Recovery of sympathetic nerve function after lumbar sympathectomy is slower in the hind limbs than in the torso
2017-08-07ZhifangZhengYishuLiuXuanMinJianbingTangHongweiLiuBiaoCheng
Zhi-fang Zheng, Yi-shu Liu,, Xuan Min, Jian-bing Tang, Hong-wei Liu, Biao Cheng,,
1e Graduate School of Southern Medical University, Guangzhou, Guangdong Province, China
2 Department of Plastic Surgery, Guangzhou General Hospital of Guangzhou Military Command, Guangzhou, Guangdong Province, China
3e Graduate School ofird Military Medical University, Chongqing, China
4 Department of Plastic Surgery, the First A ffi liated Hospital of Jinan University, Guangzhou, Guangdong Province, China
5 Center of Wound Treatment, Guangzhou General Hospital of Guangzhou Military Command, Guangzhou, Guangdong Province, China
6e Key Laboratory of Trauma Treatment & Tissue Repair of Tropical Area of Chinese PLA, Guangzhou, Guangdong Province, China
Recovery of sympathetic nerve function after lumbar sympathectomy is slower in the hind limbs than in the torso
Zhi-fang Zheng1,2, Yi-shu Liu2,3, Xuan Min2, Jian-bing Tang2, Hong-wei Liu4, Biao Cheng1,2,3,5,6,*
2 Department of Plastic Surgery, Guangzhou General Hospital of Guangzhou Military Command, Guangzhou, Guangdong Province, China
4 Department of Plastic Surgery, the First A ffi liated Hospital of Jinan University, Guangzhou, Guangdong Province, China
5 Center of Wound Treatment, Guangzhou General Hospital of Guangzhou Military Command, Guangzhou, Guangdong Province, China
How to cite this article:Zheng ZF, Liu YS, Min X, Tang JB, Liu HW, Cheng B (2017) Recovery of sympathetic nerve function aer lumbar sympathectomy is slower in the hind limbs than in the torso. Neural Regen Res 12(7):1177-1185.
Graphical Abstract

orcid: 0000-0003-3383-1671 (Biao Cheng)
Local sympathetic denervation by surgical sympathectomy is used in the treatment of lower limb ulcers and ischemia, but the restoration of cutaneous sympathetic nerve functions is less clear.is study aims to explore the recovery of cutaneous sympathetic functions aer bilateral L2–4sympathectomy.e skin temperature of the lefeet, using a point monitoring thermometer, increased intraoperatively aer sympathectomy.e cytoplasm of sympathetic neurons contained tyrosine hydroxylase and dopamine β-hydroxylase, visualized by immuno fl uorescence, indicated the accuracy of sympathectomy. Iodine starch test results suggested that the sweating function of the hind feet plantar skin decreased 2 and 7 weeks aer lumbar sympathectomy but had recovered by 3 months. Immuno fl uorescence and western blot assay results revealed that norepinephrine and dopamine β-hydroxylase expression in the skin from the sacrococcygeal region and hind feet decreased in the sympathectomized group at 2 weeks. Transmission electron microscopy results showed that perinuclear space and axon demyelination in sympathetic cells in the L5sympathetic trunks were found in the sympathectomized group 3 months after sympathectomy. Although sympathetic denervation occurred in the sacrococcygeal region and hind feet skin 2 weeks aer lumbar sympathectomy, the skin functions recovered gradually over 7 weeks to 3 months. In conclusion, sympathetic functional recovery may account for the recurrence of hyperhidrosis aer sympathectomy and the normalization of sympathetic nerve trunks aer incomplete injury.e recovery of sympathetic nerve function was slower in the limbs than in the torso aer bilateral L2–4sympathectomy.
nerve regeneration; lumbar sympathectomy; sympathetic nerve; skin; recovery of function; neural regeneration
Introduction
Sympathetic denervation is associated with many diseases. Sympathetic neuropathy is frequently observed in patients with diabetes, and re fl ects the severity of diabetes (Faerman et al., 1982). Patients with a spinal cord injury or Parkinson’s disease may also show symptoms of sympathetic denervation. Many localized lesions, such as diabetic feet (Faerman et al., 1982), limb ischemia (Stumpflen et al., 2000), Raynaud’s disease (Thune et al., 2006), and hyperhidrosis (Ng and Yeo, 2003), are also strongly associated with sympatheticnervous system function. Surgical sympathectomy is an effective method for treating such localized lesions.oracic sympathectomy is useful to treat digital ischemia or ulcers (Han et al., 2008; Coveliers et al., 2011). Lumber sympathectomy improves wound healing in diabetic feet (Shor et al., 2004), chronic venous leg ulcers (Patman, 1982), and critical limb ischemia (Hatangdi and Boas, 1985; Rivers et al., 1986; van Dielen et al., 1998; Tomlinson, 2000). Digital periarterial sympathectomy is the first treatment option for Raynaud syndrome (Letamendia et al., 2016).
Objective and reliable evaluation methods are essential to detect sympathetic denervation (Navarro, 2016). Useful indicators of sympathetic denervation, include sweating, warm skin, increased blood fl ow, electrodermal activity, pain response, skin integrity, and skin wrinkling. Norepinephrine (NE), tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DβH) are commonly used as sympathetic markers that can re fl ect sympathetic nerve function in the skin. TH and DβH are key enzymes involved in NE synthesis (Lehtosalo et al., 1988; Mione et al., 1991).
The skin is the largest and most superficial organ of the body; its functions change after sympathetic denervation. There has been extensive research on nerve regeneration. However, it has mainly focused on the regeneration of motor and sensory nerves.e regeneration of sympathetic nerves has been con fi rmed in the internal organs and cardiovascular system, but seldom in skin. Navarro et al. (1997) showed sudomotor nerve regeneration in the footpad aer crush or section of the sciatic nerve in mice. However, what controls the regeneration of sympathetic nerves aer lumbar sympathectomy is still unclear. In this study, we used various methods to measure the recovery from sympathetic denervation in the skin of the sacrococcygeal region and hind feet of rats aer lumbar sympathectomy.
Materials and Methods
Animals
Thirty-six male Sprague-Dawley rats weighing 250–300 g were purchased from the Experimental Animal Center of Southern Medical University of China (SCXK (Yue) 2016-0041). Rats were acclimatized for at least 1 week before use.e rats were maintained at 22–24°C with a 12-hour light/ dark cycle and allowed free access to standard laboratory food and water. The ambient temperature was maintained at 25°C in all animal experiments. The study protocol was approved by the Ethics Committee of Guangzhou General Hospital of Guangzhou Military Command of China (approval No. 2015030901).
Rats were randomly divided into sympathectomized (n= 12), sham-operated (n= 12), and normal groups (n= 12).
Rat model establishment
All rats underwent bilateral lumbar L2–4sympathetic trunk resection as follows: the rats were intraperitoneally anesthetized with 0.6% pentobarbital sodium (40 mg/kg; Anthony Products, Arcadia, CA, USA). Aer epilation and disinfection, an incision was made along the middle of the abdomen from the xiphoid to the pubic symphysis.e lumbar sympathetic trunks were located behind the abdominal aorta and vena cava and dissociated under a surgical microscope (M300; Leica, Mannheim, Germany) from the level of the lerenal artery (L2sympathetic ganglia) to the level of the bifurcation in the descending aorta (L5sympathetic ganglia) (Hweidi et al., 1985). Bilateral L2–4sympathetic trunks were removed and used for the preparation of frozen sections to test for TH and DβH activities.e increased skin temperature of the lefeet, taken intraoperatively, and the positive staining of TH and DβH indicated the accuracy of sympathectomy. Aer complete hemostasis, the incision was closed with 5-0 sutures, and all rats were transferred to a clean cage. The rats in the sham-operated group underwent the identical surgical procedures to those in the sympathectomized group, but the bilateral lumbar sympathetic trunks were not removed. The normal group did not undergo surgery after anesthesia. All rats were transferred to a clean cage after sympathectomy. No drugs were used aer the operation. After fasting for 6 hours, the rats were fed normal diets.
Immuno fl uorescence analysis of lumbar sympathetic trunks aer operation
Freshly frozen lumbar sympathetic trunk segments from the rats were sectioned (5-μm) and fi xed with ice-cold acetone for 10 minutes.e sections were immersed in ethylenediaminetetraacetic acid bu ff er (pH 6.0) for 20 minutes at 95°C. The sections were incubated with primary goat polyclonal anti-TH antibody (1:800 dilution; Abcam, Cambridge, UK) and sheep polyclonal anti-DβH antibody (1:800 dilution; Abcam) for 12 hours at 4°C. Aer rinsing with Tris-bu ff ered saline, sections were incubated for 60 minutes at room temperature with the secondary antibody anti-goat IgG H&L antibody (Alexa Fluor®488) and anti-sheep IgG H&L antibody (Alexa Fluor®568) (Abcam).e slides were then incubated with Hoechst 33342 as the substrate for 3 minutes to visualize positively immunostained cells. Finally, the slides were mounted with 95% glycerol and observed under a fluorescence microscope (Olympus, Tokyo, Japan).
Temperature monitoring postoperatively
Iodine starch test
Apocrine (Robertshaw, 1974) and eccrine (Sokolov et al., 1980) sweat glands are innervated by the sympathetic nervous system. The sudomotor system is also controlled by sympathetic neurons. The iodine starch reaction, which is oen used to observe the appearance of sweat spots and to evaluate areas wetted by sweat, can be more sensitive than cardiovascular tests (Ryder et al., 1988). The iodine starch test was performed on the plantar skin to evaluate sweating 2,7 weeks, and 3 months aer sympathectomy on the remaining rats. The plantar skin was covered with iodine reagent (Aladdin, Shanghai; 2 g dissolved in 100 mL anhydrous ethanol). Hydrochloride adrenaline (20 μg/100 g) was injected into the thigh muscle to induce sweating. Aer the plantar skin was fully dried, soluble starch (50 g in 100 mL castor sesame oil; Tianjin Damao Chemical Reagent Factory, Tianjin, China) was applied. Starch reacts with the iodine reagent giving a blue-brown color.e number of blue sweat spots was determined and photographed using a digital camera (D3100; Nikon, Tokyo, Japan) 15 minutes after starch was added to the iodine reagent. The sweating in the hind feet with sympathetic denervation will be less than that in the front feet with normal sympathetic nerve function. We defi ned the decreased sweat spots in hind feet with sympathetic denervation as positive staining.e number of the rats with positive staining was calculated.
Sample collection
All samples were collected under anesthesia, and the rats were subsequently euthanized. The skin of the sacrococcygeal region and hind feet was removed for immunoenzyme staining and western blot assay in 4 rats per group at 2, 7 weeks, and 3 months after sympathectomy. The L5sympathetic trunks at the level of the bifurcation of the descending aorta were excised and quickly placed in 4% paraformaldehyde for transmission electron microscopy (H-7500; Hitachi, Tokyo, Japan).
Immunoenzyme staining
Para ffi n sections (5-μm) of skin from the sacrococcygeal region and hind feet were de-para ffi nized, washed three times in phosphate bu ff er saline for 5 minutes, and then blocked with 5% serum for 30 minutes.e slides were subsequently incubated with sheep polyclonal anti-DβH antibody (1:800 dilution; Abcam) and rabbit polyclonal anti-NE antibody (1:1,000 dilution; Lifespan, Cambridge, UK) at 4°C overnight. Aer rinsing three times with phosphate bu ff er saline, the slides were incubated with donkey anti-sheep IgG H&L antibody (1:1,000 dilution; Abcam) and donkey anti-rabbit IgG H&L antibody (1:1,000 dilution; Abcam) at 37°C for 30 minutes.e secondary antibodies were horseradish peroxidase-labeled antibodies, further developed with 3,3′-diaminobenzidine solution. Aer subsequent staining with hematoxylin, the sections were observed under the microscope and images were taken with the Image-Pro Plus software (version 6.0; Media Cybernetics Corp., Silver Springs, MD, USA).
Western blot assay
Transmission electron microscopy of L5sympathetic trunks
At 3 months after sympathectomy, the sympathetic trunks were prefixed in 4% paraformaldehyde at 4°C overnight, followed by post-fixation with 1% osmium tetroxide for 2 hours.e specimens were dehydrated with gradient alcohol series and acetone, incubated in propylene oxide/epoxy resin mixture, and embedded in pure resin. 40–60 nm sections were cut, double stained with uranyl acetate and lead citrate, and observed under transmission electron microscope (H-7500; Hitachi).
Statistical analysis
Quantitative data were expressed as the mean ± SD. All statistical computations were performed using the SPSS 21.0 so-ware (IBM, Armonk, NY, USA). Statistical comparisons were performed using one-way analysis of variance and the least signi fi cant di ff erence test for quantitative data and chi-square test for qualitative data.e data for analysis of variance were subjected to a Kolmogorov Smirnov Test for normal distribution, which showed that the data in every group were normally distributed.P< 0.05 was considered statistically signi fi cant.
Results
Assessment of rat models of sympathectomy
All rats survived the surgical procedures.e anatomy of the sympathetic trunks can vary (Miao et al., 1995). It is important to be accurate when cutting the bilateral paravertebral ganglia of the sympathetic chain during lumbar sympathectomy. The lumbar sympathetic trunks are pale and located in front of the spine on the inner side of the bilateral genitofemoral nerves, but behind the abdominal aorta and vena cava (Figure 1), consistent with a previous report (Hweidi et al., 1985). Care must be taken to avoid damage to large bloodvessel during surgery. If the small blood vessels around the sympathetic trunks are damaged, hemostasis must be restored. It is important to note the differences between the lumbar sympathetic trunk and genitofemoral nerve. The lumbar sympathetic trunk is gray with many ganglia branches. Genitofemoral nerves are white and have no branches between spinal segments 2 and 4.
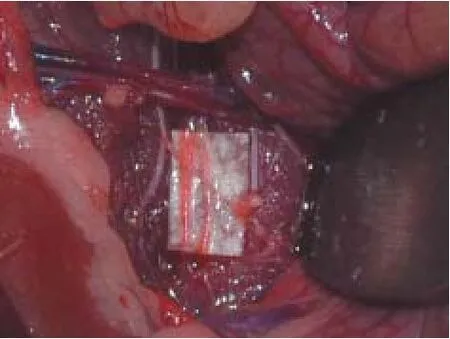
Figure 1 Anatomy of the bilateral lumbar sympathetic trunk (indicated by arrows) in a rat.

Figure 2 Immuno fl uorescence staining results in the lumbar sympathetic trunk in rats under fl uorescence microscope (× 200).

Figure 3 Skin temperature of the lefeet.
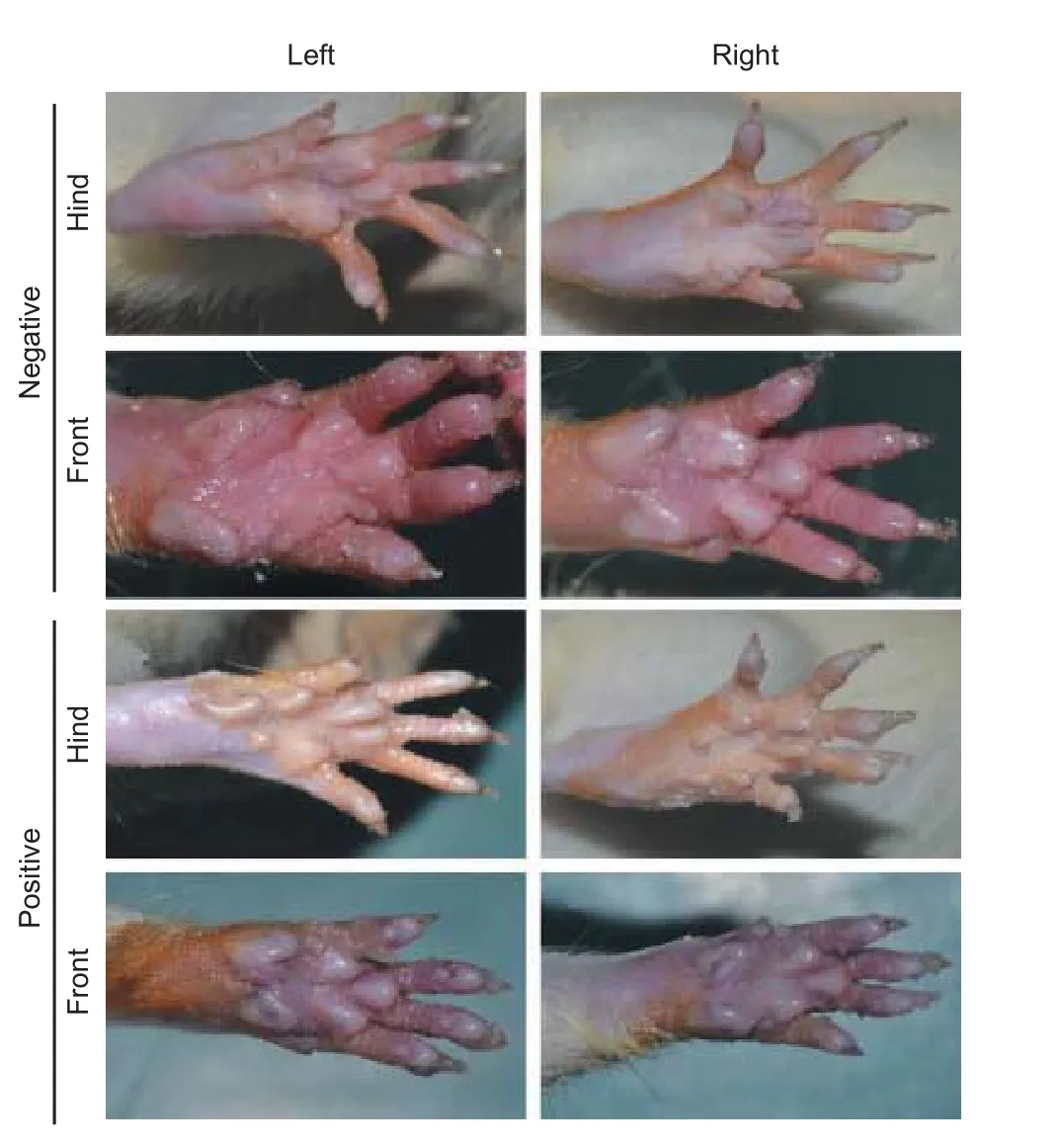
Figure 4 E ff ect of lumbar sympathectomy on the sweating of the plantar skin of the hind feet.
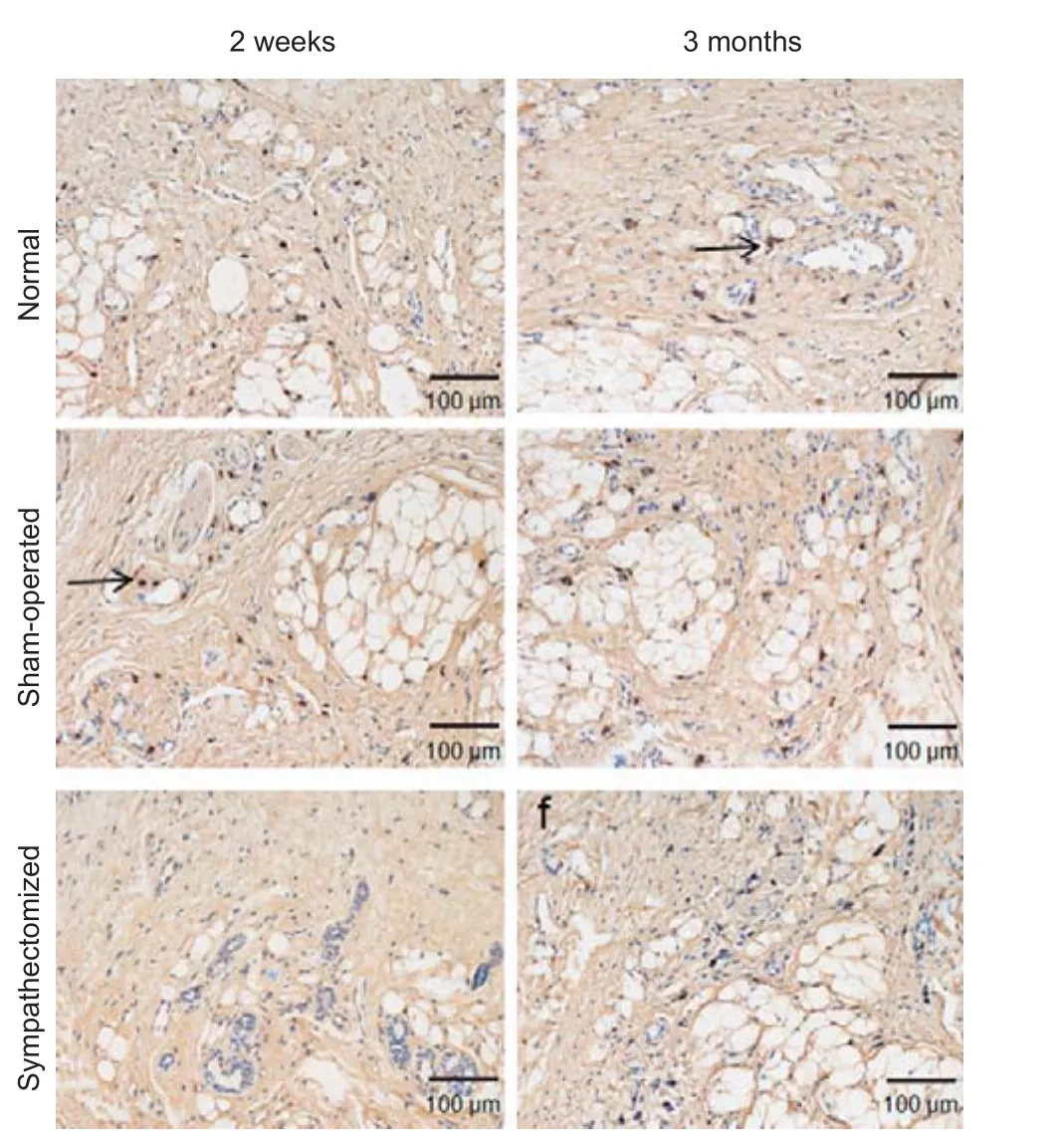
Figure 5 Immunohistochemical staining of norepinephrine under a light microscope in the plantar skin of hind feet 2 weeks and 3 months aer sympathectomy in rats.

Figure 6 Immunohistochemical staining of dopamine β-hydroxylase under a light microscope in the plantar skin of hind feet 2 weeks and 3 months aer sympathectomy in rats.

Figure 8 Immunohistochemical staining of norepinephrine under a light microscope in sacrococcygeal skin 2 and 7 weeks aer sympathectomy in rats.

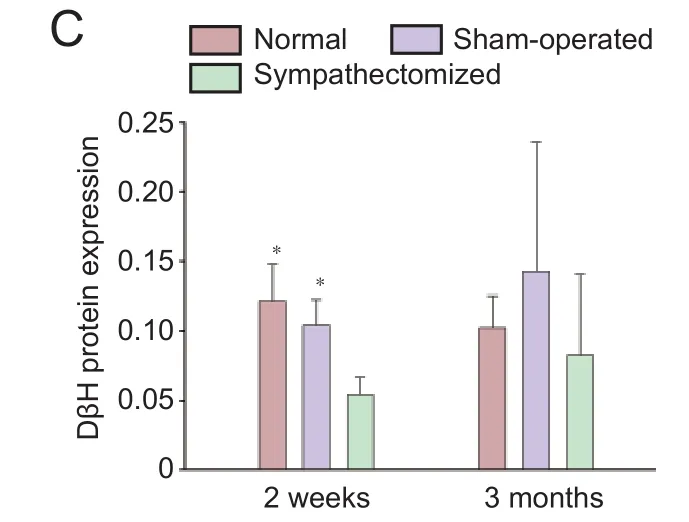
Figure 7 Protein expression of NE and DβH in the plantar skin of hind feet 2 weeks and 3 months aer sympathectomy in rats.
Expression of TH and DβH in the cytoplasm of sympathetic neurons in excised sympathetic trunks
Skin temperature
E ff ect of lumbar sympathectomy on sweating by the plantar skin of the hind feet
Fewer blue sweat spots were detected on the plantar skin of the hind feet than on the plantar skin of the front feet in rats with sympathetic denervation (positive staining; Figure 4). In contrast, blue sweat spots in the plantar skin of hind feet were not di ff erent from those in the plantar skin of the front feet in rats without sympathetic denervation (negative staining; Figure 4). Positive staining was seen in 12/12, 3/12, and 1/12 rats at 2, 7 weeks and 3 months respectively in the sympathectomized group. No rats in the control groups stained positive with the iodine starch test. The difference in the number of the rats with positive staining between the sympathectomized and other groups was signi fi cant at 2 and 7 weeks (P< 0.001) but not at 3 months (P> 0.05).
NE and DβH expression in the plantar and sacrococcygeal skin of the hind feet
NE and DβH were mainly localized in arteriovenous anastomoses, arrector pilorum muscles and arterioles, whereas few adrenergic fi bers were found around the sweat glands (Figures 5, 6); (Donadio et al., 2006). At 2 weeks post-surgery, NE and DβH expression in the plantar skin of the hind feet was signi fi cantly lower in the sympathectomized group compared with the normal group (bothP< 0.05). NE (P< 0.001 and DβH (P= 0.006) expression in the sympathectomized group significantly decreased compared with the sham-operated group at 2 weeks in the plantar skin of the hind feet. NE expression levels were not di ff erent among the normal, sham-operated and sympathectomized groups at 3 months in the plantar skin of the hind feet (P> 0.05). DβH levels were also not di ff erent among the groups at 3 months in the plantar skin of the hind feet (P> 0.05; Figure 7).
NE and DβH levels in the sacrococcygeal skin are shown in Figures 8–10. NE expression had decreased signi fi cantly at 2 weeks in the sympathectomized group compared with the normal and sham-operated groups (bothP< 0.05). DβH expression was also signi fi cantly lower in the sympathectomized group than in the normal and sham-operated groups in the sacrococcygeal skin at 2 weeks (bothP<0.05). At 7 weeks, NE expression levels in the sacrococcygeal skin were not di ff erent among the normal, sham-operated and sympathectomized groups (P> 0.05). Similarly, there was no significant difference in DβH expression levels in the sacrococcygeal skin among the normal, sham-operated and sympathectomized groups at 7 weeks (P> 0.05; Figure 10).
Ultrastructure of the L5sympathetic trunks
The L5sympathetic trunks in the sympathectomized and sham-operated groups were observed under transmission electron microscope at 3 months aer sympathectomy (Figure 11). Regeneration of the L2–4sympathetic trunks did not occur, and intestinal adhesions were found.e ultrastructure showed integral myelin in the axons and no perinuclear spaces in the sympathetic cells of the sham-operated group (Figure 11A). However, perinuclear space in sympathetic cells and axon demyelination were found in the sympathectomized group (Figure 11B).e nuclei of sympathetic neurons in the sympathectomized group were irregular, whereas those in the normal group were round. More lysosomes and rough endoplasmic reticulum were present in the sympathectomized group than in the sham-operated group.
Discussion
The sympathetic nervous system plays an important role in various skin diseases. Sympathetic denervation can be a clinical manifestation of some skin diseases, and serve as a treatment for others. Sympathetic nerve function can be restored to some extent aer sympathectomy in clinical practice; severe histological damage in the sympathetic trunk almost normalizes 12 weeks aer thoracoscopic clipping of the sympathetic trunk in patients with disabling primary hyperhidrosis or facial blushing (omsen et al., 2014). Hyperhidrosis and pain disorders may recur in patients even aer treatment by thoracic sympathectomy (Johnson et al., 1999). Plantar hyperhidrosis (Rieger et al., 2011) and neuralgia (Buche et al., 1988) can recur aer resection of the lumbar sympathetic trunk. Although recovery of sympathetic function in skin has been found in clinical patients, few animal models have been reported that observed and verified the regularity of this recovery.
Previous animal studies have shown sympathetic denervation in superficial pineal gland (Zhang et al., 1991; Hernandez et al., 1994) and the heart (Lindpaintner et al., 1987) aer surgical sympathectomy. Major portions of the hind leg (Miao et al., 1995; Catre and Salo, 1999) and femoral arteries (Peterson and Norvell, 1985) are innervated by the L2–4levels of the sympathetic chain. If the bilateral L2–4sympathetic trunks are removed, the sympathetic nerve function in the lower limbs would decline. In this experiment, the lumbar sympathetic trunks were exposed clearly and cut success-fully, as demonstrated by intraoperative monitoring of skin temperature and immuno fl uorescence analysis of the lumbar sympathetic trunks. Monitored skin temperature, sweating and NE and DβH proteins re fl ect the denervation of sympathetic nerve function in the skin 2 weeks aer lumbar sympathectomy, in accordance with the previous studies (Flotte, 1959; Mashiah et al., 1995; Shor and Chumak Iu, 2001; Dellon et al., 2012). Long-term postoperative follow-up of the skin temperature aer sympathectomy is rare (Hatangdi and Boas, 1985; Kruse, 1985; Greenstein et al., 1994). In our study, no significant change in skin temperature was observed between the lefeet and sacrococcygeal region 2 and 7 weeks after sympathectomy. Jeong et al. (2006) also reported that skin temperature of the palms gradually begins to normalize approximately 1 week after bilateral thoracic sympathectomy.
Some reports have shown that the effects of surgical sympathectomy are time-dependent in splanchnic organs, mesenteric arteries and veins (Lehtosalo et al., 1988) and the smooth muscle of myenterically and extrinsically denervated rat jejunum (Luck et al., 1993). Baroreflex regulation of renal sympathetic nerve activity may partially recover after spinal cord hemisection in rats (Zahner et al., 2011). In this paper, the recovery of sympathetic nerve function in the hind feet and sacrococcygeal skin after lumbar sympathectomy was explored. The rate of the recovery of sympathetic nerve function in the hind feet was slower than that in the sacrococcygeal skin.e recovery of sympathetic nerve function was veri fi ed by the recovery of monitored skin temperature, sweating and expressions of NE and DβH protein in skin. To determine whether the sympathetic nerve function had completely regenerated after lumbar sympathectomy, the ultrastructure of the L5sympathetic trunk was examined by transmission electron microscopy. The results showed that the L5sympathetic trunk had not returned to its normal state 3 months aer the sympathectomy.
Navarro (2009) suggested that the reinnervation of denervated segments could occurviaregeneration of injured axons or collateral branching of undamaged axons. Sympathetic preganglionic neurons consist of cells in the lateral grey column from T1to L2/3, which synapse with their postganglionic neurons, including paravertebral ganglia of the sympathetic chain, prevertebral ganglia, and chroma ffi n cells of the adrenal medulla. Kobayashi et al. (2001) reported that the sympathetic pathways from the canine hypogastric nerve to the seminal tract could be reconstructed spontaneously aer serious injury due to their cross-innervation system. In our experiment, prevertebral ganglia and chromaffin cells of the adrenal medulla remained aer resection of bilateral lumbar sympathetic trunks, and may have been involved in the compensatory recovery of sympathetic nerve function in sacrococcygeal skin and the hind feet.
In summary, sympathetic functional recovery may account for the recurrence of hyperhidrosis aer sympathectomy and normalization of sympathetic nerve trunks aer an incomplete injury.e recovery of sympathetic nerve function in the sacrococcygeal skin was quicker than that in the hind feet. The slower recovery of sympathetic nerve function in the limbs than in the torso may also be the general pattern of sympathetic functional recovery in the patients aer injury to sympathetic nerve trunks. We will investigate this future research.
Author contributions:BC designed this study. JBT, XM and HWL reviewed the manuscript. ZFZ and YSL performed experiments and collected the data. ZFZ analyzed the data and wrote the paper. All authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Buche M, Randour P, Mayne A, Joucken K, Schoevaerdts JC (1988) Neuralgia following lumbar sympathectomy. Ann Vasc Surg 2:279-281.
Catre MG, Salo PT (1999) Quantitative analysis of the sympathetic innervation of the rat knee joint. J Anat 194 (Pt2):233-239.
Coveliers HM, Hoexum F, Nederhoed JH, Wisselink W, Rauwerda JA (2011)oracic sympathectomy for digital ischemia: a summary of evidence. J Vasc Surg 54:273-277.
Dellon AL, Hoke A, Williams EH, Williams CG, Zhang Z, Rosson GD (2012)e sympathetic innervation of the human foot. Plast Reconstr Surg 129:905-909.
Donadio V, Nolano M, Provitera V, Stancanelli A, Lullo F, Liguori R, Santoro L (2006) Skin sympathetic adrenergic innervation: an immuno fl uorescence confocal study. Ann Neurol 59:376-381.
Faerman I, Faccio E, Calb I, Razumny J, Franco N, Dominguez A, Podesta HA (1982) Autonomic neuropathy in the skin: a histological study of the sympathetic nerve fi bres in diabetic anhidrosis. Diabetologia 22:96-99.
Flotte CT (1959) Evaluation of lumbar sympathectomy. Am J Cardiol 4:644-648.
Greenstein D, Brown TF, Kester RC (1994) Assessment of chemical lumbar sympathectomy in critical limb ischaemia using thermal imaging. Int J Clin Monit Comput 11:31-34.
Han KR, Kim C, Park EJ (2008) Successful treatment of digital ulcers in a scleroderma patient with continuous bilateral thoracic sympathetic block. Pain Physician 11:91-96.
Hatangdi VS, Boas RA (1985) Lumbar sympathectomy: a single needle technique. Br J Anaesth 57:285-289.
Hernandez G, Bello AR, Lopez-Coviella I, Abreu P, Fajardo N, Reiter RJ, Hernandez A, Alonso R (1994) Tyrosine hydroxylase activity in peripherally denervated rat pineal gland. Neurosci Lett 177:131-134.
Hweidi SA, Lee S, Wolf P (1985) E ff ect of sympathectomy on microvascular anastomosis in the rat. Microsurgery 6:92-96.
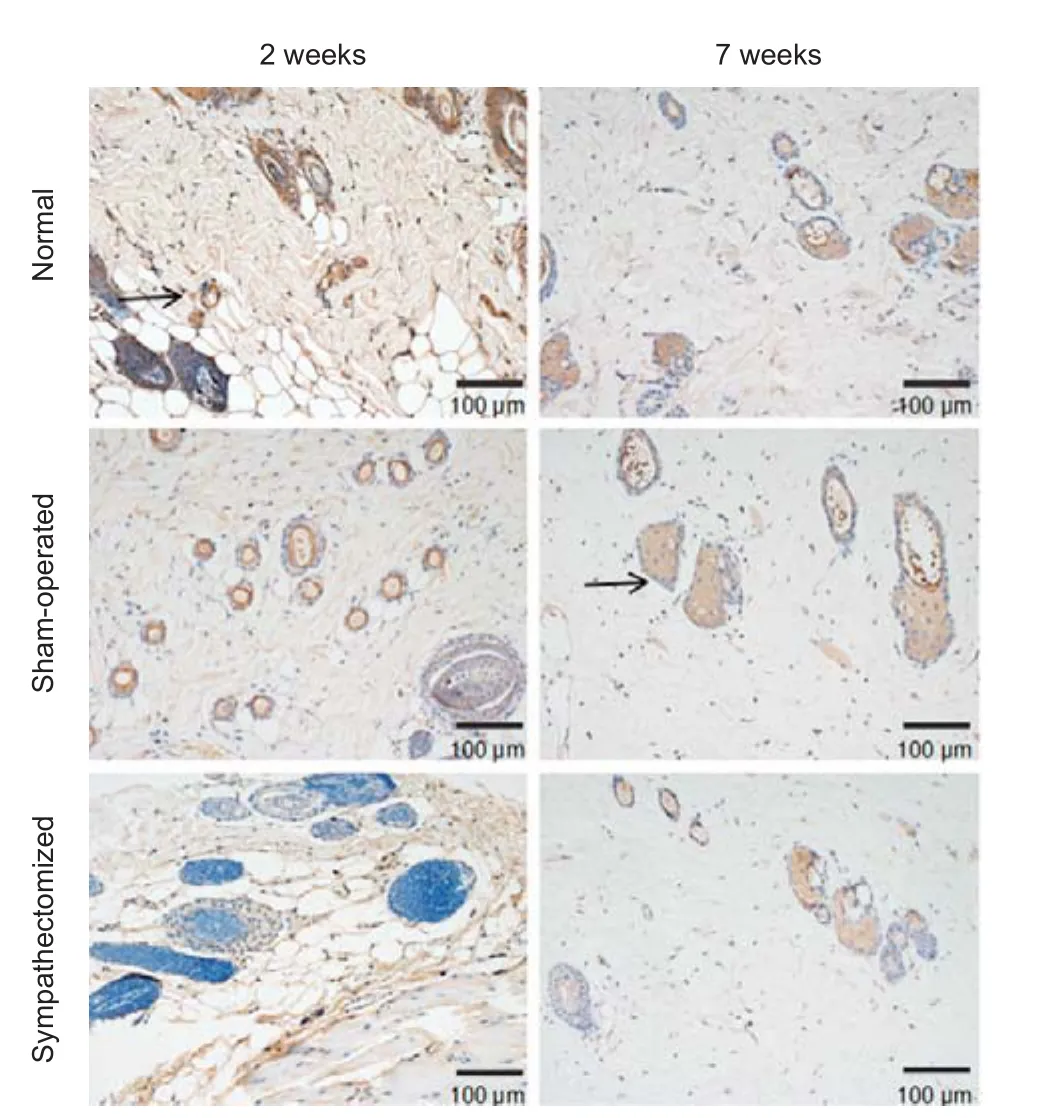
Figure 9 Immunohistochemical staining of dopamine β-hydroxylase under a light microscope in sacrococcygeal skin 2 and 7 weeks aer sympathectomy in rats.
Jeong SM, Kim TY, Jeong YB, Sim JY, Choi IC (2006)e changes of skin temperature on hands and feet during and aer T3 sympathicotomy for palmar hyperhidrosis. J Korean Med Sci 21:917-921.
Johnson JP, Obasi C, Hahn MS, Glatleider P (1999) Endoscopic thoracic sympathectomy. J Neurosurg 91:90-97.
Kobayashi T, Kihara K, Kageyama Y, Yamada T, Liu S, Sato K (2001) Spontaneous reconstruction of the canine hypogastric nerve over a long period aer removing half of its length. Auton Neurosci 86:151-162.
Lehtosalo J, Eranko L, Palkama A, Uusitalo H (1988) Nerve fibers showing immunoreactivities for thyrosine hydroxylase and dopamine-beta-hydroxylase re-appear in the guinea pig uvea aer sympathectomy. Acta Ophthalmol (Copenh) 66:419-426.
Letamendia A, Lopez-Roman J, Bustamante-Munguira J, Herreros J (2016) Digital periarterial sympathectomy in the management of post-traumatic Raynaud syndrome. J Vasc Surg 63:459-465.
Lindpaintner K, Lund DD, Schmid PG (1987) Role of myocardial hypertrophy in trophic stimulation of indices of sympathetic cardiac innervation. J Cardiovasc Pharmacol 10 Suppl 12:S211-220.
Luck MS, Dahl JL, Boyeson MG, Bass P (1993) Neuroplasticity in the smooth muscle of the myenterically and extrinsically denervated rat jejunum. Cell Tissue Res 271:363-374.
Mashiah A, Soroker D, Pasik S, Mashiah T (1995) Phenol lumbar sympathetic block in diabetic lower limb ischemia. J Cardiovasc Risk 2:467-469.
Miao FJ, Kinnman E, Janig W, Levine JD (1995) Variation in the anatomy of the lumbar sympathetic chain in the rat. J Auton Nerv Syst 56:115-118.
Mione MC, Sancesario G, D’Angelo V, Bernardi G (1991) Increase of dopamine beta-hydroxylase immunoreactivity in non-noradrenergic nerves of rat cerebral arteries following long-term sympathectomy. Neurosci Lett 123:167-171.
Navarro X (2009) Chapter 27: Neural plasticity aer nerve injury and regeneration. Int Rev Neurobiol 87:483-505.
Navarro X (2016) Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: a critical overview. Eur J Neurosci 43:271-286.
Navarro X, Verdu E, Wendelschafer-Crabb G, Kennedy WR (1997) Immunohistochemical study of skin reinnervation by regenerative axons. J Comp Neurol 380:164-174.
Ng I, Yeo TT (2003) Palmar hyperhidrosis: intraoperative monitoring with laser Doppler blood fl ow as a guide for success aer endoscopic thoracic sympathectomy. Neurosurgery 52:127-130; discussion 130-121.


Figure 10 Expression of NE and DβH in sacrococcygeal skin 2 and 7 weeks aer sympathectomy in rats.
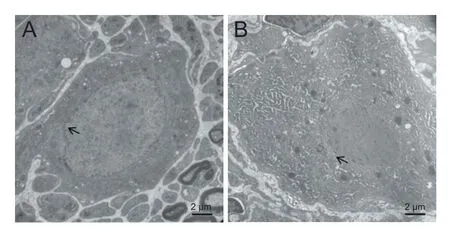
Figure 11 E ff ects of lumbar sympathectomy on the ultrastructure of L5sympathetic trunks of rats (transmission electron microscopy, uranyl acetate staining).
Patman RD (1982) Sympathectomy in the treatment of chronic venous leg ulcers. Arch Surg 117:1561-1565.
Peterson DF, Norvell JE (1985) Sympathetic postganglionic pathways to the hind-limb of the rat: a fl uorescent histochemical study. J Auton Nerv Syst 13:171-174.
Rieger R, Loureiro Mde P, Pedevilla S, de Oliveira RA (2011) Endoscopic lumbar sympathectomy following thoracic sympathectomy in patients with palmoplantar hyperhidrosis. World J Surg 35:49-53.
Rivers SP, Veith FJ, Ascer E, Gupta SK (1986) Successful conservative therapy of severe limb-threatening ischemia: the value of nonsympathectomy. Surgery 99:759-762.
Robertshaw D (1974) Proceedings: Neural and humoral control of apocrine glands. J Invest Dermatol 63:160-167.
Ryder RE, Marshall R, Johnson K, Ryder AP, Owens DR, Hayes TM (1988) Acetylcholine sweatspot test for autonomic denervation. Lancet 1:1303-1305.
Shor NA, Chumak Iu F (2001) Application of lumbar sympathectomy for the lower extremities diabetic angiopathy in presence of purulent necrotic changes on the foot. Klin Khir 59-61.
Shor NA, Chumak Iu F, Reuka VP, Zhukov OA (2004) Revascularization of the lower limbs for ischemic diabetic foot with pyonecrotic tissue lesions. Angiol Sosud Khir 10:85-89.
Sokolov VE, Shabadash SA, Zelikina TI (1980) Innervation of eccrine sweat glands. Biol Bull Acad Sci USSR 7:331-346.
Stump fl en A, Ahmadi A, Attender M, Gschwandtner M, Hofmann S, Maca T, Schnurer G, Minar E (2000) E ff ects of transvenous regional guanethidine block in the treatment of critical fi nger ischemia. Angiology 51:115-122.
Tomlinson L (2000) Case study to illustrate a multidisciplinary approach to a case of critical limb ischaemia and the role of chemical lumbar sympathectomy. J Tissue Viability 10:140-143.
van Dielen FM, Kurvers HA, Dammers R, oude Egbrink MG, Slaaf DW, Tordoir JH, Kitslaar PJ (1998) Effects of surgical sympathectomy on skin blood fl ow in a rat model of chronic limb ischemia. World J Surg 22:807-811.
Yamada M, Terayama R, Bando Y, Kasai S, Yoshida S (2006) Regeneration of the abdominal postganglionic sympathetic system. Neurosci Res 54:261-268.
Zahner MR, Kulikowicz E, Schramm LP (2011) Recovery of barore fl ex control of renal sympathetic nerve activity aer spinal lesions in the rat. Am J Physiol Regul Integr Comp Physiol 301:R1584-1590.
Zhang ET, Mikkelsen JD, Moller M (1991) Tyrosine hydroxylase- and neuropeptide Y-immunoreactive nerve fi bers in the pineal complex of untreated rats and rats following removal of the superior cervical ganglia. Cell Tissue Res 265:63-71.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211200
Accepted: 2017-05-20
*Correspondence to: Biao Cheng, M.D., chengbiaocheng@163.com.
杂志排行
中国神经再生研究(英文版)的其它文章
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves
- Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/ reperfusion injury
- Long-term acupuncture treatment has a multitargeting regulation on multiple brain regions in rats with Alzheimer’s disease: a positron emission tomography study
- E ff ect of glycosides of Cistanche on the expression of mitochondrial precursor protein and keratin type II cytoskeletal 6A in a rat model of vascular dementia
- How does conserved dopamine neurotrophic factor protect against and rescue neurodegeneration of PC12 cells?
