新疆红枣枣果主要致病菌交链格孢菌产生毒素种类及黑斑病病果毒素含量测定
2017-08-30何丽郭开发艾尼古丽依明赵思峰
何丽,郭开发,艾尼古丽·依明,赵思峰
(新疆绿洲农业病虫害治理与植保资源利用自治区高校重点实验室/石河子大学农学院,新疆石河子 832003)
新疆红枣枣果主要致病菌交链格孢菌产生毒素种类及黑斑病病果毒素含量测定
何丽,郭开发,艾尼古丽·依明,赵思峰
(新疆绿洲农业病虫害治理与植保资源利用自治区高校重点实验室/石河子大学农学院,新疆石河子 832003)
【目的】研究新疆红枣缩果病和枣果黑斑病两种病害的主要致病菌交链格孢菌(Alternariaalternate)产生的主要毒素种类,以及栆果黑斑病病果中的毒素种类和含量,为红枣加工和食用的安全性评价提供依据。【方法】采用超高效液相色谱-串联质谱法,测定10株链格孢菌产生的毒素种类及含量,并对枣果黑斑病不同严重度发病枣果和人工接种果实中毒素种类及含量进行测定。【结果】10株交链格孢供试菌株可产生4种毒素,分别为链格孢酚(alternariol,AOH)、交链格孢酚单甲醚(alternariol monomethyl ether,AME)、交链孢烯(altenuene,ALT)和细交链格孢菌酮酸(tenuazonic acid,TeA)。TeA、ALT、AME和AOH在不同病级发病枣果和人工培养条件下均有检出,其中TeA检出含量最高,范围为3.1×103~5.5×103mg/kg;AME、AOH和ALT的含量范围分别为7.2×102~6.4×102mg/kg,1.2~3.8×102mg/kg和0.09~5.08 mg/kg。人工接种链格孢菌后,无伤、有伤枣果内均检测到大量TeA。不止在发病枣果中检测到了毒素,无伤接种未发病枣果亦发现大量毒素,且未接种健康枣果中亦有少量链格孢霉毒素。【结论】新疆红枣枣缩果病和枣果黑斑病病原菌两种病害的主要致病菌交链格孢菌(Alternariaalternate)均可产生链格孢霉毒素,且产毒量高,危害严重,影响了新疆红枣的产量及商品价值,患病枣果无法安全食用。在红枣鲜食加工和风险评估中应引起关注和重视,并在红枣种植和贮存期间减少病原菌侵染,以避免更多的污染。
红枣;枣果缩果病;枣果黑斑病;链格孢霉;毒素
0 引 言
【研究意义】链格孢属真菌已报道约500种,其广泛存在于水果、蔬菜和田间作物上导致多种农作物病害,同时该属真菌还可以产生多种真菌毒素,主要种类有链格孢酚(AOH)、交链格孢酚单甲醚(AME)、交链孢烯(ALT)和细交链格孢菌酮酸(TeA)等[1-3],这些毒素具有诱变性、致癌性、基因毒性等多种毒性作用[4]。【前人研究进展】目前已在苹果、柑橘、番茄、土豆、小麦、葵花籽等农产品中检测到AME、AOH等链格孢毒素[5-8]。Dong等[9]从林县的谷物上测出多种链格孢霉毒素,并认为AOH和AME两种毒素很有可能与中国河南林县的食道癌发生密切相关。AOH能引起食道上皮细胞增殖,并能引起胚胎食道鳞状细胞癌变,用AOH处理老鼠皮下,可诱导鳞状上皮细胞癌[10]。AME作为直接诱变剂,可使姊妹染色体交换率上升,诱导NIH 3T3细胞转化,转化后的细胞易癌变[11];AME也可选择性作用于特定的基因组或DNA序列引发诱变[12]。TeA在链格孢霉毒素中毒性最强,具多种生物特性,如抗病毒[13]、抗肿瘤、抑菌[14]、细胞毒性、植物毒性[15],抑制蛋白质的合成[16]等。TeA对哺乳动物(小鼠、大鼠等[17])具有急性毒性,其钠盐、镁盐与一种发生在非洲的人类出血性疾病-奥尼赖病(Onyalai)密切相关[18]。目前欧盟已就食品与饲料中链格孢霉毒素对人畜的健康进行风险发布,其中TeA已列入美国食品药物管理局(FDD)有毒化学物质登记册。【本研究切入点】新疆枣缩果病和枣果黑斑病的主要病原菌均为交链格孢菌(Alternariaalternate)[19,20],而其是否产生链格孢菌毒素以及毒素的种类和量目前尚未有明确报道。【拟解决的关键问题】研究采用超高效液相色谱-串联质谱法,对分离自新疆枣缩果病和枣果黑斑病的交链格孢菌毒素种类及含量进行测定和分析,并测定及分析枣果黑斑病不同发病病级的果实和人工接种交链格孢菌后红枣中毒素种类和含量,为新疆红枣果实的安全食用风险评估提供依据。
1 材料与方法
1.1 材 料
1.1.1 供试菌株
链格孢菌代表菌株(Alternariaalternate)10株,分离自新疆红枣主产区的枣缩果病和枣果黑斑病病样上,经形态学和分子生物学方法鉴定为交链格孢菌(A.alternate),菌种保藏于石河子大学新疆绿洲农业病虫害治理与植保资源利用自治区高校重点实验室。
1.1.2 发病枣果样品
采自新疆阿拉尔市周边骏枣发病果实,按发病面积进行分级:0级,果实表面没有病斑;1级,病斑面积<25%;2级,病斑面积25%~50%;3级,病斑面积50%~75%;4级,病斑面积>75%[21]。
1.1.3 接种用枣果
采自阿拉尔市周边骏枣膨大期果实,快递至石河子市实验室内供接种用。
1.1.4 培养基
DRYES培养基[22]:酵母膏 20 g、蔗糖150 g、虎红0.5 g、氯霉素0.1 g(0.2%溶于乙醇)、水1 L,pH 6.8。
PDA培养基[23]:马铃薯(去皮) 200 g、葡萄糖 20 g、琼脂粉 15 g、水1 L,pH 6.8。
交链格孢酚单甲醚(AME,纯度>98%)和链格孢酚(AOH,纯度>98%)标准品购买于美国Sigma公司;细交链格孢菌酮酸(TeA,纯度>98%)和交链孢烯(ALT,纯度>98%)标准品购买于北京莱耀生物公司;其余乙酸乙酯、乙二胺-N-丙基硅烷(PSA)、乙腈等试剂购买于石河子市试剂公司。
Xevo TQ/MS超高效液相色谱串联质谱仪(美国Waters公司),配有Acquity UPLC EH C18液相色谱柱(2.1 mm×150 mm,1.7 μm)。
1.2 方 法
1.2.1 超高效液相色谱-串联质谱条件
色谱柱:Acquity UPLC BEH C18液相色谱柱(1.7 μm,50 mm×2.1 mm);流动相:乙腈-水(0.1%甲酸);离子化模式:电喷雾离子源,正离子模式(ESI+);质谱扫描方式:多反应监测(MRM);毛细管电压3.5 kV,锥孔电压40 kV,离子源温度150℃,雾化温度500℃,脱溶剂气流量1 000 L/h,锥孔气流速50 L/h。列出4种链格孢霉毒素的监测离子、锥孔电压和碰撞电压等质谱参数。表1
表1 4种链格孢霉毒素的串联质谱测定参数
Table 1 MS/MS parameters for the 4 Alternaria mycotoxins
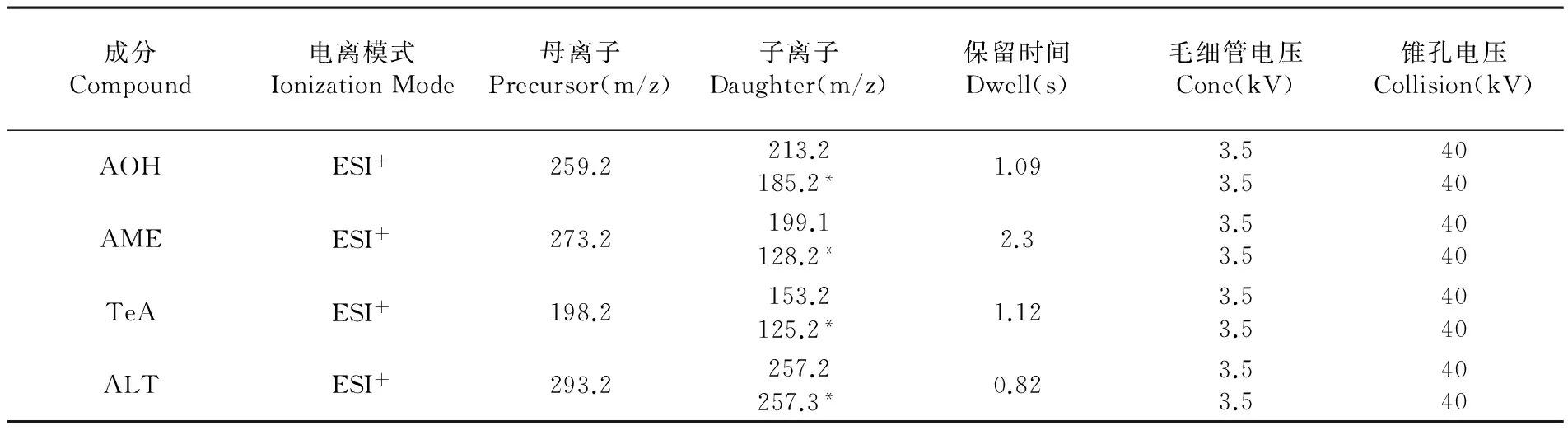
成分Compound电离模式IonizationMode母离子Precursor(m/z)子离子Daughter(m/z)保留时间Dwell(s)毛细管电压Cone(kV)锥孔电压Collision(kV)AOHESI+259.2213.2185.2*1.093.53.54040AMEESI+273.2199.1128.2*2.33.53.54040TeAESI+198.2153.2125.2*1.123.53.54040ALTESI+293.2257.2257.3*0.823.53.54040
注:*定量离子
Note:*Quantitative ion
1.2.2 10株交链格孢菌毒素种类及含量测定
将10株交链格孢菌菌株在PDA培养基上活化后,用打孔器打5 mm菌饼,接种到DRYES培养基上,三次重复,以空白为对照,28℃恒温、12 h昼夜交替培养14 d后,分别从三次重复中取1 g菌饼,用液氮研磨充分,于2 mL离心管中。离心管内加入1 mL乙酸乙酯(含1%甲酸),震荡1 h,在4 000 r/min下离心5 min,将提取液转入一个新2 mL离心管内,以氮吹器蒸发干燥,将冻干粉末溶解到400 μL甲醇,取上清液过0.45 μm有机滤膜,滤液经UPLC-MS/MS分析。
1.2.3 不同病级发病枣果样品处理
将采集的不同发病级数红枣栆果样品用清水洗净晾干,用75%酒精表面消毒,再用无菌水将果实冲洗干净,最后用灭菌滤纸吸干果实上残留的水分,分别称取健康枣果和发病果实病斑附近果肉2 g(精确至0.01 g),于50 mL尖底具塞离心管中,加入10 mL 1%甲酸乙腈溶液,7.5 mL无菌水,震荡4 min;再加入4 g无水MgSO4和1 g NaCl,剧烈震荡2 min 后,在4 000 r/min下离心5 min;取上清液转入15 mL离心管,加入0.2 g PSA和0.6 g无水MgSO4,剧烈震荡2 min后,在4 000 r/min下离心5 min;取上清液以氮吹器蒸发干燥,将冻干粉末溶解到400 μL甲醇,过0.45 μm有机滤膜,滤液经UPLC-MS/MS分析。10株枣缩果病和枣果黑斑病代表性的致病菌株在DRYES培养基上培养14 d后,提取粗提取物,测量4种链格孢霉毒素。
1.2.4 人工接种链格孢后发病枣果样品处理
将活化的10株采用离体菌块贴接法[20],对健康枣果进行无伤、有伤接种,将活化后的供试菌株,用打孔器打5 mm菌饼,将采集的健康无伤的红枣果实用清水洗净晾干,用75%酒精表面消毒,再用无菌水将果实冲洗干净,最后用灭菌滤纸吸干果实上残留的水分,用灭菌牙签刺孔,在超净工作台中将菌饼菌面朝下接种到红枣果实上,以DRYES培养基块为对照,然后放入铺有湿润滤纸的方形发芽盒内(12 cm×12 cm×1.5 cm)中,每个代表菌株接种3个果实,28℃条件下12 h光照、12 h黑暗交替培养,5 d后称取病斑附近果肉2 g,按1.2.3方法处理后测定4种链格孢霉毒素种类及含量。
试验分别准确称取健康果肉、DRYES培养基,在20、100、500和1 000 ppb 4个水平下加标,每个水平重复测定6次,按1.3.2样品处理方法处理并测定,以进样质量浓度X(ng/mL)为横坐标、峰面积Y为纵坐标绘制标准曲线。
2 结果与分析
2.1 4种链格孢霉毒素总离子流
在乙腈-水(0.1%甲酸)流动相体系中质量浓度为100 ng/mL的混标溶液(AOH、AME、ALT和TeA混合物)在3 min内达到有效分离且峰型良好。保留时间为0.81和2.3 mim的物质分别为ALT和AME,1.09和1.12 min分别为AOH和TeA。4种链格孢霉毒素的总离子流中有3个显示峰,AOH和TeA聚集在一个峰上,但试验采用超高效液相色谱质谱连用技术,结合浓度为100 ng/mL标准溶液中4种链格孢霉毒素(AOH、AME、ALT和TeA)的MRM定量离子对质谱图,以离子比为参照确定混合物中组分,并不影响试验结果,试验可行。图1,图2

图1 4种链格孢霉毒素的混合标准溶液(100 ng/mL)总离子流
Fig.1 Total ion current of 4 mycotoxins mixed standard solution(100 ng/mL)
2.2 方法验证
4种链格孢霉毒素在2~100 ng/mL范围内均有良好的线性关系,R2>0.99,平均回收率在70%~95%,相对标准偏差均小于8.9%在S/N=3时,ALT、AME、AOH和TeA的检出限在5~20 ppb。研究表明,该方法对红枣样品的不同含量的4种链格孢霉毒素的测定均具有较高的回收率和精密度,满足检测要求。表2,表3
2.3 10株链格孢菌菌株中毒素种类及含量
研究表明,10株菌均可产生TeA,在4种链格孢霉毒素中TeA含量最高,其中菌株21-HB含量最高,可达5.39×103mg/kg,hm-16产毒最低,含量为35.9 mg/kg;菌株92-3-1和hm-24产生的毒素种类最多,4种链格孢霉毒素均可产生;菌株30-HB-2和36-3-3产生毒素种类最少,仅产生TeA;菌株hm-16、hm-31和hm-35可产生AOH、AME、ALT,产毒范围分别为5.52~3.82×102mg/kg,1.96~7.16×102mg/kg,0.22~2.53 mg/kg。表3
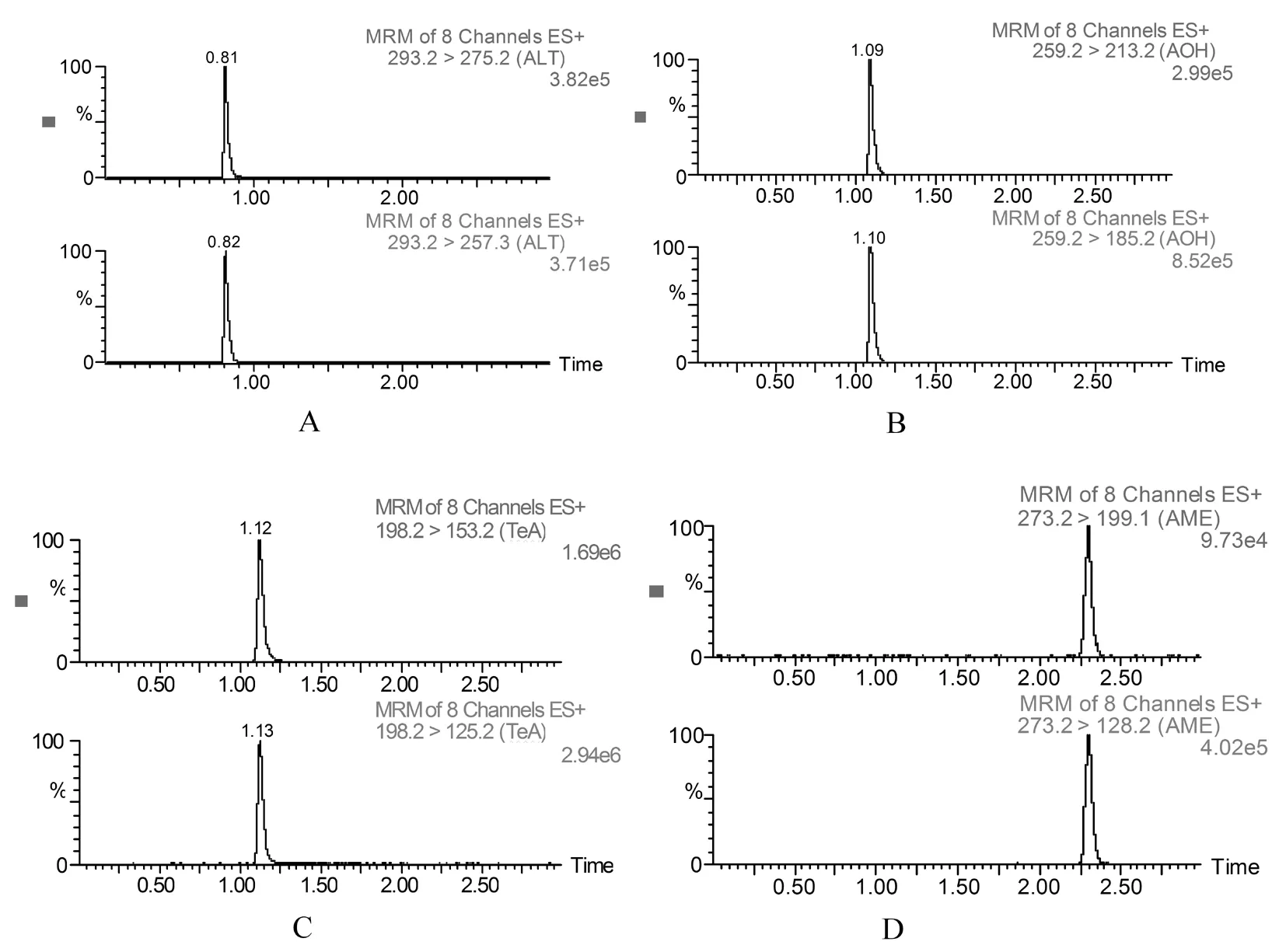
注:A.ALT;B.AOH;C.TeA;D.AME
Notes:A.ALT; B.AOH; C.TeA; D.AME
图2 4种链格孢霉毒素混合标准溶液在ESI+模式下MRM色谱
Fig.2 Mutiple reaction monitoring (MRM) chromatograms of mixed solution
of 4 mycotoxins standards in ESI+
表2 4种链格孢霉毒素的线性范围、线性方程、R2、检出限(LOD)
Table 2 Linear equations, correlation coefficients, linear ranges, detections limi, recoveries and precisions for the 4Alternariamycotoxins

基质成分Components线性方程R2线性范围(ng/mL)检出限(LOD)回收率Recovery(%)RSD(%)CKAOHAMETeAALTY=402.347X+2014.14Y=240.212X+1292.26Y=889.202X+12622.6Y=125.01X+227.1710.98510.99130.97630.99262~1001020510847688864.54.73.28.9果肉AOHAMETeAALTY=253.954X+2974.11Y=204.011X+1456.73Y=720.905X+4860.95Y=171.612X+7196.060.98510.99490.99060.98912~1001020510707295844.46.55.97.1培养基AOHAMETeAALTY=253.371X+1116.79Y=191.023X+1620.98Y=728.674X+20711Y=192.937X+302.4590.99790.9980.94840.92942~1001020510736987795.25.96.16.3
表3 DRYES培养基中10株链格孢菌产生毒素种类及含量
Table 3 The species and content of 10Alternariastrains produce mycotoxins in DRYES medium (mg/kg)

菌号成分CK34T11L30-HB-2hm-35hm-1692-3-1hm-2421-HB36-3-3hm-60hm-31AOH40.13381.5811.895.5212.8955.70AME12.0264.9836.561.96716.10375.42TeA1.751261.5744.06429.935.882396.634647.965389.21537.31724.404943.01ALT2.530.220.52
2.4 不同病级发病枣果中4种链格孢霉毒素含量
研究表明,不同发病程度发病枣果中4种链格孢霉毒素均有检出。随着枣果发病程度增加,链格孢霉毒素含量逐渐升高,3级时毒素含量最高;4种毒素中TeA含量最高,最高可达3.1×103mg/kg,AME、AOH、ALT分别可达6.4×102mg/kg,94.5 mg/kg,16.5 mg/kg。且0级果实中也检测到了4种毒素,但检出量较低,可能是运输储藏过程中健康枣果受到病果污染所致。表4
表4 不同病级发病枣果中4种链格孢霉毒素含量
Table 4 The content of 4Alternariamycotoxins in effected jujube fruit (mg/kg)

病级TeAAOHAMEALT030.910.040.620.031126.970.401.790.182524.9977.22638.643.3333102.2794.51995.3816.484186.463.092.131.68
2.5 人工接种枣果后4种链格孢霉毒素含量
研究表明,10株菌人工接种健康枣果后,无伤、有伤枣果内均产生大量链格孢霉毒素,其中TeA含量最高,4种毒素含量范围分别为4.6~2.1×103mg/kg,4.7~1.4×103mg/kg,1.2~122 mg/kg,0.09~5.08 mg/kg。用菌株hm-24接种的产毒量最高,TeA为2 102.90 mg/kg,AME为1 390.86 mg/kg;hm-16产毒量最低,TeA仅为5.40 mg/kg;34T11L、hm-35、hm-24、hm-604个菌株接种后可产生4种链格孢霉毒素;30-HB-2、92-3-1、21-HB、和36-3-3接种后在红枣果肉中仅检测出了TeA。且CK果肉中也检测到TeA,可能是样品在运输储藏的过程中健康枣果受到病果污染。表5
表5 枣果接种后4种链格孢霉毒素含量测定
Fig.5 The content of four Alternaria mycotoxins after inoculated withA.alternateon Jujube fruit (mg/kg)

成分菌号TeAAOHAMEALTCK-+1.202.8034T11L-+4.6046.407.1032.000.391.2230-HB-2-+16.1041.50hm-35-+54.00429.904.7030.100.09hm-16-+4.505.401.20122.20249.1892-3-1-+5.6070.40hm-24-+17.002102.900.4999.101390.860.685.0821-HB-+6.60151.7036-3-3-+6.2071.10hm-60-+11.8012.207.2922.021.19hm-31-+10.601455.402.90
注:空白表示未检测到毒素
Note:Blank said undetectedAlternaria Mycotoxins
3 讨 论
链格孢菌种类繁多,适应性强,寄主范围广,95%以上的种能兼性寄生于植物上,可引起多种植物尤其是粮食作物、蔬菜、水果等农作物和经济作物病害。A.tenuissima、A.alternata、Alternariasp、A.triticina等可侵染引起小麦叶斑病等[24],A.arborescens、A.tenuissima、A.mali、A.alternata/A.longipes等[25]多种链格孢菌可引起苹果叶斑病和果斑病。A.tenuissima侵染引起的小麦黑胚病(Black point of wheat)是一种世界性籽粒病害,在中国、美国、墨西哥、加拿大、印度、英国、前苏联、秘鲁等国均有发生[26,27]。A.alternata侵染引起的梨黑斑病(裂果病)是梨的三大病害之一,是梨树上广泛发生的世界性病害,尤其在亚洲的日本、韩国和中国发病十分严重[28]。梨黑斑病主要侵染叶片,造成大量落叶;有时也可危害果实和新梢,能造成较大的经济损失,发生严重时可使梨采后损失达50%以上[29]。2004 年我国鸭梨曾因黑斑病被停止出口美国和加拿大[30]。史文景[31]、程月萌等[32]测定了苹果、桃和梨中链格孢霉毒素,检测结果表明,苹果中检测到AOH和AME,含量分别为49和30 μg/kg。Birgitte等[33]运用超高液相色谱质谱串联技术测定了小麦、番茄、核桃、蓝莓中的链格孢霉毒素,结果表明均存在AOH、AME、ALXII、ALT、TeA、TEN等毒素。Peter 等[34]测定了谷物中链格孢霉毒素,检测结果表明83份样品中均检测到AOH、AME,含量分别为0.34和0.13 ng/g。Miller等[17]对小鼠分别经静脉注射、腹腔、皮下、口腔单次给TeA钠盐的LD50分别为(125±10)、(150±10)、(145±20)、(225±25) mg/kg;AOH和AME对实验动物的急性毒性较弱,小鼠的LD50>100 mg/kg。链格孢霉毒素在全球多种农产品中均已检测到,对人类和动物健康的潜在危害已不容忽视。
红枣种植目前已成为新疆近年来发展最快、效益最突出和惠民成效最为显著的一项林果产业[35,36],然而枣果黑斑病在很多地区均有发生。研究以新疆红枣缩果病和枣果黑斑病病果分离的10株交链格孢菌以及不同发病级数的枣果作为研究对象,10株供试菌株均能产生链格孢菌毒素,不同菌株产生的毒素种类以及含量有差异,其中从哈密分离到的hm-24菌株可以产生4种毒素,且产生毒素的量也最高,用其接种健康枣果后也可以在果肉中检测到4种毒素。不同病级枣果中4种链格孢霉毒素均有检出,TeA、AME、AOH、ALT含量分别为30.9~3.1×103mg/kg,0.6~6.4×102mg/kg,0.3~94.5 mg/kg,0.2~16.5 mg/kg。这与Magnani等[30]蒋黎艳等[31]检测到链格孢霉毒素中TeA含量最高而相一致。其中在健康枣果上也检测到了微量的TeA毒素,可能是运输过程中污染所致,也有可能健康果实表面也有链格孢菌的污染产生的毒素。
4 结 论
链格孢菌毒素分布面广,对人以及哺乳动物均有诱变性、致癌性、基因毒性等多种毒性作用,研究采用超高效液相色谱-串联质谱法,明确了引起枣缩果病和枣果黑斑病的主要致病菌交链格孢菌可以产生TeA、AME、AOH和ALT 4种毒素,毒素含量分别为1.2~2 102.9、22.02~1 390.86、1.2~122.2和0.39~5.08 mg/kg,其中TeA毒素的产生量最高,最高可达2 102.9 mg/kg。用其接种健康枣果5 d后,可在接种部位检测到大量链格孢菌毒素;同时对田间自然条件下采集的不同发病级数的枣果黑斑病果实进行检测后也能检测到4种链格孢菌毒素,在健康未发病的果实上也检测到了少量链格孢菌毒素。因此,安全、有效、及时控制枣果黑斑病已成为新疆红枣种植过程中的一个影响红枣食用安全性的关键问题,采收时应当将健康果实与病果分开,防止健康果实被病果污染。
References)
[1] Logrieco, A., Bottalico, A., Mulé, G., Moretti, A., & Perrone, G. (2003). Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops.EuropeanJournalofPlantPathology, 109(7): 645-667.
[2] Thomma, B. P. H. J. (2003). Alternaria, spp.: from general saprophyte to specific parasite.MolecularPlantPathology, 4(4): 225-236.
[3] Patriarca, A., Azcarate, M. P., Terminiello, L., & Fernández, P. V. (2007). Mycotoxin production by alternaria strains isolated from argentinean wheat.InternationalJournalofFoodMicrobiology, 119(3): 219-222.
[4] Janardhanan, K. K., & Husain, A. (1983). Studies on isolation, purification and identification of tenuazonic acid, a phytotoxin produced by alternaria alternata, (fr.) keissler causing leaf blight of datura innoxia, mill.Mycopathologia, 83(3): 135-140.
[5] Logrieco, A., Moretti, A., & Solfrizzo, M. (2009). Alternaria toxins and plant diseases: an overview of origin, occurrence and risks.WorldMycotoxinJournal, 2(2): 129-140.
[6] O Ostry, V. (2008). Alternaria mycotoxins: an overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs.WorldMycotoxinJournal, 1(2): 175-188.
[7] Magnani, R. F., Souza, G. D. D., & Rodrigues-Filho, E. (2007). Analysis of alternariol and alternariol monomethyl ether on flavedo and albedo tissues of tangerines ( citrus reticulata ) with symptoms of alternaria brown spot.JournalofAgricultural&FoodChemistry, 55(13): 4,980-4,986.
[8] Logrieco, A., Visconti, A., & Bottalico, A. (1990). Mandarin fruit rot caused by alternaria alternata and associated mycotoxins.PlantDisease, 74(6): 415-417.
[9] Dong, Z. G., Liu, G. T., Dong, Z. M., Qian, Y. Z., An, Y. H., & Miao, J. A., et al. (1987). Induction of mutagenesis and transformation by the extract of alternaria alternata isolated from grains in linxian, china.Carcinogenesis, 8(7): 989-991.
[10] Liu, G. T., Qian, Y. Z., Zhang, P., Dong, W. H., Qi, Y. M., & Guo, H. T. (1992). Etiological role of alternaria alternata in human esophageal cancer.ChineseMedicalJournal, 105(5): 394-400.
[11] Lehmann, L., Wagner, J., & Metzler, M. (2006). Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells.Food&ChemicalToxicology, 44(3): 398-408.
[12] An, Y. H., Zhao, T. Z., Miao, J., Liu, G. T., Zheng, Y. Z., & Xu, Y. M., et al. (1989). Isolation, identification, and mutagenicity of alternariol monomethyl ether.JournalofAgricultural&FoodChemistry, 37(5): 1,341-1,343.
[13]M Miller, F. A., Rightsel, W. A., Sloan, B. J., Ehrlich, J., French, J. C., & Bartz, Q. R., et al. (1964). Antiviral activity of tenuazonic acid.Nature,200(4913): 1,338-1,339.
[14] Gitterman, C. O. (1965). Antitumor, cytotoxic, and antibacterial activities of tenuazonic acid and congeneric tetramic acids.JournalofMedicinalChemistry, 8(4): 483-486.
[15] Lebrun, M. H., Nicolas, L., Boutar, M., Gaudemer, F., Ranomenjanahary, S., & Gaudemer, A. (1988). Relationships between the structure and the phytotoxicity of the fungal toxin tenuazonic acid.Phytochemistry, 27(1): 77-84.
[16] Shigeura, H. T., & Gordon, C. N. (1963). The biological activity of tenuazonic acid.Biochemistry, 2(2): 1,132-1,137.
[17]吴春生,马良,江涛,等.链格孢霉毒素细交链格孢菌酮酸的研究进展[J].食品科学,2014,35(19): 295-301.
WU Chun-sheng, MA Liang, JIANG Tao, et al. (2014). A review on tenuazonic acid, a toxic produced byAlternaria[J].FoodScience, 35(19):295-301.(in Chinese)
[18] Steyn, P. S., & Rabie, C. J. (1976). Characterization of magnesium and calcium tenuazonate from phoma sorghina.Phytochemistry, 15(12): 1,977-1,979.
[19]董宁,冯宏祖,王兰,等.南疆骏枣黑斑病症状表现及病原菌鉴定[J].植物保护学报,2016,43(6): 922-927.
DONG Ning, FENG Hong-zu, WANG Lang, et al. (2016). The disease symptom and the pathogen identification of jujube black spot in southern Xinjiang [J].JournalofPlantProtection, 52(7):53-58. (in Chinese)
[20]向征,钟聪慧,胡军,等.新疆枣果黑斑病病原鉴定[J].新疆农业科学,2013,50(5): 845-850.
XIANG Zheng, ZHONG Cong-hui, HU Jun, et al. (2013). Identification of Jujube black spot pathogens in Xinjiang, China [J].XinjiangAgriculturalSciences, 50(5):845-850. (in Chinese)
[21]马荣,刘晓琳,梁英梅,等. 枣果黑斑病发生相关因素分析及田间药效试验[J]. 中国农学通报,2015,(16): 182-189.
MA Rong, LIU Xiao-lin, LIANG Ying-mei, et al. (2015). Analysis of the influencing factors on disease occurrence of Jujube black pot and field efficacy Test [J].ChineseAgriculturalScienceBulletin, (16):182-189. (in Chinese)
[22] Andersen, B., Hansen, M. E., & Smedsgaard, J. (2005). Automated and unbiased image analyses as tools in phenotypic classification of small-spored alternaria spp.Phytopathology, 95(9): 1,021-1,029.
[23]方中达.植病研究方法[M].第三版. 北京:中国农业出版社,1998.
FANG Zhong-da. (1998).Methodofplantpathologyresearch[M]. Third Edition. Beijing: China Agriculture Press, 1998.(in Chinese)
[24] Zhao, K., Shao, B., Yang, D., Li, F., & Zhu, J. (2015). Natural occurrence of alternaria toxins in wheat-based products and their dietary exposure in China.PLOSOne. 10(6), e0132019.
[25] Harteveld, D. O. C., Akinsanmi, O. A., & Drenth, A. (2013). Multiple alternaria, species groups are associated with leaf blotch and fruit spot diseases of apple in australia.PlantPathology, 62(2): 289-297.
[26] Machacek, J. E., & Greaney, F. J. (2011). The "black-point" or "kernel smudge" disease of cereals.CanadianJournalofResearch, (16): 84-113.
[27]Y B Kang, Y J Zhang , H J Li , et al. (1999). State of the field of wheat on black point disease (in Chinese) [J].TriticalCrops, 19(2): 58- 60. (in Chinese)
[28]杨晓平,胡红菊,王友平,等. 梨黑斑病病原菌的生物学特性及其致病性观察[J].华中农业大学学报,2009,(12):680-684.
YANG Xiao-ping, HU Hong-ju, WANG You-ping, et al. (2009). Biological characteristics and pathogencity of pear black spot byAlternariaalternate(Fr.) Keissl [J].JournalofHuazhongAgriculturalUniversity, (12):680-684. (in Chinese)
[29]谢莉,张铎,张丽萍,等. 拮抗梨黑斑病菌的链霉菌的筛选及鉴定[J].河北师范大学学报(自然科学版),2008,32(4):526-529.
XIE Li, ZHANG Duo, ZHANG Li-ping, et al. (2008). Isolation and identification of an antagonisticStreptomycesstrain againstAlternariaalternate. [J].JournalofHebeiNormalUniversity(NaturalScienceEd.) , 32(4): 526-529. (in Chinese)
[30]严进,施宗伟,宋福,等. 河北和山东鸭梨果实上链格孢菌鉴定[J].植物保护学报,2009,36(1) :37-43.
YAN Jin, SHI Zong-wei, SONG Fu, et al. (2009). Identification ofAlternariaisolates from Ya-pear fruits in Hebei and Shandong provinces [J].JournalofPlantProtection, 36( 1) :37-43. (in Chinese)
[31]史文景,赵齐阳,张耀海,等. 超高效液相色谱结合分散液液微萃取同时测定果汁中的7种真菌毒素[C]//. 中国仪器仪表学会分析仪器分会、中国仪器仪表行业协会分析仪器分会、中国仪器仪表学会检验检疫仪器应用技术分会第三届中国食品与农产品质量安全检测技术国际论坛暨展览会论文集.北京: 中国北京雄鹰国际展览有限公司,2014:13-19.
SHI Wen-jing, ZHAO Qi-yang, ZHANG Yao-hai, et al. (2014).SimultaneousDeterminationofSevenMycotoxinsinFruitJuicebyUltraPerformanceLiquidchromatographyCombinedwithModifiedDispersiveLiquid-LiquidMicroextraction[C]//. China Instrument Society Analysis Instrument Branch, China Instrument Industry Association Analysis Instrument Branch, China Instrument Society inspection and quarantine instrument application technology branch. The third Chinese food and agricultural products quality and safety testing technology international forum and Exhibition proceedings. Beijing, Beijing Lanneret International Exhibition Co, Ltd. : 13-19. (in Chinese)
[32]陈月萌,李建华,张静,等. 高效液相色谱-荧光检测法同时测定水果中的3种链格孢霉毒素[J]. 分析试验室,2012,(6):70-73.
CHEN Yue-meng, LI Jian-hua, ZHANF Jing, et al. (2016). Simultaneous determination of threeAlternariasin fruits by HPLC with fluorescence detection [J].ChineseJournalofAnalysisLaboratory, (6):70-73. (in Chinese)
[33] Andersen, B., Nielsen, K. F., Pinto, V. F., & Patriarca, A. (2015). Characterization of alternaria, strains from argentinean blueberry, tomato, walnut and wheat.InternationalJournalofFoodMicrobiology: (196): 1-10.
[34] Scott, P. M., Zhao, W., Feng, S., & Lau, P. Y. (2012). Alternaria, toxins alternariol and alternariol monomethyl ether in grain foods in canada.MycotoxinResearch, 28(4):261-266.
[35]张栋海,李克福,赵思峰.新疆南疆矮化密植枣园三种红枣病害发生规律及其影响因素研究[J].北方园艺,2015,(3):105-108.
ZHANG Dong-hai, LI Ke-fu, ZHAO Si-feng. (2015). Study on law and its influence factors of three main jujube disease in south xinjiang under dwarf and close-planting managements [J].NorthernHorticulture, (3):105-108. (in Chinese)
[36]赵燕,郭庆元,王洪凯,等.新疆红枣病害种类及田间发生[J].新疆农业科学, 2015,52(3):511-516,589.
ZHAO Yan, GUO Qin-yuan, WANG Hong-kai, et al. (2015). Investigation of Chinese jujube diseases and their field occurrence in Xinjiang [J],XinjiangAgriculturalSciences, 52(3):511-516,589. (in Chinese)
[37]王志霞,孙洁,赵思峰,等.新疆矮化密植枣园红枣叶斑病病原鉴定[J].中国森林病虫,2013,32(4):1-5.
WANG Zhi-xia, SUN Jie, ZHAO Si-feng, et al. (2013). Identification of the pathogenic fungus causing leaf spot ofZizyphusjujubein dwarf and close-planting orchards in Xinjiang [J].ForestPestandDisease, 32(4):1-5. (in Chinese)
[38]蒋黎艳,赵其阳,龚蕾,等.超高效液相色谱串联质谱法快速检测柑橘中的5种链格孢霉毒素[J].分析化学,2015,(12): 1 851-1 858.
JIANG Li-yan, ZHAO Qi-yang, GONG Lei, et al. (2015). Rapid Determination of fiveAlternariaMycotoxins in citrus by ultra-high performance liquid chromatography-tandem mass spectrometry [J].AnalyticalChemistry, (12):1,851-1,858. (in Chinese)
Mycotoxin Analysis of Main PathogenAlternariaalternateof Jujube Fruit Shrink and Determination of Black Spot Disease Toxin Content in Xinjiang
HE Li,GUO Kai-fa,Ainiguli Yiming,ZHAO Si-feng
(KeyLaboratoryforOasisAgriculturalPestManagementandPlantResourceUtilizationatUniversitiesofXinjiangUygurAutonomousRegion/CollegeofAgronomy,ShiheziUniversity,ShiheziXinjiang832003,China)
【Objective】 In order to make clear mycotoxins species and content of mainly pathogenAlternariaalternateof jujube fruit shrink, jujube black spot, and mycotoxins species and content of jujube black spot diseased fruits, this study aims to provide the basis for the safety evaluation of jujube processing and consumption in Xinjiang.【Method】Utra-performance liquid chromatography-tandem mass spectrometry was established as the method to determine the variation and content of mycotoxins from 10Alternariastrains and the onset fruit of jujube black spot in different severities and the species and content of toxin in artificially inoculated fruits.【Result】The results showed that 10Alternariastrains could produce 4 kinds ofAlternariamycotoxins: alternariol (AOH), alternariol monomethylether (AME), altenuene(ALT) and tenuazonic acid (TeA).TeA, ALT, AME and AOH were detected in different disease onset of jujube fruit which were affected byA.alternateand artificial cultivation conditions, the highest content of TeA, range of 3.1×103-5.5×103mg/kg; AME, AOH and ALT were 7.2×102-6.4×102mg/kg, 1.2-3.8×102mg/kg and 0.09-5.08 mg/kg. After inoculation withA.alternaria, alarge number of TeA were not only detected injuring jujube fruit, but also detected in the uninjured jujube fruit. Not only the toxin was detected in the onset jujube fruit, but also a large amount of toxin was found in the jujube without injury, although there was a small amount ofAlternariatoxin in healthy jujube fruit.【Conclusion】The main pathogens (Alternariaalternate) leading to jujube fruit shrink and jujube black spot in Xinjiangcan produce a lot ofAlternariamycotoxins, which have such great influence on the production and value of Xinjiang red jujube that it was inedible. Therefore, it should be paid more attention to in fresh jujube processing and risk assessment, and to reduce the infection of pathogens in jujube planting and storage, so as to avoid more pollution. So in jujube planting and storage period, the pathogen infection must be reduced in order to avoid more contamination.
jujube; short ultivars; growth stages; major gene plus polygene inheritance; genetic analysis
ZHAO Si-feng (1975- ), male, native place: Bazhong Sichuan. Professor, PhD. research field: Biological control of plant pest. (E-mail)Zhsf_agr@shzu.edu.cn
10.6048/j.issn.1001-4330.2017.06.014
2017-03-09
国家星火计划“南疆红枣主要病虫害绿色防控技术示范与推广”(2015GA891007)
何丽(1991-),女,四川南充人,硕士研究生,研究方向为植物病理学,(E-mail)602157044@qq.com
赵思峰(1975-),男,教授,博士,博士生导师,研究方向为植物病虫害生物防治,(E-mail)zhsf_agr@shzu.edu.cn
S432.4+2
A
1001-4330(2017)06-1076-09
Supported by: The National Spark Program of China "Demonstration and Popularization of Green Prevention and Control Technology for Main Diseases and Pests of Jujube in Southern Xinjiang"(2015GA891007)
