous peroxide enzyme. Sections were blocked with 1% bovine serum albumin (in 0.5% Triton-X-100) for 30 minutes at 37°C, and then incubated at 4°C overnight with primary antibodies: rabbit polyclonal anti-DCX (1:600; Abcam, Cambridge, MA, USA) and rabbit monoclonal anti-GAP43 (1:300; Abcam). On the second day, sections were rinsed three times in 0.01 M PBS and incubated for 40 minutes at 37°C with secondary antibodies: horseradish peroxidase AffiniPure goat anti-rabbit IgG (1:500; EARTHOX, San Francisco, CA, USA). The peroxidase reaction was performed using diaminobenzidine (Boster, Wuhan, China) for 2–8 minutes. Color change was observed under a microscope (BX-51; Olympus, Tokyo, Japan). Sections were dehydrated and mounted in neutral resin after counterstaining with hematoxylin. Hippocampal sections were examined under a microscope.
Imaging analysis
Brain sections were examined and photographed under a microscope. Brains from four mice were chosen from each group. The total number of cells from six coronal sections per hippocampus, spanning −1.8 to −3.2 mm posterior to bregma, were used as the sample volume for cell counting. Four high-power (400×) fi elds were taken of the hippocampal dentate gyrus from each section. Image Pro-Plus 6.0 soware (Media Cybernetics, Rockville, MD, US) was used to count immunopositive cells and measure optical density.
Western blot assay
Hippocampi of four mice from each group were homogenized in phenylmethyl sulfonylfluoride radioimmune precipitation assay lysis buf f er and centrifuged for 15 minutes at 4°C. Supernatant protein was removed and the concentration determined using the bicinchoninic acid Protein Assay Kit (Beyotime, Shanghai, China). Protein samples were separated by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis under the same conditions, and transferred onto polyvinylidene fl uoride membranes at 300 mA for 2 hours. Membranes were blocked with 5% nonfat milk for 1 hour at room temperature, and then incubated at 4°C overnight with primary antibodies: rabbit polyclonal anti-DCX (1:600; Abcam), rabbit monoclonal anti-GAP-43 (1:300; Abcam), and rabbit monoclonal anti-BDNF (1:250; Abcam). On the second day, membranes were rinsed four times with Tris-buf fered saline with Tween, and incubated for 1 hour at room temperature with secondary antibodies: horseradish peroxidase Af fi niPure goat-anti rabbit IgG (1:10,000; EARTHOX). Membranes were washed four times with Tris-buf f ered saline with Tween and detected by enhanced chemiluminescence (super ECL Assay Kit, EARTHOX). Blots were incubated with β-tubulin (1:1,000; Millipore, Billerica, MA, USA) as a loading control. All western blot analyses were made in triplicate.e optical density of each labeled band was measured using Quantity One soware (Bio-Rad, CA, USA).
Statistical analysis
All experimental data were presented as the mean ± SD and analyzed by SPSS 17.0 software (SPSS, Chicago, IL, USA). Differences between groups were compared by one-way analysis of variance followed by Dunnett’spost hoctest.P<0.05 was considered statistically signif i cant.
Results
EGb ef f ect on motor function in mice exposed to ACR
Gait score significantly increased in mice of the ACR group (3.75 ± 0.46) compared with the control group (P<0.01; Table 1). Altogether, these results suggest that mice in the ACR group exhibit a severe gait abnormality. However, in the EGb therapeutic groups, this abnormal gait showed a marked improvement, indicated by an visibly decreased gait score. Mice in the EGb groups showed considerably lower gait scores compared with the ACR group. Statistical analysis showed that all EGb groups were signif i cantly decreased compared with the ACR group (P< 0.01; Table 1).
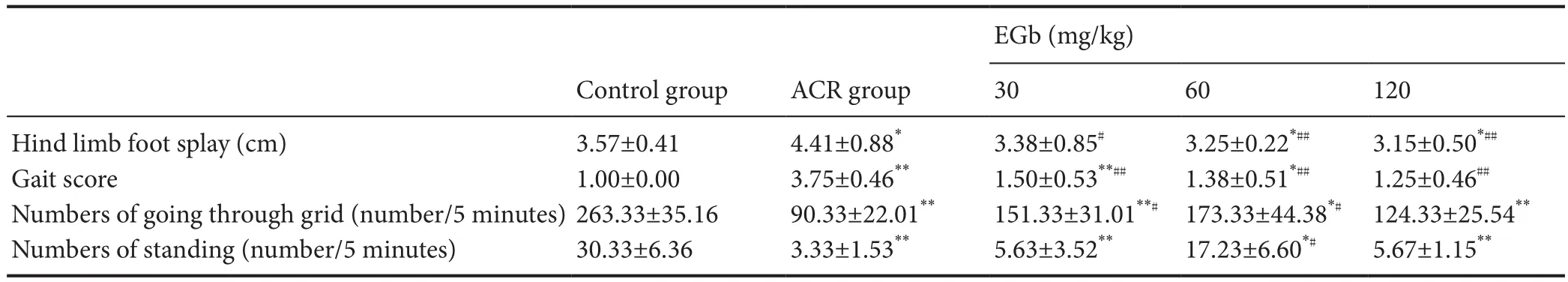
Table 1 Ef f ect of dif f erent EGb doses on abnormal gait in mice treated with ACR
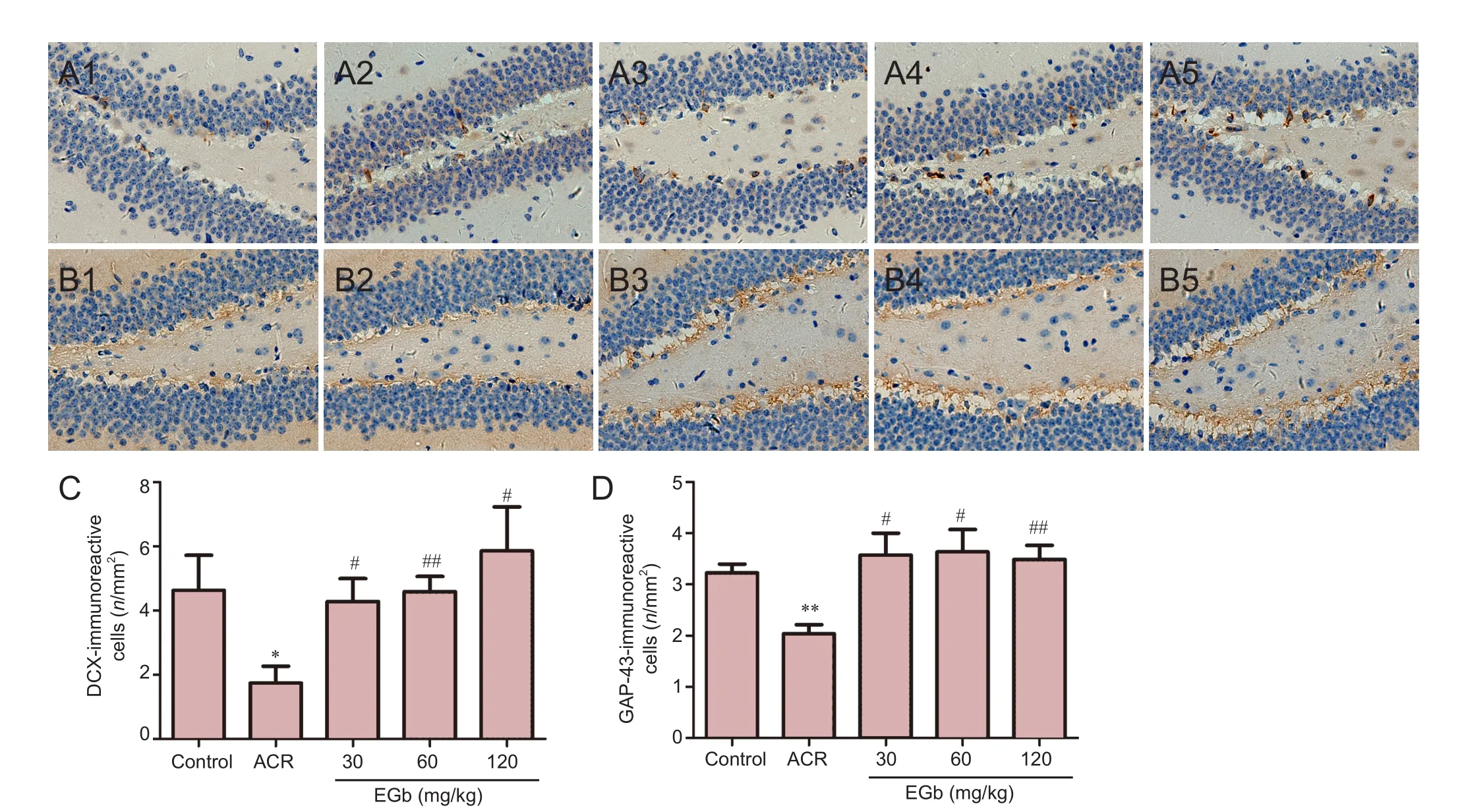
Figure 1 EGb ef f ect on DCX and GAP-43 immunoreactivity in the hippocampus of mice treated with ACR.
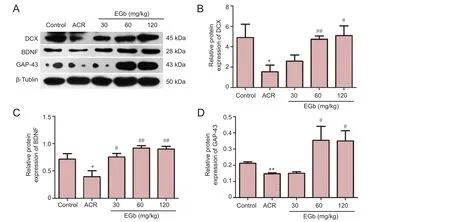
Figure 2 EGb ef f ect on protein expression of DCX, BDNF, and GAP-43 in mice treated with ACR.
EGb ef f ect on DCX and GAP-43 immunoreactivities
EGb administration increased DCX immunoreactivity in the hippocampus of mice. DCX-immunoreactive cells were mainly distributed in the subgranular layer of the dentate gyrus of the mouse hippocampus. DCX is a neuronal precursor marker, which can be used to study neuronal proliferation, migration, and dif f erentiation (Sánchez-Farías et al., 2015). Hence, we determined if EGb has benef i cial ef f ects on DCX immunoreactivity. DCX immunoreactivity decreased in the ACR group. DCX-immunoreactive cells were round or oval. Synaptic morphological and structural changes were found in the mouse hippocampus. Terminal branches were swollen and the number of synapses decreased. However, aer 4 weeks of EGb administration, DCX immunoreactivity nearly returned to normal levels. Synapses maintained good morphology, with promotion of neurite outgrowth and increased synapse number in DCX-immunoreactive cells (Figure 1A). Quantitative analysis showed a signif i cantly decreased number of DCX-immunoreactive cells in the ACR group compared with the control group (P< 0.05; Figure 1C). Aer 4 weeks of EGb administration, ACR and EGb significantly increased DCX immunoreactivity (P< 0.05; Figure 1C).erefore, EGb supplementation may be useful against neuronal damage induced by ACR.
EGb administration increased GAP-43 immunoreactivity in the hippocampus. GAP-43 is a specific phosphoric acid protein that is highly expressed in active brain regions. During neural development, GAP-43 plays an important role in synaptogenesis, as well as in synaptic connections between nerve cells (Li et al., 2002). As a crucial molecule for neuronal growth, GAP-43 has a signif i cant ef f ect on neuronal development (Phatak et al., 2015). We found that ACR administration decreased GAP-43 immunoreactivity in the hippocampus in the ACR group compared with the control group. However, GAP-43 immunoreactivity increased at dif f erent concentrations of EGb administrated (Figure 1D). Quantitative analyses revealed signif i cant dif f erences in ACR mice compared with control mice (P< 0.01; Figure 1D). In addition, there were significant differences between the 30 mg/kg and 60 mg/kg EGb groups, and ACR group (P< 0.05, Figure 1D). Additionally, there were signif i cant dif f erences between the 120 mg/kg EGb group and ACR group (P< 0.01; Figure 1D).
Western blot assay for DCX, BDNF, and GAP-43 protein expression in the hippocampus
BDNF plays a crucial role in neuronal differentiation, growth, and development. Lack of central BDNF interferes with neuronal differentiation in the hippocampus of adult mice (Schmitz et al., 2014). Consistent with previous results, ACR treatment reduced protein expression of DCX, BDNF, and GAP-43 (Figure 2). However, in the 30, 60, and 120 mg/kg EGb groups, protein expression increased to dif f ering levels. Quantitative analyses showed that DCX, BDNF, and GAP-43 showed significant differences between the ACR group and control group (P< 0.05; Figure 2B–D). In contrast, DCX protein expression increased in the 120 mg/kg EGb group compared with the ACR group (P< 0.05; Figure 2B). A more significant increase was observed in the 60 mg/kg EGb group compared with the ACR group (P< 0.01; Figure 2B). Similarly, there was a significant difference in BDNF protein expression between the 30 mg/kg EGb group and ACR group (P< 0.05; Figure 2C), with more marked differences between the 60 and 120 mg/kg EGb groups and ACR group (bothP< 0.01; Figure 2C). GAP-43 protein expression was significantly higher in the 60 and 120 mg/kg EGb groups compared with the ACR group (P< 0.05; Figure 2D).
Discussion
ACR is a highly hydrophilic chemical that shows medium toxicity to nerves, and which can damage the nervous systemviamany routes. Previous studies have shown that ACR produces similar neurotoxicity at low and high doses, with low doses only requiring longer exposure (Erkekoglu et al., 2014). Furthermore, symptoms of peripheral and central nervous system damage appear in ACR poisoned mice (Pennisi et al., 2013; Mehri et al., 2015). ACR can lead to pathological lesions and remodeling in nerve terminals with the presence of distal axonal swellings and degeneration (LoPachin et al., 2015). Symptoms of ACR-mouse neurotoxicity include severe gait abnormalities. In the present study, ACR-treated mice showed symptoms of paralyzed hind limbs, which could not support their body weight, and decreased movement capacity. We proposed that ACR might destroy the central nervous system through inhibition of signal transmission between synapses, or alternatively, changes in function of the efferent system. At present, there are no specif i c drugs to relieve ACR toxicity, therefore many studies have focused on the active ingredients in herbal plants. EGb is a traditional Chinese medicine that contains flavonoids and other active components with important medical properties. EGb shows a protective effect against cerebral ischemia injury, neuroplasticity, and neurodegenerative diseases (Müller et al., 2012; Zhang et al., 2012, 2017).us, we used EGb to relieve ACR poisoning.
To investigate the ef f ect of ACR poisoning on movement disorders, and in turn the therapeutic ef f ect of EGb, we performed gait analysis and the open-f i eld test. We found that ACR-treated mice presented with tremors during walking and weakness or paralysis in posterior limbs, consistent with a previous study (DeGrandchamp et al., 1990). We also observed that particular neurotoxicity symptoms were produced in a certain way when ACR enters the body, such asbehavioral changes and abnormal gait. Nevertheless, not all our experimental fi ndings agree with this assumption. For example, in the open-f i eld test, administration of 120 mg/kg EGb had no effect on number of grid crossings, while 30 mg/kg and 120 mg/kg EGb had no ef f ect on frequency of rearing.ese results suggest that the therapeutic effect of EGb has a strict concentration range. We will address the most ef f ective concentration of EGb for a therapeutic ef f ect in future experiments.
DCX is associated with the normal brain development processes of neuronal cell birth and migration (Rao et al., 2004; Reiner et al., 2013; Yoo et at., 2016). BDNF plays an important role in prevention of neurobiological changes and neuronal protection (Garraway et al., 2016; Gonzalez et al., 2016; Shrivastava et al., 2016; Cheah et al., 2017). GAP-43 is essential for promoting denervation-induced sprouting, maintaining normal climbing fi ber structure, and remodeling axon terminals (Wang et al., 2001; Erkekoglu et al., 2014; Hou and Kang, 2016). We investigated the mechanism of ACR neurotoxicity by analyzing structural changes in the hippocampus. We found decreased DCX, BDNF, and GAP-43 expression in the hippocampus of ACR-treated mice.ese results are consistent with other studies (Ogawa et al., 2012; Song et al., 2013), and suggest that neuronal regeneration is blocked by ACR administration. Altogether, this indicates that the brain is particularly vulnerable to the neurotoxic ef f ects of ACR, which are associated with behavioral changes in mice. Encouragingly, we found increased DCX expression in the hippocampus in all three EGb-treated groups, as well as increased BDNF and GAP-43 expression levels. Thus, inhibition of neuronal regeneration is associated with decreased BDNF and GAP-43 levels in the mouse hippocampus, while EGb caused an increase in BDNF and GAP-43 expression, which promotes neural growth.
DCX is a microtubule-associated protein expressed by neuronal precursor cells, and is associated with neuronal regeneration (Ryu et al., 2016). Nerve injury and neurotoxicity prevent DCX expression (Ko et al., 1999). However, nerve injury can cause increased DCX expression (Ma et al., 2015). Therefore, it is likely that different protection mechanisms deal with distinct types of damage. Some injuries stimulate the brain’s repair system and others inf l uence neurotrophic factors or neuronal survival. Hence, treatment measures should be selected according to dif f erent damage mechanisms. Our results show that DCX expression can be reduced by ACR administration. Simultaneously, BDNF and GAP-43 expression are decreased, which means that neurogenesis is blocked in the mouse hippocampus. However, EGb administration reversed the damage induced by ACR.ese observations conf i rm that EGb has a protective ef f ect by promoting neuronal regeneration.
It is well known that EGb is particularly ef f ective on promoting brain blood circulation and antioxidants (Rojas et al., 2012). Here, the mechanisms of how ACR injures the central nervous system, and how EGb inf l uences DCX, BDNF, and GAP-43 expression are not clear.us, we still do not know how EGb influences ACR neurotoxicity, whether ACR is cleared in brain blood, or EGb only increases DCX, BDNF, and GAP-43 expression, or both.is needs further research to address these issues. Nonetheless, our findings demonstrate that there is a strong relationship between increased DCX, BDNF, and GAP-43 expression and protection af f orded by EGb.
In conclusion, EGb administration improves ACR-induced neuronal damage, mainly by promoting neuronal regeneration, which is shown by increased DCX, BDNF, and GAP-43 expression. Thus, EGb has a therapeutic effect on ACR neurotoxicity. EGb may promote neuronal regeneration in the hippocampus of ACR-treated mice, and can be exploited to improve ACR damage, although its mechanism still needs further investigation.
Author contributions:WLH and YXM designed the study, performed experiments and wrote the paper. YBF, SML and JL participated in the experimental implementation and data analysis. HQL and LL prepared animal models. GYL and SMT supervised the study and modif i ed the paper. All authors approved the fi nal version of the manuscript.
Conf l icts of interest:None declared.
Research ethics:
Data sharing statement:
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Aydin D, Peker E, Karakurt M, Gurel A, Ayyildiz M, Cevher Ş, Agar E, Dane S (2016) Ef f ects of Ginkgo biloba extract on brain oxidative condition aer cisplatin exposure. Clin Invest Med 39:27511.
Cheah SY, McLeay R, Wockner LF, Lawford BR, Young RM (2017) Expression and methylation of BDNF in the human brain in schizophrenia. World J Biol Psychiatry doi: 10.1080/15622975.2016.1245443.
DeGrandchamp RL, Reuhl KR (1990) Lowndes HE Synaptic terminal degeneration and remodeling at the rat neuromuscular junction resulting from a singleexposure to acrylamide. Toxicol Appl Pharmacol 105:422-433.
Dybing E, Sanner T (2003) Risk assessment of acrylamide in foods. Toxicol Sci 75:7-15.
Eckert A, Keil U, Scherping I, Hauptmann S, Müller WE (2005) Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann N Y Acad Sci 1056:474-85.
Erkekoglu P, Baydar T (2014) Acrylamide neurotoxicity. Nutr Neurosci 17:49-57.
Friedman M, Rochelle W, Tyl R, Marr M, Myers C, Gerling F (1999) Ef f ects of lactational administration of acrylamide on rat dams and of f spring. Reprod Toxicol 13: 511-520.
Garraway SM, Huie JR (2016) Spinal plasticity and behavior: BDNF-induced neuromodulation in uninjured and injured spinal cord. Neural Plast 2016:9857201.
Gonzalez A, Moya-Alvarado G, Gonzalez-Billaut C, Bronfman FC (2016) Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton (Hoboken) 73:612-628.
Grasselli G, Strata P (2013) Structural plasticity of climbing fi bers and the growth-associated protein GAP-43. Front Neural Circuit 7:25.
Hashiguchi M, Ohta Y, Shimizu M, Maruyama J, Mochizuki M (2015) Meta-analysis of the ef fi cacy and safety of Ginkgo biloba extract for the treatment of dementia. J Pharm Health Care Sci 1:14.
Hou YJ, Kang HP (2016) Effects of acupuncture and rehabilitation therapy on the expression of growth associated protein-43 and synaptophysin at the injury site of cerebral palsy rats. Zhongguo Zuzhi Gongcheng Yanjiu 20:3999-4005.
Kim MS, Bang JH, Lee J, Han JS, Balk TG, Jeon WK (2016) Ginkgo biloba L. extract protects against chronic cerebral hypoperfusion by modulating neuroinf l ammation and the cholinergic system. Phytomedicine 23:1356-1364.
Ko MH, Chen WP, Lin-Shiau SY, Hsieh ST (1999) Age-dependent acrylamide neurotoxicity in mice: morphology, physiology, and function. Exp Neurol 158:37-46.
Krishna G, Muralidhara (2015) Inulin supplementation during gestation mitigates acrylamide-induced maternal and fetal brain oxidative dysfunctions and neurotoxicity in rats. Neurotoxicol Teratol 49:49-58.
Li H, Dokas LA, Godfrey DA, Rubin AM (2002-2003) Remodeling of synaptic connections in the deaf f erented vestibular nuclear complex. J Vestib Res 12:167-183.
LoPachin RM (2005) Acrylamide neurotoxicity: neurological, morhological and molecular endpoints in animal models. Adv Exp Med Biol 561:21-37.
LoPachin RM, Ross JF, Reid ML, Das S, Mansukhani S, Lehning EJ (2002) Neurological evaluation of toxic axonopathiesin rats: Acrylamide and 2,5-Hexanedione. Neurotoxicology 23:95-110.
LoPachin RM, Gavin T (2015) Toxic neuropathies: mechanistic insights based on a chemical perspective. Neurosci Lett 596:78-83.
Ma Y, Shi J, Zheng M, Liu J, Tian S, He X, Zhang D, Li G , Zhu J (2011) Toxicological effects of acrylamide on the reproductive system of weaning male rats. Toxicol Ind Health 27:617-627.
Ma Y, Tian S, Sun L, Yao S, Liang Z, Li S, Liu J, Zang L, Li G (2015)e ef f ect of acori graminei rhizoma and extract fractions on spatial memory and hippocampal neurogenesis in amyloid Beta 1-42 injected mice. CNS Neurol Disord Drug Targets 14:411-420.
Massieu L, Morán J, Christen Y (2004) Ef f ect of Ginkgo biloba (EGb 761) on staurosporine-induced neuronal death and caspase activity in cortical cultured neurons. Brain Res 1002:76-85.
Mehri S, Abnous K, Khooei A, Mousavi SH, Shariaty VM, Hosseinzadeh H (2015) Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress. Iran J Basic Med Sci 18:902-908.
Müller WE, Heiser J, Leuner K (2012) Effects of the standardized Ginkgo biloba extract EGb 761 on neuroplasticity. Int Psychogeriatr 1:S21-24.
Neto J, de Almeida A, da Silva O, dos Santos P, de Sousa D, de Freitas R (2013) Antioxidant ef f ects of nerolidol in mice hippocampus aer open fi eld test. Neurochem Res 38:1861-1870.
Ogawa B, Wang L, Ohishi T, Taniai E, Akane H, Suzuki K, Mitsumori K, Shibutani M (2012) Reversible aberration of neurogenesis targeting late-stage progenitor cells in the hippocampal dentate gyrus of rat offspring after maternal exposure to acrylamide. Arch Toxicol 86:779-790.
Pennisi M, Malaguarnera G, Puglisi V, Vinciguerra L, Vacante M, Malaguarnera M (2013) Neurotoxicity of acrylamide in exposed workers. Int J Environ Res Public Health 10:3843-3854.
Phatak NR, Stankowska DL, Krishnamoorthy RR (2015) Transcription factor brn-3b overexpression enhances neurite outgrowth in PC12 cells under condition of hypoxia. Cell Mol Neurobiol 35:769-783.
Rao MS, Shetty AK (2004) Ef fi cacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19:234.
Reiner O (2013) LIS1 and DCX: implications for brain development and human disease in relation to microtubules. Scientifica (Cairo) 2013:393975.
Ribeiro ML, Moreira LM, Arcari DP, Santos LF, Marques AC, Jr JP, Cerutti SM (2016) Protective ef f ects of chronic treatment with a standardized extract of Ginkgo biloba L. in the prefrontal cortex and dorsal hippocampus of middle-aged rats. Behav Brain Res 313:144-150.
Rojas P, Montes P, Rojas C, Serrano-Gaecia N, Rojas-Castaneda JC (2012) Effect of a phytopharmaceutical medicine, Ginko biloba extract 761, in an animal model of Parkinson’s disease: therapeutic perspectives. Nutrition 28:1081-1088.
Rosen J, Hellenas KE (2002) Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry. Analyst 127:880-882.
Ryu S, Lee SH, Kim SU, Yoon BW (2016) Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res 11:298-304.
Sánchez-Farías N, Candal E (2015) Doublecortin is widely expressed in the developing and adult retina of sharks. Exp Eye Res 134:90-100.
Santhanasabapathy R, Vasudevan S, Anupriya K, Pabitha R, Sudhandiran G (2015) Farnesol quells oxidative stress, reactive gliosis and inf l ammation during acrylamide-induced neurotoxicity: behavioral and biochemical evidence. Neuroscience 308:212-27.
Schmitz F, Pierozan P, Rodrigues AF, Biasibetti H, Grunevald M, Pettenuzzo LF, Scaini G, Streck EL, Netto CA, Wyse ATS (2016) Methylphenidate causes behavioral impairments and neuron and astrocyte loss in the hippocampus of juvenile rats. Mol Neurobiol 54:4201-4216.
Schneider R, Welt , Aust , Kluge R, Löster H, Fitzl G (2010) Cardiovascular autonomic neuropathy in spontaneously diabetic rats with and without application of EGb 761. Histol Histopathol 25:1581-90.
Sen E, Tunali Y, Erkan M (2015) Testicular development of male mice offsprings exposed to acrylamide and alcohol during the gestation and lactation period. Hum Exp Toxicol 34:401-414.
Sener G, Sehirli O, Tozan A, Velioglu-Ovunc A, Gedik N, Omurtag GZ (2007) Ginkgo biloba extract protects against mercury (II)-induced oxidative tissue damage in rats. Food Chem Toxicol 45:543-550.
Shrivastava A, De Sousa A Rao GP (2016) Brain-derived neurotrophic factor and suicide in schizophrenia: critical role of neuroprotective mechanisms as an emerging hypothesis. Indian J Psychol Med 38:499-504.
Song M, Mohamad O, Gu X, Wei L, Yu SP (2013) Restoration of intracortical and thalamocortical circuits after transplantation of bone marrow mesenchymal stem cells into the ischemic brain of mice. Cell Transplant 22:2001-2015.
Stackman R, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn J (2003) Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp Neurol 184:510-520.
Tan M, Yu J, Tan C, Wang H, Meng X, Wang C, Jiang T, Zhu X, Tan L (2015) Efficacy and adverse effects of Ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimers Dis 43:589-603.
Wang XY, Zhang JT (2001) Progress in synaptic plasticity and related proteins. Chin Pharmacol Bull 17:369-372.
Yoo DY, Lee KY, Park JH, Jung HY, Kim JW, Yoon YS, Won MH, Choi JH, Hwang IK (2016) Glucose metabolism and neurogenesis in the gerbil hippocampus aer transient forebrain ischemia. Neural Regen Res 11:1254-1259.
Zhang CY, Chen R, Wang F, Ren C, Zhang P, Li Q, Li H, Guo K, Geng D, Liu C (2017) EGb-761 attenuates the anti-proliferative activity of fl uoride via DDK1 in PC-12 cells. Neurochem Res 42:606-614.
Zhang Z, Peng D, Zhu H (2012) Experimental evidence of Ginkgo biloba extract EGB as a neuroprotective agent in ischemia stroke rats. Brain Res Bull 87:193-198.
Zhu X, Wang K, Zhang K, Lin X, Zhu L, Zhou F (2016) Puerarin protects human neuroblastoma SH-SY5Y cells against glutamateinduced oxidative stress and mitochondrial dysfunction. J Biochem Mol Toxicol 30:22-28.
Copyedited by James R, Stow A, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Su-min Tian, M.D., guangyaosumin@sina.com.
Su-min Tian, M.D., guangyaosumin@sina.com.
#
orcid: 0000-0002-2707-1402 (Su-min Tian)
10.4103/1673-5374.213548
Accepted: 2017-07-25
杂志排行
中国神经再生研究(英文版)的其它文章
- Transcriptional inhibition in Schwann cell development and nerve regeneration
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- Effects of estrogen receptor modulators on cytoskeletal proteins in the central nervous system
- Optogenetics and its application in neural degeneration and regeneration
- Live-cell imaging: new avenues to investigate retinal regeneration
- Neurotrophic factors and corneal nerve regeneration
