超支化聚合物作为金属纳米粒子稳定剂的研究进展
2017-08-31沈燕宇何桂金郭永胜方文军
沈燕宇, 何桂金, 郭永胜, 方文军
(浙江大学 化学系, 浙江 杭州 310058)
超支化聚合物作为金属纳米粒子稳定剂的研究进展
沈燕宇, 何桂金, 郭永胜, 方文军
(浙江大学 化学系, 浙江 杭州 310058)
在本研究中,着重介绍了用作金属纳米粒子稳定剂的聚酰胺-胺、聚缩水甘油和聚乙烯亚胺等几类超支化聚合物的研究进展。聚酰胺-胺类超支化聚合物可用于堵水剂和化学驱油剂,用于金属纳米粒子反应器时既作还原剂,又作分散剂,能稳定分散金属纳米粒子,还能提高纳米复合材料的循环再生性能;聚缩水甘油类含有大量的端羟基,经修饰可得到两亲性的纳米胶囊,具有良好的生物相容性,可用作优质的原油破乳剂,金属纳米粒子的粒径可通过其相对分子质量来调控;聚乙烯亚胺-胺类具有众多的胺官能团,为金属离子的配位提供了丰富的位点,其包裹的金属纳米粒子可用于温敏材料等。结合超支化聚合物的结构可控性以及纳米金属优秀的催化性能,这类物质在石油工程领域中会有较大的应用前景。
超支化聚合物; 金属纳米粒子; 稳定剂
1 超支化聚合物
超支化聚合物从一个中心核出发,由支化单体ABn逐级伸展形成具有高度支化的三维球状立体结构,并具有丰富的末端基团(如图1)。显示出与相应线型分子截然不同的性质,如低黏度、良好的溶解性以及高流变性。同时,其合成相对简单、成本较低。因此,超支化聚合物具有广阔的应用前景,在石油工程领域中,已用于堵水剂[1]、化学驱油剂[2-3]、原油破乳剂[4-5]以及钻井液处理剂[6]等。
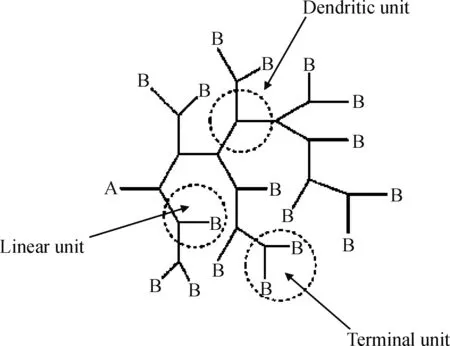
图1 超支化聚合物结构示意图Fig.1 Schematic structure of hyperbranched polymers
1.1 超支化聚合物的合成
自20世纪中期首次成功合成超支化聚合物以来,其合成方法得到了极大丰富和完善。依据聚合机理,主要有以下几类合成方法:(1)缩聚法。由具有两个或两个以上官能团的单体,通过缩聚反应生成超支化聚合物,同时产生简单分子(如H2O、HX、醇等)。缩聚法可在本体或溶液中进行,反应简单,所合成聚合物的相对分子质量具有多分散性。目前,通过一步缩聚法已经成功制备了聚苯类[7]、聚酯类[8]、聚酰胺类[9]和聚硅烷类[10]等多种类型的超支化聚合物。(2)加聚法。在引发基团上通过烯烃加成反应生成超支化聚合物,可赋予超支化聚合物以C—C骨架,从而使其具有比杂原子骨架更好的稳定性。参与该合成过程的单体数较多,可以制备相对分子质量较大的超支化聚合物。比如,Fréchet等[11]采用自缩合乙烯基聚合法制备超支化聚合物,乙烯基单体既是引发剂也是支化点,它在外激发作用下可被活化,产生多个活性自由基,形成新的反应中心,从而引发下一步的反应,生成相对分子质量很大的超支化聚合物。(3)开环聚合。将具有环状结构的单体(如图2)引发后,通过开环反应聚合成超支化聚合物。反应过程中不需要除去小分子化合物且能得到相对分子质量高的超支化聚合物。目前,采用开环聚合法制备了超支化的聚胺[9]、聚酯[12]和聚醚[13]等,但与其他结构超支化聚合物的报道相比仍较少。
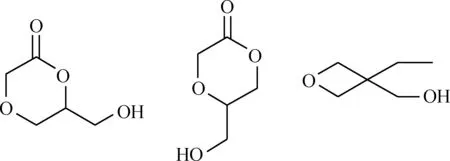
图2 常见开环聚合的单体Fig.2 Common monomers of ring opening polymerization
1.2 超支化聚合物的应用
超支化聚合物具有较低的黏度和良好的流变性能,通过结构改造或修饰,还具有两亲性和一定的反应活性,在石油化工、涂料、油墨等领域已有应用[14-16]。He等[17]发现,超支化聚缩水甘油改性物在碳氢燃料升温过程中能发挥“自由基仓库”的作用,从而促进碳氢燃料的裂解;Bruchmann等[18]通过调控超支化聚酯的亲水性和亲油性,得到了一种高效的油溶性破乳剂;Zhang等[19]用3-(丙烯酰氧乙基)磷酸酯(TAEP)和哌嗪通过迈克尔加成反应制备了超支化多聚磷酸盐丙烯酸酯(HPPAs),可以用于紫外光固化涂料。超支化聚合物还可用于主-客体封装,制备有机-无机杂化材料,甚至可以在反应中直接用作纳米反应器。超支化聚合物也能用于形状记忆材料[20-22]、自我修复材料[23]、CO2捕集材料[24]、多微孔材料[25]、弹性体[26]、黏合剂[27]和催化剂[28]等等。
2 金属纳米粒子的合成方法
纳米粒子是尺寸为1~100 nm的超细粒子,由几十到几百个原子或分子构成。纳米粒子具有独特的物理、化学和生物学特性[29],目前已被广泛用于石油化工、医药、电子工业及农业生产等领域[30]。尤其是金属纳米粒子具有良好的光学、电学、磁学以及催化特性,在石油开采、催化、光学器件、生物传感等领域中呈现出广阔的应用前景[31]。纳米金属粒子作为催化剂和助燃剂已成功地应用到碳氢燃料的催化裂解和燃烧中[32];还可以掺杂到高能密度材料(如炸药)中,作为引爆剂使用。金属纳米粒子的特殊性质和潜在应用价值均与它的纳米级尺寸和形貌密切相关。因此,建立一个可以对金属纳米粒子尺寸和形貌有效调控的制备方法尤为重要,也是纳米材料领域的关键技术。迄今为止,人们已经深入研究和发展了多种可控合成纳米金属粒子的物理或化学方法,包括相转移法、光照还原法、激光烧蚀法、绿色生物学法等[33-34]。
2.1 相转移法
相转移法主要用于制备贵金属(如Au、Ag等)纳米粒子。常用的方法是在相转移剂的作用下,将金属盐从水溶液中萃取到含有稳定剂(如硫醇等)的非极性有机溶剂中,然后缓慢加入还原试剂(如NaBH4等),在有机相中还原制备具有一定纳米尺度的金属粒子。相转移法的关键在于选择恰当的相转移剂或稳定剂,使纳米金属粒子能够高效转移并且稳定存在。常用的相转移试剂有烷基铵类表面活性剂、氨基化合物、硫醇、油酸、柠檬酸和聚合物等。超支化聚合物作为相转移剂时分为两类:一类是需要外加还原剂(如NaBH4等);另一类是不需要外加还原剂,利用自身所带官能团还原金属离子,原位生成金属纳米粒子。
2.2 光照还原法
光照还原法是利用光照将金属离子还原成零价金属的方法,可以通过控制光照时间来控制金属粒子的尺寸、形貌等[35]。Rodriguez等[36]在紫外光照射下,用肝素钠还原HAuCl4合成了金纳米粒子,通过改变肝素钠的浓度和光照时间等可以得到粒径为20~300 nm的各向异性的纳米金,如椭圆形、三角形、六边形以及棒状纳米粒子等。Prakash等[37]用络氨酸为光还原剂,不需要添加额外的稳定剂,在水相中通过光照合成了粒径分布窄的银纳米粒子。
2.3 激光烧蚀法
激光烧蚀法是利用脉冲激光束将靶材瞬间(<10 ms)加热到气化温度以上,产生由靶材原子、离子和原子簇组成的蒸气,在飞行过程中与环境气体原子碰撞减速而形成纳米颗粒。Smalley等[38]用激光照射铜靶,在超声速气体的作用下获得了铜纳米粒子,这是利用激光烧蚀法首次制得的纳米材料。Mohamed等[39]报道了一种在聚乙烯醇(PVA)水溶液中用激光烧蚀法制备银纳米粒子的方法,其中PVA既作为还原剂,又能通过分子骨架保护金纳米粒子。在质量分数为1%、3%和4% 的PVA水溶液中,所得到的纳米金粒径可分别控制为6.13 nm、6.86 nm和3.99 nm。
2.4 绿色生物学法
近年来,很多生物学模板被用于合成金属纳米粒子,比如植物[40]、藻类、真菌[41]、细菌以及病毒[42]等。Kuber等[43]通过培养真菌的方法来合成银纳米粒子。Richa等[44]用了18种碳酸钙不动杆菌做研究,发现鲍曼-醋酸钙不动杆菌LRVP54可以在70℃条件下还原AgNO3,生成粒径为8~12 nm单分散的球形纳米银。
3 用于合成金属纳米粒子的超支化聚合物
在金属纳米粒子的各种合成方法中,相转移法是最为简便、应用最多的方法。由于金属纳米粒子在相转移过程中容易发生聚集[45],因此,在制备过程中需用稳定剂来辅助分散[46]。树枝状聚合物和超支化聚合物是典型的稳定剂,树枝状大分子可作为金属纳米粒子的反应器,金属离子首先与聚合物配位富集,然后再在还原剂的作用下被原位还原成稳定的纳米粒子[47]。然而,树枝状大分子由于结构完美,合成条件比较复杂,成本相对较高。相比之下,超支化聚合物合成方法简单,成本低廉,且化学、物理性质与树枝状大分子非常接近。因此,近年来超支化聚合物作为金属纳米粒子稳定剂的研究受到重视[48-50],已有3类超支化聚合物较多用于金属纳米粒子的稳定剂,下面分别阐述。
3.1 聚酰胺-胺类超支化聚合物
聚酰胺-胺大分子主链重复单元中含有酰胺-胺基团,端基以胺基为多(如图3)。胺基或酰胺的孤对电子能与金属离子配位,起到捕集、固定金属离子的作用;它还具有还原性,在一定条件下可将金属离子还原成金属,达到原位还原的目的,减少金属离子在还原过程中聚集。这类超支化聚合物既作还原剂,又作分散剂,克服了纳米金属溶胶制备工艺复杂、适用性差的缺点[51]。因此,越来越多的聚酰胺-胺类聚合物用作金属纳米粒子反应器[52-54]。
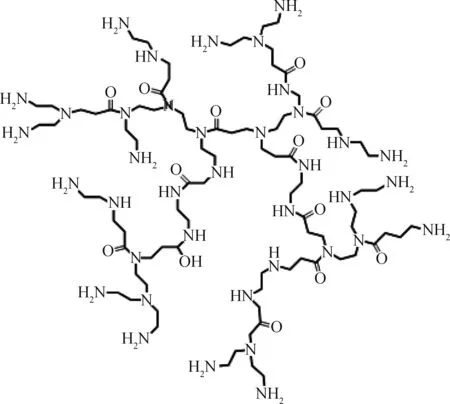
图3 聚酰胺-胺的结构Fig.3 Schematic structure of poly(amidoamine)
Nelly等[55]用具有超支化结构的聚酰胺-胺(HYPAM)(如图4)及其葡萄糖胺衍生物(如图5)合成了水溶性金纳米粒子。纳米粒子在水溶液中的稳定性主要受聚合物相对分子质量以及溶液pH值的影响。聚合物相对分子质量越大,纳米粒子的稳定性越好,而聚合物相对分子质量可以通过改变聚合条件来调控。HYPAM的葡萄糖胺功能化可进一步阻止金纳米粒子的聚集,对金纳米粒子稳定性的影响要大于聚合物尺寸效应带来的影响。
图4 HYPAM超支化聚合物的结构Fig.4 Schematic structure of HYPAM
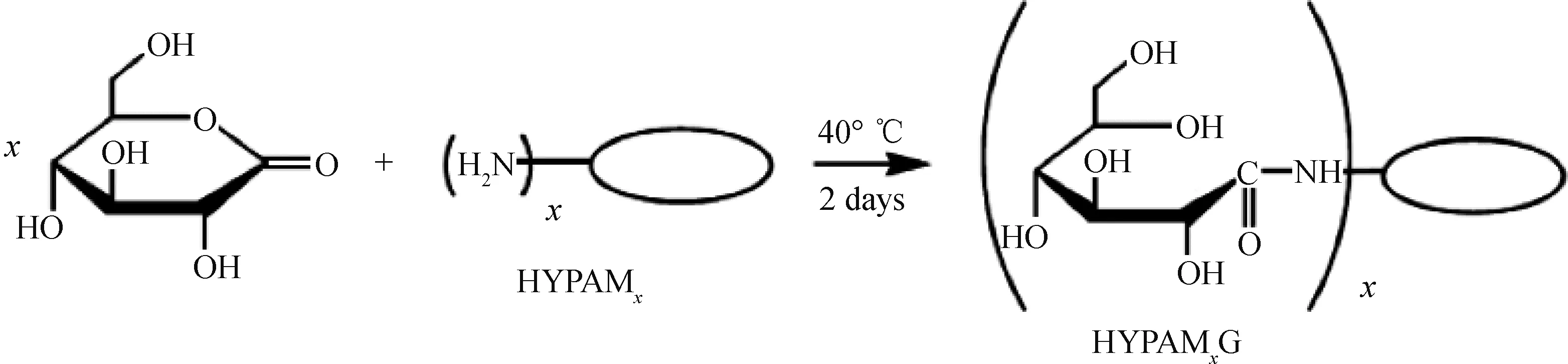
图5 葡萄糖胺功能化的HYPAM超支化聚合物Fig.5 Functionalization of HYPAM with gluconolactone
Nitul等[56]用聚丙烯酰胺(PA)和超支化聚胺/聚丙烯酰胺混杂体(HB-PA)来合成银纳米粒子,用HB-PA合成的银纳米粒子比用PA合成的银纳米粒子要稳定。以HB-PA为母体时,银纳米粒子的粒径为8.5 nm;以PA为母体时,银纳米粒子的粒径为9.9 nm。HB-PA包裹的银纳米粒子对枯草芽孢杆菌的抗菌性要高于用PA合成的银纳米粒子。
Roozbe等[57]用超支化聚酰胺(PAMAM)在聚乙烯胺功能化的介孔氧化硅(PVAm/SBA-15)表面发生聚合,生成杂化材料。通过PAMAM和金属离子的配位作用诱捕水溶性的金属离子(如Ni2+等),然后再用NaBH4将金属离子还原得到由这种杂化材料包裹的镍纳米粒子。所得到的纳米复合材料可作为拟均相催化剂催化NaBH4还原芳硝基物的反应,且具有良好的循环再生性能,经循环10次后其催化活性仍未明显下降。
3.2 聚缩水甘油改性物
超支化聚缩水甘油(HPG)是一种分子内部为醚键,分子周围有大量羟基的超支化聚合物[58-59](如图6)。自Frey等[60]合成出低分散性的HPG以来, 关于HPG合成与应用的研究越来越受到重视。HPG的合成方法主要有阳离子聚合与阴离子聚合两种,Wang等[61]以丙三醇为核,以BF3O(C2H5)2为催化剂,对缩水甘油进行阳离子开环聚合,得到了相对分子质量为2000~3000、支化度为0.5~0.6的超支化聚缩水甘油醚。但是,阳离子聚合反应过程中容易出现环化副产物,反应产物相对分子质量较小且分布较宽。目前主要采用阴离子开环聚合结合单体缓慢滴加技术,以1,1,1-三羟甲基丙烷(TMP)为核,用甲醇钾对其进行质子化,引发缩水甘油开环聚合,可避免成环副反应的发生,使产物的相对分子质量分布很窄,且能得到相对分子质量较大的聚合物。
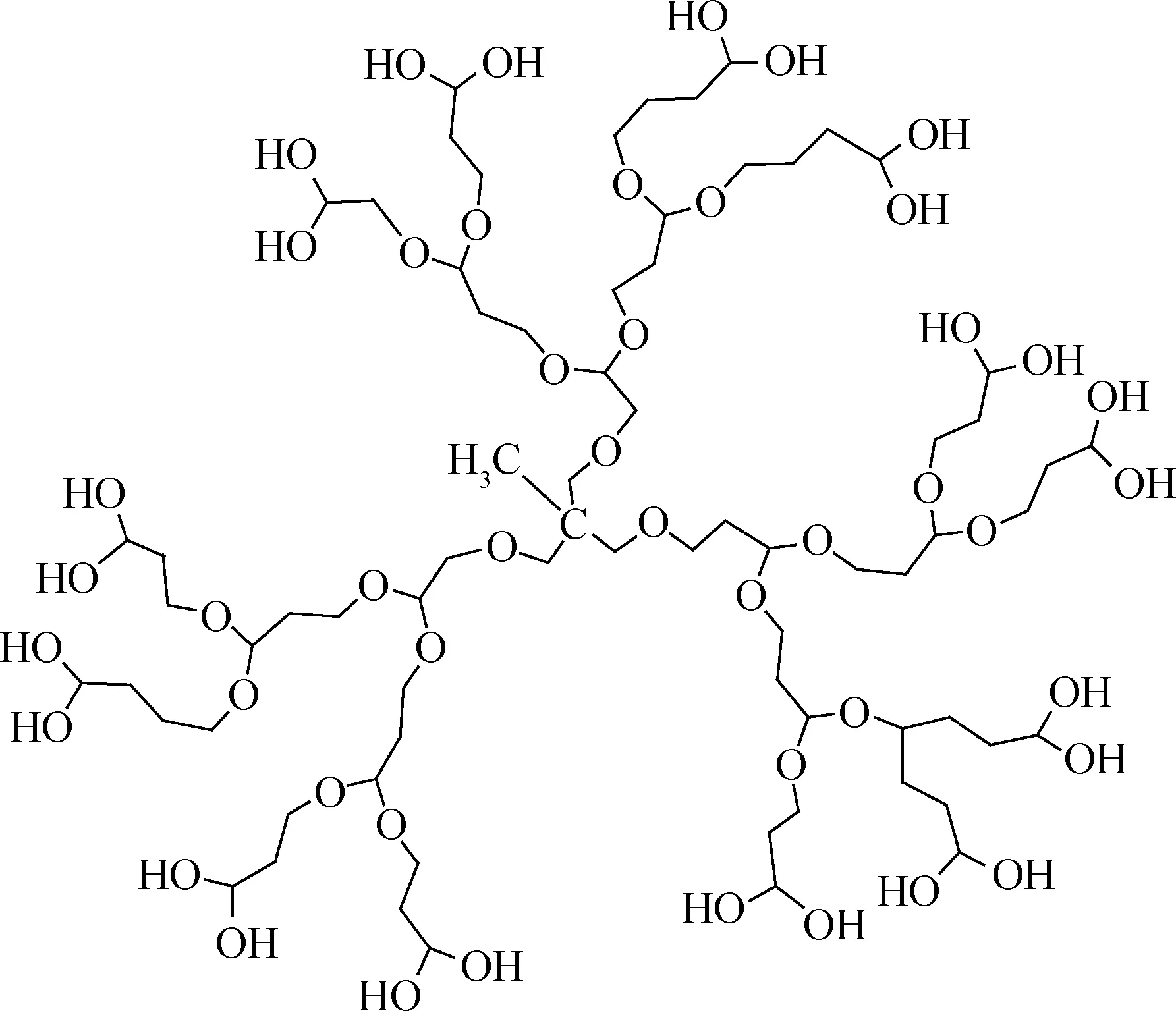
图6 超支化聚缩水甘油的结构Fig.6 Schematic structure of hyperbranched polyglycerol(HPG)
HPG含有大量的端羟基,是原子转移自由基聚合(ATRP)或者点击化学来修饰HPG的活性位点[62],比如用长链酰氯[63]与羟基反应可制得油溶性的HPG(如图7)。Slagt等[64]将HPG经烷基酰氯部分酯化后,得到一种内部亲水、外部疏水的核-壳结构型超支化聚合物,其亲水的核能通过氢键作用与过渡金属结合,从而实现过渡金属催化剂的负载与富集。HPG改性物用作金属纳米粒子稳定剂主要有以下优势[65]:HPG外围是树枝状结构,中间含有空腔,有利于包裹金属纳米粒子;HPG的端羟基对金属离子有一定的还原作用,所以HPG不仅可以作为稳定剂还可以作为还原剂;包裹纳米金属后,外围的羟基能使金属纳米粒子进一步得到修饰,形成需要的纳米复合物。
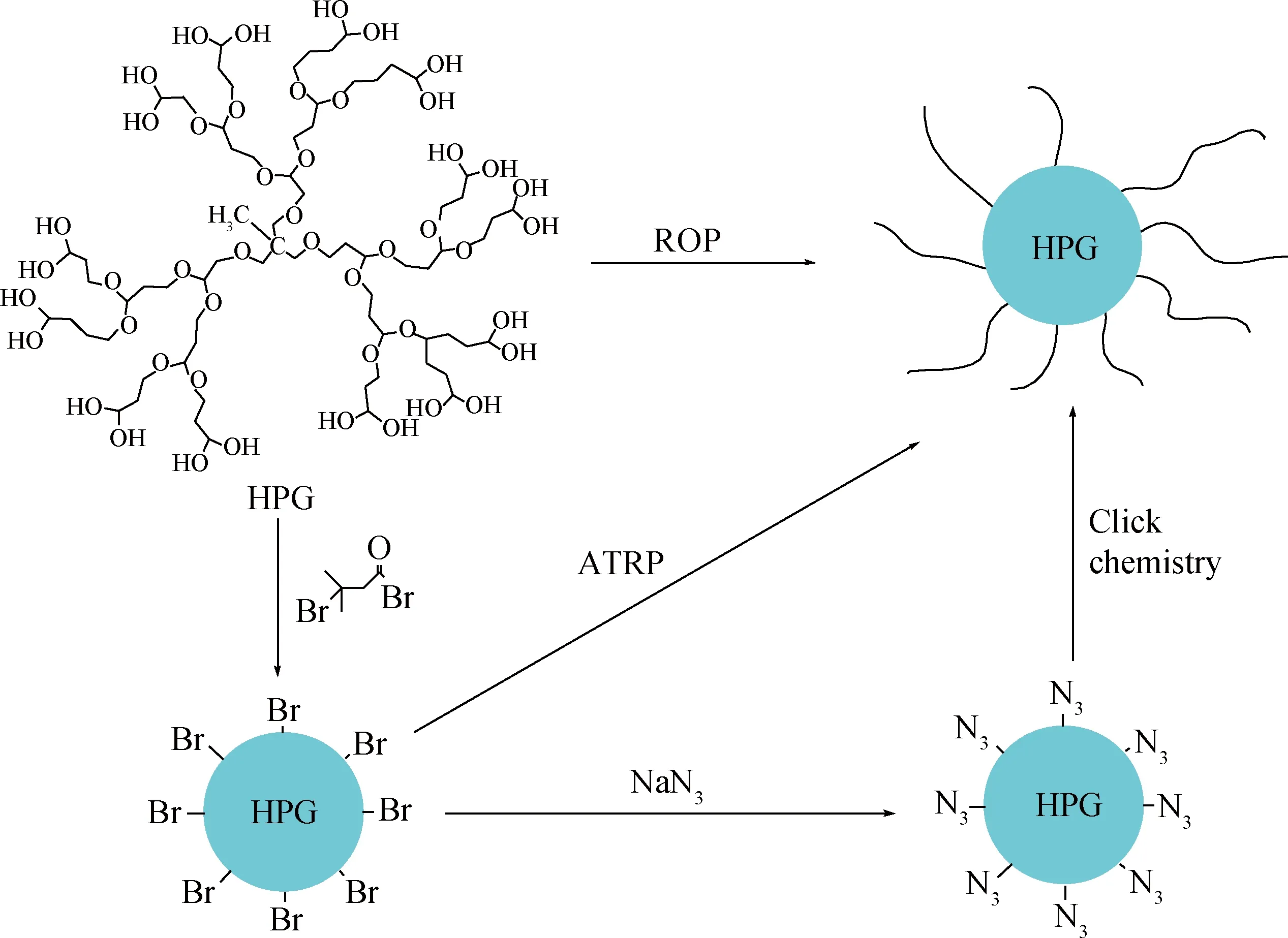
图7 HPG的羟基功能化Fig.7 Hydroxyl functionalization of HPG
Martijn等[66]用两亲性的HPG包裹铂螯合物,将带有疏水烷基链的聚醚多元醇的羟基部分酯化,形成具有反胶束结构的两亲性纳米胶囊,这种纳米胶囊能在其亲水内部包裹亲水性的磺化铂螯合物(如图8)。这种包裹铂螯合物的纳米胶囊可作为均相催化剂用于迈克尔加成,通过透析的方式分离产物,催化剂的回收率高达97%。
在HPG骨架上引入巯基对纳米粒子的合成十分有利。Sunny等[67]用3-巯基丙酸先与HPG发生酯化反应,再用1-溴代十二烷使外围巯基烷基化,生成目标超支化聚合物(如图9),这种超支化聚合物能溶于氯仿、二氯甲烷和二甲基甲酰胺而不溶于甲醇。然后再分别与CuCl2·2H2O、AgNO3、HAuCl4配位制备Cu、Ag、Au纳米粒子。Decheng等[68]用HPG做为模板合成金纳米颗粒,用1-溴-3-氯丙烷使HPG的羟基烷基化,31.6%的羟基转化成烯丙基,22.4%的羟基转化成3-氯丙基,3-氯丙基与十二硫醇硫烷基化,形成了1个两亲性的模板分子(如图10):亲水的PG为核心、疏水的硫醚构成外壳,合成了两种相对分子质量的模板分子,其包裹的金纳米粒子平均尺寸分别为(3.0±1.6) nm和(5.1±2.4) nm,分散到有机溶剂(如氯仿、四氢呋喃)中形成透明胶体,可稳定存放180 d。
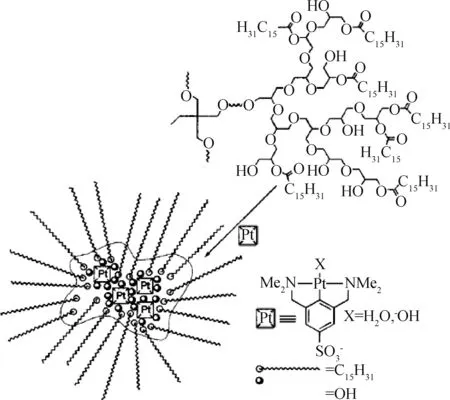
图8 两亲性HPG胶囊包裹铂螯合物Fig.8 Encapsulation of platinum pincer complexes in the amphiphilic HPG
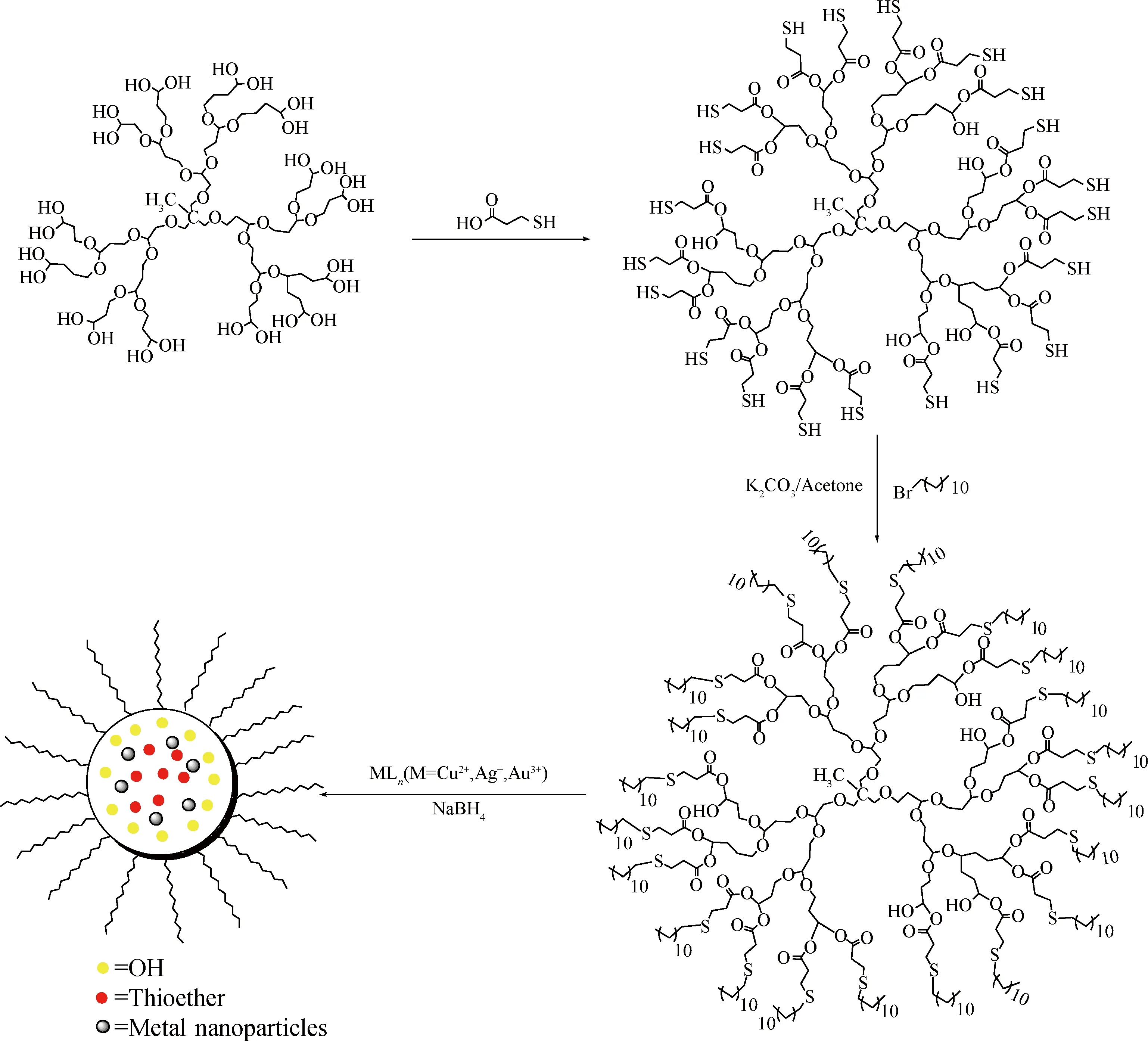
图9 具有硫醚结构的HPG及其包裹金属纳米粒子的示意图Fig.9 Preparation of HPG with thioether shells and its encapsulation on metal NPs
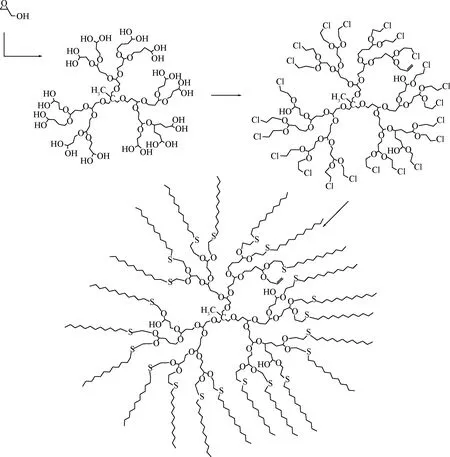
图10 含硫HPG改性物合成示意图Fig.10 Synthesis of sulfur-bearing HPG
3.3 聚乙烯亚胺类超支化聚合物
通过氮杂环丙烷(乙烯亚胺)的催化开环聚合可以获得超支化聚乙烯亚胺(HPEI)(如图11)。胺官能团的多功能性为金属离子的配位提供了理想的配合位点。
Tang等[69]用棕榈酰氯修饰的聚乙烯亚胺(HPEI),将柠檬酸盐保护的金纳米粒子从水相中转移到氯仿中。研究表明,超支化聚合物作为金属纳米粒子稳定剂,其效果要优于线型聚合物,主要表现在:发生有效萃取需要的量更少;含有超支化聚合物的金纳米粒子体系较少出现聚沉现象;超支化聚合物分散的金纳米颗粒更均一、堆积更密集,可以干燥稳定存放长达210 d。
Liu等[70]用乙酸酐(ACAm)、丙酸酐(PRAm)、丁酸酐(BUAm)和异丁酸酐(IBAm)修饰HPEI,分别得到对应的酰胺化产物HPEI-ACAm、HPEI-PRAm、HPEI-BUAm以及HPEI-IBAm(如图12),然后分别合成金纳米粒子,通过聚合物和纳米金的非共价键作用得到了纳米金复合材料,该纳米金复合材料可以催化NaBH4还原4-硝基苯酚的反应。Aymonier等[71]用带长链烷基的酰胺与PEI反应,用来包裹银纳米粒子(如图13),生成了平均粒径为5nm的具有抗菌性能的纳米银。刘训恿等[72]通过对不同相对分子质量的超支化聚乙烯亚胺的端基进行部分或完全异丁酰胺化,制备了一系列具有不同低临界溶解温度(LCST)的超支化温敏聚合物。通过离子键或氢键之间的相互作用,所得超支化温敏聚合物可以吸附于经柠檬酸钠还原并稳定的金纳米粒子的表面,从而得到具有温敏性质的金纳米粒子。
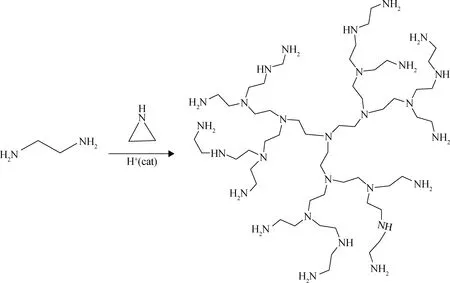
图11 HPEI的合成Fig.11 Synthesis of HPEI

图12 不同氨基化合物修饰的聚乙烯亚胺(HPEI)
Anja等[73]用麦芽糖修饰的超支化聚乙烯亚胺合成金纳米粒子,在金纳米粒子形成的过程中,超支化聚合物既作为还原剂又作为稳定剂。在PEI核的区域,Au3+被还原形成紧密堆积的金核,同时聚合物外围的分子链发生塌陷,金纳米粒子和聚合物的分子链结合形成新的壳-核结构。通过调控PEI的相对分子质量,合成出了粒径为3.6 nm的金纳米粒子。
3.4 其它超支化聚合物
除了以上3类超支化聚合物,还有超支化聚酯、超支化聚苯乙烯、超支化聚醚胺等也可以用作金属纳米粒子稳定剂。
超支化聚酯(HBPE)高度支化的结构和大量的端基官能团使它容易被接枝改性,HBPE 可有效地解决热固性树脂因其高度交联结构而产生的韧性差的问题,还能改善纳米粒子的团聚现象,促进纳米粒子在树脂中的分散。Zhu等[74]采用光照还原法,用端基含羟基和羧基的超支化聚酯合成了粒径为3~19 nm的银纳米粒子。Joshua等[75]将水黄皮油(PO)羟基化形成POH,再与亚麻酸发生酯化反应,生成超支化聚酯,然后与苯乙烯共聚形成共聚物,用于合成银纳米粒子的稳定剂。
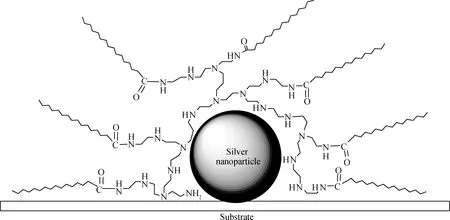
图13 PEI酰胺化及包裹银纳米粒子Fig.13 Silver NPs encapsulated by amidated PEI
Gao等[76]用超支化聚苯乙烯的氨盐(HPS-NR3+Cl-)稳定过渡金属(M)纳米粒子(如图14)。研究表明,1~3 nm的钌、铑、铱、铂和钯纳米粒子能稳定分散于聚合物基体中形成M@HPS-NR3+Cl-,其 分散性可以通过改变R基团来调控,M@HPS-NR3+Cl-对烯烃和芳香烃的加氢反应具有良好的催化作用。Keisuke等[77]用亲-疏水性可调的超支化聚苯乙烯合成铂纳米粒子,用外围带—Cl的超支化聚苯乙烯(HPS-Cl)合成HPS-NR3+Cl-。HPS-NR3+Cl-的一部分覆盖在铂纳米粒子上,其带有C12H25或者(CH2CH2O)2Me链的氨基可以通过静电作用稳定铂纳米粒子,形成多个HPS-NR3+Cl-分子包裹1个铂纳米粒子的结构(如图15),并且能通过改变R基团来调节其溶解性能。
Yu等[78]用超支化聚醚胺(HPEA)自组装合成了边缘长度为1~2 μm、厚度为4~5 nm的超支化聚醚胺的纳米片(HPEA-NSs),以笼状倍半硅氧烷(POSS)和蒽(AN)为封端剂,合成了金纳米粒子(如图16)。金的前驱体通过金原子与氨基配位吸附在HPEA-NSs的亲水表面上,通过氨基的还原作用,转变成金纳米粒子。

图14 M@HPS-NR3+Cl-的形成示意图Fig.14 Schematic diagram for the formation of M@HPS-NR3+Cl-
4 结论与展望
(1) 具有特殊表面界面效应、小尺寸效应的纳米粒子与具有密度小、耐腐蚀、易加工等优良特性的超支化聚合物结合后,呈现出不同于常规聚合物复合材料的性能。
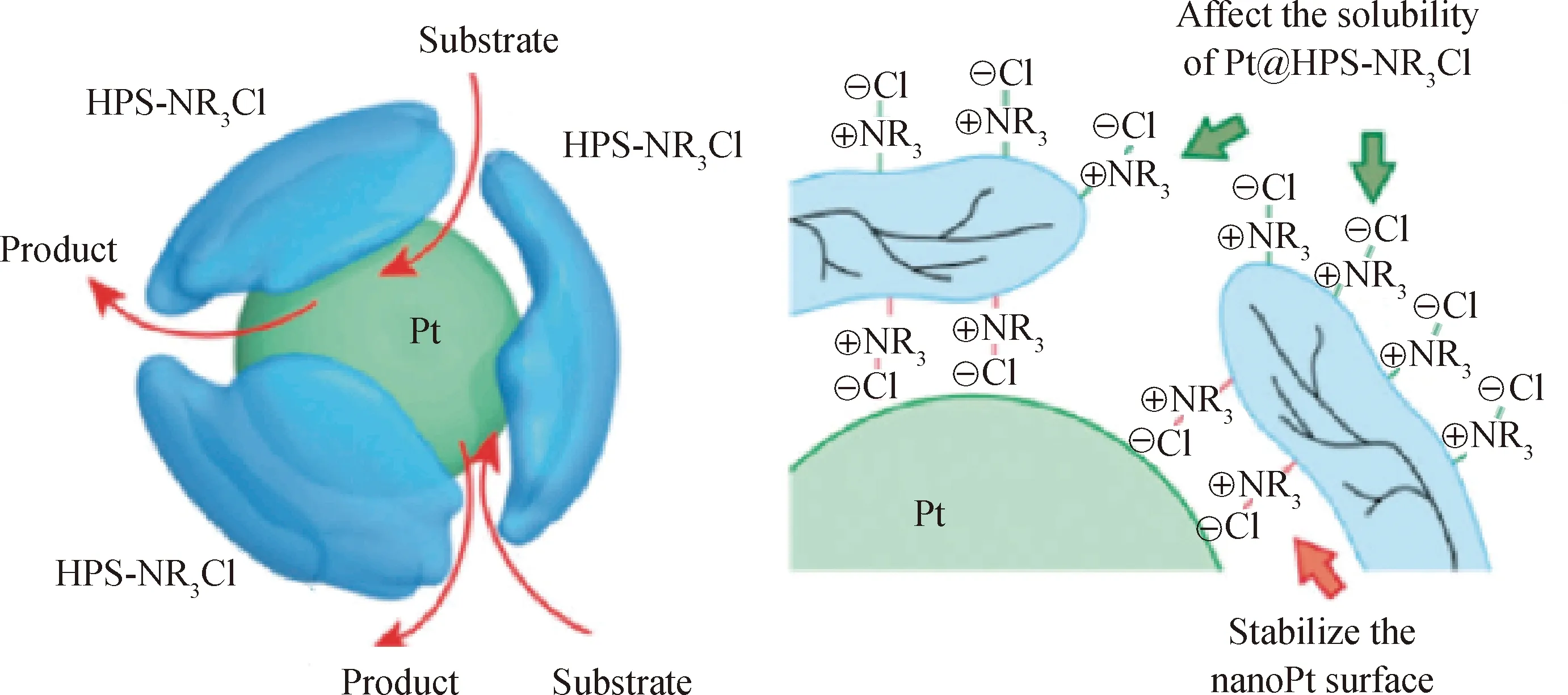
图15 HPS-NR3+Cl-包裹铂纳米粒子示意图Fig.15 Schematic diagram for stabilization of a Pt nanoparticle by HPS-N(C12H25)3+Cl-
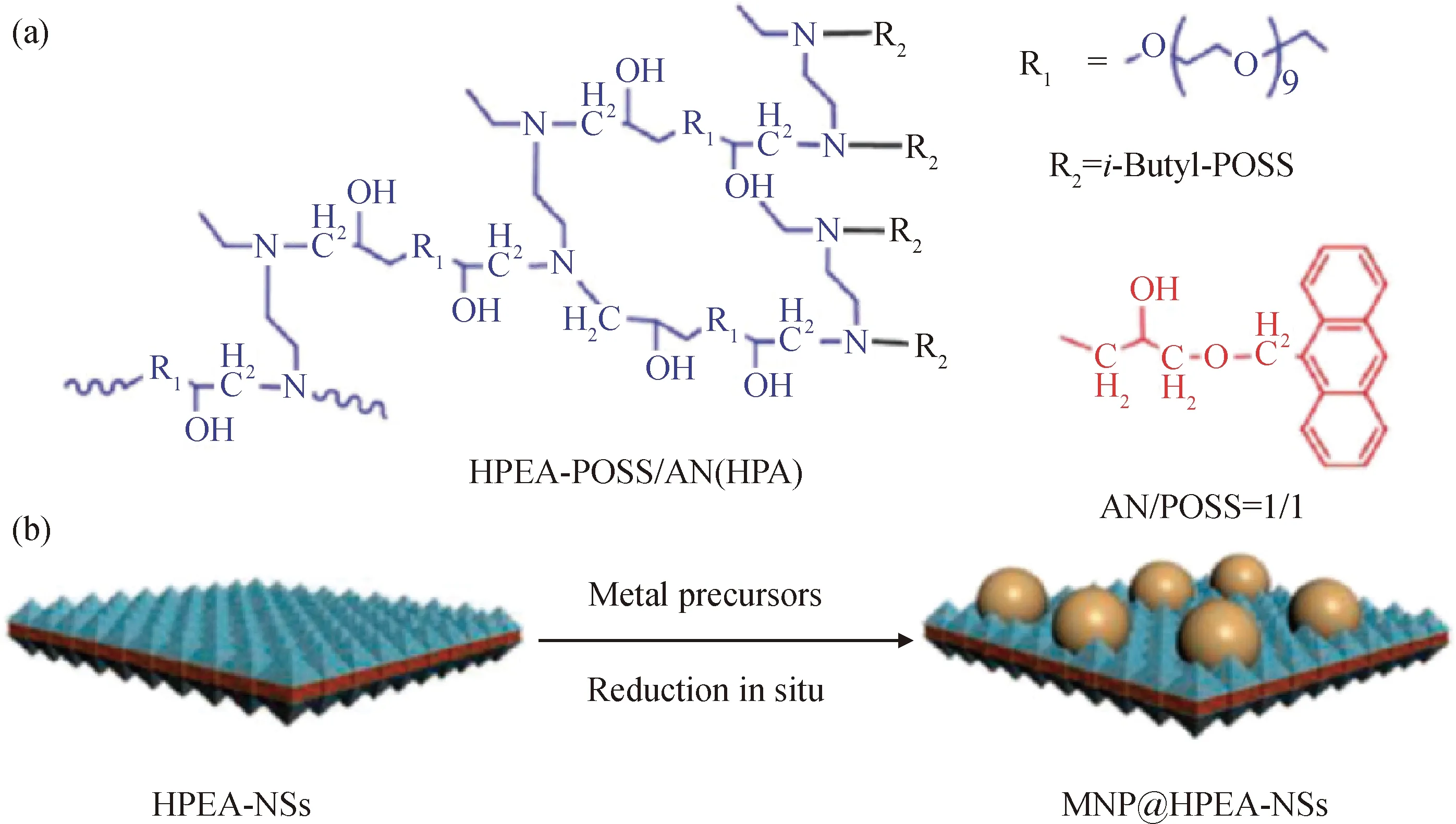
图16 以POSS/AN为封端剂的HPEA-NSs结构与HPEA-NSs负载金属纳米粒子的机理Fig.16 Structure of POSS/AN ended hyperbranched poly(ether amine) and the proposed mechanism of metal nanoparticle decoration on HPEA-NSs(a) HPEA-POSS/AN(HPA); (b) HPEA-NSs
(2) 超支化聚合物具有超支化分子拓扑和“核壳”结构,可作为纳米材料的模板,产生精致的纳米粒子复制品。
(3) 纳米粒子一般通过配位键或者分子间相互作用被封装在超支化聚合物中,通过调控聚合物分子表面的基团可控制纳米复合材料的聚集-分散行为,超支化聚合物修饰的纳米粒子可稳定地分散在介质中,不易聚沉。
(4) 通过对超支化聚合物结构的控制,调整其两亲性、溶解性和黏度等,有望在原油开采、破乳、降凝输送、油田污水处理和燃油加工等方面发挥作用。
[1] LIU Q, SUN A, FAN Z, et al. Synthesis and performance evaluation of organic bentonite modifier dimethyldistearylammonium bromide (DODMAB)[J].Open Journal of Composite Materials, 2014, 4(4): 220-223.
[2] 徐骏祺, 黄通, 吕鑫, 等. 水溶性高抗剪切超支化聚丙烯酰胺的合成和表征[J].上海应用技术学院学报(自然科学版), 2014, 14(4): 277-282. (XU Junqi, HUANG Tong, LÜ Xin, et al. Synthesis and characterization of water-soluble hyperbranched polyacrylamide with high shear-resistance[J].Journal of Shanghai Institute of Technology(Natural Science), 2014, 14(4): 277-282.)
[3] 张红艳, 康万利, 孟令伟, 等. 一种驱油用疏水缔合聚丙烯酰胺的乳化性能[J].石油学报(石油加工), 2010, 26(4): 628-634. (ZHANG Hongyan, KANG Wanli, MENG Lingwei, et al. Emulson characteristics of the hydrophobically associating polyacrylamid used for oil flooding[J].Acta Petrolei Sinica(Petroleum Processing Section), 2010, 26(4): 628-634.)
[4] ZHANG Lifeng, HE Guijin, YE Dengfeng, et al. Methacrylated hyperbranched polyglycerol as a novel high-efficient demulsifier for oil-in-water emulsions[J].Energy Fuels, 2016, 30(11): 9939-9946.
[5] 严峰, 张建, 付天宇, 等. 疏水缔合聚合物驱体系中原油乳状液性质及破乳规律[J].石油学报(石油加工), 2016, 32(3): 546-555. (YAN Feng, ZHANG Jian, FU Tianyu, et al. Properties and demulsification laws of crude oil emulsions in hydrophobically associating polymer flooding system[J].Acta Petrolei Sinica(Petroleum Processing Section), 2016, 32(3): 546-555.)
[6] 王中华. 2011~2012年国内钻井液处理剂进展评述[J].中外能源, 2013, 18(4): 28-35. (WANG Zhonghua. Review on development progress of the drilling fluid additives in China between 2011 and 2012[J].Sino-Global Energy, 2013, 18(4): 28-35.)
[7] KUSHAKOVA N S, SHAPOVALOV A V, RUD D A, et al. Synthesis of hyperbranched polyphenylenes by suzuki reaction and their spectral characteristics[J].Polymer Science Series B, 2009, 51(9): 409-415.
[8] FAN Z R, LEDERER A, VOIT B. Synthesis and characterization of A2+B3-type hyperbranched aromatic polyesters with phenolic end groups[J].Polymer, 2009, 50(15): 3431-3439.
[9] OHTA Y, FUJII S, YOKOYAMA A, et al. Synthesis of well-defined hyperbranched polyamides by condensation polymerization of AB(2) monomer through changed substituent effects[J].Angewandte Chemie-International Edition, 2009, 48(32): 5942-5945.
[10] ZHU X M, JAUMANN M, PETER K, et al. One-pot synthesis of hyperbranched polyethoxysiloxanes[J].Macromolecules, 2006, 39(5): 1701-1708.
[11] GU A. High performance bismaleimide/cyanate ester hybrid polymer networks with excellent dielectric properties[J].Composites Science & Technology, 2006, 66(11-12): 1749-1755.
[12] PARZUCHOWSKI P G, GRABOWSKA M, JAROCH M, et al. Synthesis and characterization of hyperbranched polyesters from glycerol-based AB(2) monomer[J].Journal of Polymer Science Part A-Polymer Chemistry, 2009, 47(15): 3860-3868.
[13] BEDNAREK M, PLUTA M. Oligomeric branched polyethers with multiple hydroxyl groups by cationic ring-opening polymerization for inorganic surface modification[J].Macromolecular Symposia, 2010, 287(1): 119-126.
[14] 陈泽华, 赵修太, 王增宝, 等. 乙二胺-HPAM与NaOH-HPAM体系提高稠油采收率的对比[J].石油学报(石油加工), 2015, 31(5): 1156-1163. (CHEN Zehua, ZHAO Xiutai, WANG Zengbao, et al. A comparison between the enhanced heavy oil recoveries of ethylenediamine-HPAM and NaOH-HPAM systems[J].Acta Petrolei Sinica(Petroleum Processing Section), 2015, 31(5): 1156-1163.)
[15] 杨敏, 汪健, 张宏明, 等. 纳米聚(二乙烯苯-甲基丙烯酸十八酯)降黏剂的合成及其降黏效果[J].石油学报(石油加工), 2013, 29(5): 881-884. (YANG Min, WANG Jian, ZHANG Hongming, et al. Synthesis and viscosity reducing properties of poly (divinyl benzene-methyl octadecyl acrylate) nanoviscosity reducer[J].Acta Petrolei Sinica(Petroleum Processing Section), 2013, 29(5): 881-884.)
[16] 林梅钦, 辛见, 李明远, 等. 交联聚合物溶液的热氧化及剪切安定性对其封堵性能的影响[J].石油学报(石油加工), 2009, 25(6): 807-811. (LIN Meiqin, XIN Jian, LI Mingyuan, et al. The effect of thermo-oxidizing and shear stabilities of linked polymer solution on its plugging efficiency[J].Acta Petrolei Sinica(Petroleum Processing Section), 2009, 25(6): 807-811.)
[17] HE G J, LI G Q, YING H, et al. Palmitoyl hyperbranched polyglycerol as a nanoscale initiator for endothermic hydrocarbon fuels[J].Fuel, 2015, 161: 295-303.
[18] BRUCHMANN B, EICHHOEN A, GUZMANN M.Hyperbranched polyesters and polycarbonates as demulsifiers for cracking crude oil emulsions: US, 8618180 B2[P]. 2013.
[19] ZHANG D H, LIANG E B, LI T C, et al. Environment-friendly synthesis and performance of a novel hyperbranched epoxy resin with a silicone skeleton[J].Rsc Advances, 2013, 3(9): 3095-3102.
[20] SIVAKUMAR C, NASAR A S. Poly(epsilon-caprolactone)-based hyperbranched polyurethanes prepared via A2+B3approach and its shape-memory behavior[J].European Polymer Journal, 2009, 45(8): 2329-2337.
[21] DEKA H, KARAK N,KALITA R D, et al. Biocompatible hyperbranched polyurethane/multi-walled carbon nanotube composites as shape memory materials[J].Carbon, 2010, 48(7): 2013-2022.
[22] THAKUR S, KARAK N. Bio-based tough hyperbranched polyurethane-graphene oxide nanocomposites as advanced shape memory materials[J].Rsc Advances, 2013, 3(24): 9476-9482.
[23] DOHLER D, ZARE P, BINDER W H. Hyperbranched polyisobutylenes for self-healing polymers[J].Polymer Chemistry, 2014, 5(3): 992-1000.
[24] HE H K, ZHONG M J, KONKOLEWICZ D, et al. Carbon black functionalized with hyperbranched polymers: synthesis, characterization, and application in reversible CO2capture[J].Journal of Materials Chemistry A, 2013, 1(23): 6810-6821.
[25] KOBAYASHI N, KIJIMA M. Microporous materials derived from two- and three-dimensional hyperbranched conjugated polymers by thermal elimination of substituents[J].Journal of Materials Chemistry, 2007, 17(40): 4289-4296.
[26] MAHAPATRA S S, YADAV S K, CHO J W. Nanostructured hyperbranched polyurethane elastomer hybrids that incorporate polyhedral oligosilsesquioxane[J].Reactive & Functional Polymers, 2012, 72(4): 227-232.
[27] ZHANG H, BRE L P, ZHAO T Y, et al. Mussel-inspired hyperbranched poly(amino ester) polymer as strong wet tissue adhesive[J].Biomaterials, 2014, 35(2): 711-719.
[28] HAUSSLER M, LAM J W Y, QIN A J, et al. Metallized hyperbranched polydiyne: A photonic material with a large refractive index tunability and a spin-coatable catalyst for facile fabrication of carbon nanotubes[J].Chemical Communications, 2007, (25): 2584-2586.
[29] LEE S H, SALUNKE B K, KIM B S. Sucrose density gradient centrifugation separation of gold and silver nanoparticles synthesized using magnolia kobus plant leaf extracts[J].Biotechnology and Bioprocess Engineering, 2014, 19(1): 169-174.
[30] BORASE H P, SALUNKE B K, SALUNKHE R B, et al. Plant extract:A promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles[J].Applied Biochemistry and Biotechnology, 2014, 173(1): 1-29.
[31] FERRANDO R, JELLINEK J, JOHNSTON R L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles[J].Chemical Reviews, 2008, 108(3): 845-910.
[32] LI D, FANG W J, WANG H Q, et al.Gold/oil nanofluids stabilized by a gemini surfactant and their catalytic property[J].Industrial & Engineering Chemistry Research, 2013, 52(24): 8109-8113.
[33] 杨柯利, 刘全生, 宋银敏, 等. 相转移法制备金属纳米粒子研究进展[J].化学通报, 2012, 75(6): 514-518. (YANG Keli, LIU Quansheng, SONG Yinmin, et al. Development of phase transfer methods in the synthesis of metal nanoparticles[J].Chemistry, 2012, 75(6): 514-518.)
[34] SASTRY M. Phase transfer protocols in nanoparticle synthesis[J].Current Science, 2003, 85(12): 1735-1745.
[35] KROL-GRACZ A, MICHALAK E, NOWAK P M, et al. Photo-induced chemical reduction of silver bromide to silver nanoparticles[J].Central European Journal of Chemistry, 2011, 9(6): 982-989.
[36] RODRIGUEZ-TORRES M D, DIAZ-TORRES L A, SALAS P, et al. UV photochemical synthesis of heparin-coated gold nanoparticles[J].Gold Bulletin, 2014, 47(1-2): 21-31.
[37] KSHIRSAGAR P, SANGARU S S, MALVINDI M A, et al. Synthesis of highly stable silver nanoparticles by photoreduction and their size fractionation by phase transfer method[J].Colloids and Surfaces A-Physicochemical and Engineering Aspects, 2011, 392(1): 264-270.
[38] DIETZ T G, DUNCAN M A, POWERS D E, et al. Laser production of supersonic metal cluster beams[J].Journal of Chemical Physics, 1981, 74(11): 6511-6512.
[39] MOHAMED K H, NASERI M G, SADROLHOSSEINI A R, et al. Silver nanoparticle fabrication by laser ablation inpolyvinyl alcohol solutions[J].Chinese Physics Letters, 2014, 31(7): 077803.
[40] ARUNACHALAM K D, ANNAMALAI S K, ARUNACHALAM A M, et al. Green synthesis of crystalline silver nanoparticles using indigofera aspalathoides-medicinal plant extract for wound healing applications[J].Asian Journal of Chemistry, 2013, 25(S): 311-314.
[41] SHARMA V K, YNGARD R A, LIN Y. Silver nanoparticles: Green synthesis and their antimicrobial activities[J].Advances in Colloid and Interface Science, 2009, 145(1-2): 83-96.
[42] AHMAD A, MUKHERJEE P, SENAPATI S, et al. Extracellular biosynthesis of silver nanoparticles using the fungus fusarium oxysporum[J].Colloids and Surfaces B-Biointerfaces, 2003, 28(4): 313-318.
[43] BFILAINSA K C, D’SOUZA S F. Extracellular biosynthesis of silver nanoparticles using the fungus aspergillus fumigatus[J].Colloids and Surfaces B-Biointerfaces, 2006, 47(2): 160-164.
[44] SINGH R, WAGH P, WADHWANI S, et al. Synthesis, optimization, and characterization of silver nanoparticles from acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics[J].International Journal of Nanomedicine, 2013, 8(1): 4277-4290.
[45] 李曼璐, 姜玥璐. 人工纳米颗粒在水体中的行为及其对浮游植物的影响[J].环境科学, 2015, 36(1): 365-372. (LI Manlu, JIANG Yuelu. Behaviors of engineered nanoparticles in aquatic environments and impacts on marine phytoplankton[J].Environmental Science, 2015, 36(1): 365-372.
[46] NAGARAJAN R. Nanoparticles: Building Blocks for Nanotechnology[M].Springer: Plenum Publishers, 2004: 2-14.
[47] ARYABADIE S, SADEGHI-KIAKHANI M, ARAMI M. Antimicrobial and dyeing studies of treated cotton fabrics by prepared chitosan-PAMAM dendrimer/Ag nano-emulsion[J].Fibers and Polymers, 2016, 16(12): 2529-2537.
[48] GAO L, KOJIMA K, NAGASHIMA H. Transition metal nanoparticles stabilized by ammonium salts of hyperbranched polystyrene: Effect of metals on catalysis of the biphasic hydrogenation of alkenes and arenes[J].Tetrahedron, 2015, 71(37): 6414-6423.
[49] TANG Q, CHENG F, LOU X L, et al. Comparative study of thiol-free amphiphilic hyperbranched and linear polymers for the stabilization of large gold nanoparticles in organic solvent[J].Journal of Colloid and Interface Science, 2009, 337(2): 485-491.
[50] YU B, JIANG X, YIN J. Responsive hybrid nanosheets of hyperbranched poly(ether amine) as a 2D-platform for metal nanoparticles[J].Chemical Communications, 2013, 49(6): 603-605.
[51] 朱鹏飞, 吴明华, 李琰琦, 等. 超支化聚合物的改性及其在纳米银溶胶制备中的应用[J].纺织学报, 2015, 36(9): 55-60. (ZHU Pengfei, WU Minghua, LI Yanqi, et al. Modification of hyperbranched polymer and its application in preparation of nano-silver sol[J].Journal of Textile Research, 2015, 36(9): 55-60.)
[52] PERIGNON N, MINGOTAUD A F, MARTY J D, et al. Formation and stabilization in water of metal nanoparticles by a hyperbranched polymer chemically analogous to PAMAM dendrimers[J].Chemistry of Materials, 2004, 16(24): 4856-4858.
[53] KAVITHA M, PARIDA M R, PRASAD E, et al. Generation of Ag nanoparticles by PAMAM dendrimers and their size dependence on the aggregation behavior of dendrimers[J].Macromolecular Chemistry and Physics, 2009, 210(16): 1310-1318.
[54] HABA Y, KOJIMA C, HARADA A, et al. Preparation of poly(ethylene glycol)-modified poly(amido amine) dendrimers encapsulating gold nanoparticles and their heat-generating ability[J].Langmuir, 2007, 23(10): 5243-5246.
[55] PERIGNON N, MARTY J D, MINGOTAUD A F, et al. Hyperbranched polymers analogous to PAMAM dendrimers for the formation and stabilization of gold nanoparticles[J].Macromolecules, 2007, 40(9): 3034-3041.
[56] KAKATI N, MAHAPATRA S S, KARAK N. Silver nanoparticles in polyacrylamide and hyperbranched polyamine matrix[J].Journal of Macromolecular Science Part A-Pure and Applied Chemistry, 2008, 45(8): 658-663.
[57] KALBASI R J, ZAMANI F. Synthesis and characterization of Ni nanoparticles incorporated into hyperbranched polyamidoamine-polyvinylamine/SBA-15 catalyst for simple reduction of nitro aromatic compounds[J].Rsc Advances, 2014, 4(15): 7444-7453.
[58] WILMS D, STIRIBA S E, FREY H. Hyperbranched polyglycerols: From the controlled synthesis of biocompatible polyether polyols to multipurpose applications[J].Accounts of Chemical Research, 2010, 43(1): 129-141.
[59] CALDERON M, QUADIR M A, SHARMA S K, et al. Dendritic polyglycerols for biomedical applications[J].Advanced Materials, 2010, 22(2): 190-218.
[60] SUNDER A, HANSELMANN R, FREY H, et al. Controlled synthesis of hyperbranched polyglycerols by ring-opening multibranching polymerization[J].Macromolecules, 1999, 32(13): 4240-4246.
[61] WANG X L, CHEN J J, HONG L, et al. Synthesis and ionic conductivity of hyperbranched poly(glycidol)[J].Journal of Polymer Science Part B-Polymer Physics, 2001, 39(19): 2225-2230.
[62] YING H, HE G J, ZHANG L F, et al. Hyperbranched polyglycerol/poly(acrylic acid) hydrogel for the efficient removalof methyl violet from aqueous solutions[J].Journal of Applied Polymer Science, 2016, 133(5): 42951.
[63] CHENG H X, WANG S G, YANG J T, et al. Synthesis and self-assembly of amphiphilic hyperbranched polyglycerols modified with palmitoyl chloride[J].Journal of Colloid and Interface Science, 2009, 337(1): 278-284.
[64] SLAGT M Q, STIRIBA S E, KAUTZ H, et al.Optically active hyperbranched polyglycerol as scaffold for covalent and noncovalent immobilization of platinum(II) NCN-pincer complexes. Catalytic application and recovery[J].Organometallics, 2004, 23(7): 1525-1532.
[65] LI H, JO J K, ZHANG L D, et al. Hyperbranched polyglycidol assisted green synthetic protocols for the preparation of multifunctional metal nanoparticles[J].Langmuir, 2010, 26(23): 18442-18453.
[66] SLAGT M Q, STIRIBA S-E, KLEIN GEBBINK R J M, et al. Encapsulation of hydrophilic pincer—Platinum(Ⅱ) complexes in amphiphilic hyperbranched polyglycerol nanocapsules[J].Macromolecules, 2002, 35(15): 5734-5737.
[67] SKARIA S, THOMANN R, GOMEZ-GARCIA C J, et al. A convenient approach to amphiphilic hyperbranched polymers with thioether shell for the preparation and stabilization of coinage metal (Cu, Ag, Au) nanoparticles[J].Journal of Polymer Science Part A-Polymer Chemistry, 2014, 52(10): 1369-1375.
[68] WAN D C, FU Q, HUANG J L. Synthesis of amphiphilic hyperbranched polyglycerol polymers and their application as template for size control of gold nanoparticles[J].Journal of Applied Polymer Science, 2006, 101(1): 509-514.
[69] TANG Q, CHENG F, LOU X L, et al. Comparative study of thiol-free amphiphilic hyperbranched and linear polymers for the stabilization of large gold nanoparticles in organic solvent[J].Journal of Colloid and Interface Science, 2009, 337(2): 485-491.
[70] LIU Y, XU L, LIU X Y, et al. Hybrids of gold nanoparticles with core-shell hyperbranched polymers: Synthesis, characterization, and their high catalytic activity for reduction of 4-nitrophenol[J].Catalysts, 2016, 6(1): 3-17.
[71] AYMONIER C, SCHLOTTERBECK U, ANTONIETTI L, et al. Hybrids of silver nanoparticles with amphiphilic hyperbranched macromolecules exhibiting antimicrobial properties[J].Chemical Communications, 2002, 24(24): 3018-3019.
[72] 刘训勇, 刘华姬, 李文刚, 等. 基于超支化温敏聚合物制备温敏金纳米粒子[J].高分子通报, 2010, (6): 67-70. (LIU Xunyong, LIU Huaji, LI Wengang, et al. Thermoresponsive gold nanoparticles based on the hyperbranched thermoresponsive polymers[J].Polymer Bulletin, 2010, (6): 67-70.)
[73] KOTH A, TIERSCH B, APPELHANS D, et al. Synthesis of core-shell gold nanoparticles with maltose-modified poly(ethyleneimine)[J].Journal of Dispersion Science and Technology, 2012, 33(1-3): 52-60.
[74] ZHU Z D, KAI L, WANG Y C. Synthesis and applications of hyperbranched polyesters-preparation and characterization of crystalline silver nanoparticles[J].Materials Chemistry and Physics, 2006, 96(2-3): 447-453.
[75] ALAM M, SHAIK M R, ALANDIS N M. Vegetable-oil-based hyperbranched polyester-styrene copolymer containing silver nanoparticle as antimicrobial and corrosion-resistant coating materials[J].Journal of Chemistry, 2013, Article ID 962316, 11 pages.
[76] GAO L, KOJIMA K, NAGASHIMA H. Transition metal nanoparticles stabilized by ammonium salts of hyperbranched polystyrene: Effect of metals on catalysis of the biphasic hydrogenation of alkenes and arenes[J].Tetrahedron, 2015, 71(37): 6414-6423.
[77] KOJIMA K, CHIKAMA K, ISHIKAWA M, et al. Hydrophobicity/hydrophilicity tunable hyperbranched polystyrenes as novel supports for transition-metal nanoparticles[J].Chemical Communications, 2012, 48(86): 10666-10668.
[78] YU B, JIANG X S, YIN J.Responsive hybrid nanosheets of hyperbranched poly(ether amine) as a 2D-platform for metal nanoparticles[J].Chemical Communications, 2013, 49(6): 603-605.
The Progress of Hyperbranched Polymers as Stabilizers for Metal Nanoparticles
SHEN Yanyu, HE Guijin, GUO Yongsheng, FANG Wenjun
(DepartmentofChemistry,ZhejiangUniversity,Hangzhou310058,China)
Several kinds of hyperbranched polymers including hyperbranched poly(amido-amine) (HPAM), hyperbranched polyglycerol (HPG), and hyperbranched polyethylene imine (HPEI), as metal nanoparticle stabilizers are summarized in this work. HPAM is a good candidate as a plugging agent and a chemical oil displacement agent. It can serve as both reductant and dispersant, which can not only stabilize the dispersion of metal nanoparticles but also improve the reproducibility of nanocomposites. With a large amount of hydroxyl groups, HPG can be modified to be amphiphilic nanocapsules, which has good biocompatibility and can be used as high-quality crude oil demulsifier. Furthermore, the size of metal nanoparticles can be controlled by the relative molecular mass of HPG.HPEI owns a large number of amine functional groups, which provides the ideal coordination sites for metal ions. As an example, the HPEI-coated metal nanoparticles have been found as potential thermo-sensitive materials. With the combination of the structural controllability of hyperbranched polymer and excellent catalytic activity of metal nanoparticles, metalized hyperbranched polymers show great promises in the petrochemical industry.
hyperbranched polymer; metal nanoparticle; stabilizer
2016-12-30
国家自然科学基金项目(91441109)资助
沈燕宇,女,硕士研究生,从事航空航天推进剂化学研究;E-mail:mlhkdzxsyy@163.com
方文军,教授,博士,主要从事航空航天推进剂化学研究;E-mail:fwjun@zju.edu.cn
1001-8719(2017)04-0605-14
O63
A
10.3969/j.issn.1001-8719.2017.04.002
