E ff ect of glycosides of Cistanche on the expression of mitochondrial precursor protein and keratin type II cytoskeletal 6A in a rat model of vascular dementia
2017-08-07YanmeiZhangWeiWuWeiMaFangWangJunYuan
Yan-mei Zhang, Wei Wu, Wei Ma, Fang Wang, Jun Yuan,
1 Department of Neurology, Inner Mongolia Autonomous Region People’s Hospital, Hohhot, Inner Mongolia Autonomous Region, China
2 Department of Brain Center,e Second A ffi liated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, China
3 First Hospital of Wuhan, Wuhan, Hubei Province, China
4 Jianghan University, Wuhan, Hubei Province, China
E ff ect of glycosides of Cistanche on the expression of mitochondrial precursor protein and keratin type II cytoskeletal 6A in a rat model of vascular dementia
Yan-mei Zhang1, Wei Wu2, Wei Ma3, Fang Wang4, Jun Yuan1,*
1 Department of Neurology, Inner Mongolia Autonomous Region People’s Hospital, Hohhot, Inner Mongolia Autonomous Region, China
2 Department of Brain Center,e Second A ffi liated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, China
3 First Hospital of Wuhan, Wuhan, Hubei Province, China
4 Jianghan University, Wuhan, Hubei Province, China
How to cite this article:Zhang YM, Wu W, Ma W, Wang F, Yuan J (2017) E ff ect of glycosides of Cistanche on the expression of mitochondrial precursor protein and keratin type II cytoskeletal 6A in a rat model of vascular dementia. Neural Regen Res 12(7):1152-1158.
Graphical Abstract

orcid: 0000-0002-0469-1203 (Yan-mei Zhang)
Glycosides ofCistanche(GC) is a preparation used extensively for its neuroprotective e ff ect against neurological diseases, but its mechanisms of action remains incompletely understood. Here, we established a bilateral common carotid artery occlusion model of vascular dementia in rats and injected the model rats with a suspension of GC (10 mg/kg/day, intraperitoneally) for 14 consecutive days. Immunohistochemistry showed that GC signi fi cantly reduced p-tau and amyloid beta (Aβ) immunoreactivity in the hippocampus of the model rats. Proteomic analysis demonstrated upregulation of mitochondrial precursor protein and downregulation of keratin type II cytoskeletal 6A aer GC treatment compared with model rats that had
nerve regeneration; vascular dementia; glycosides of Cistanche; mitochondrial precursor protein; keratin type II cytoskeletal 6A; proteomics; neuroprotection; neural regeneration
Introduction
Vascular dementia is the second most common type of dementia (Neltner et al., 2014; Sachdev et al., 2014; Tatlisumak et al., 2014; Sinclair et al., 2015;omas et al., 2015; Reijmer et al., 2016).e unremitting and irreversible memory damage that occurs in patients with vascular dementia leads to a severe deterioration in quality of life and places a heavy economic burden on the patient’s family (Barker et al., 2014; Burke et al., 2014; Chen et al., 2014; Brandenburg et al., 2016). Prevention and treatment of the disease is increasingly important in countries with aging populations.erefore, there is increasing research interest in the search for e ff ective drugs for the treatment of vascular dementia.
Cistancheis a plant used as a nootropic in traditional Chinese medical theory (Chen et al., 2014; Szalárdy et al., 2015; You et al., 2015). Glycosides ofCistanche(GC) is a preparation extracted fromCistanche.ere is considerable evidence supporting the idea that GC can improve axonal regeneration (Procaccio et al., 2014; Love et al., 2015; Brandenburg et al., 2016; Gu et al., 2016). Numerous animalstudies have shown that GC enhances cognitive function (Procaccio et al., 2014; Talarowska et al., 2014; Fischer and Maier, 2015).
We previously used bilateral common carotid artery occlusion to establish rat models of vascular dementia. In this model, spatial learning and memory are significantly improved after intervention with GC (Chen et al., 2015). GC can also regulate and improve immunity, but is best known as a nerve tonic and memory enhancer (Chen et al., 2014; Procaccio et al., 2014; Szalárdy et al., 2015; You et al., 2015; Brandenburg et al., 2016).
However, few studies have investigated the molecular mechanisms underlying the effects of GC in vascular dementia. Here, we use proteomics to explore the relationship between proteins and vascular dementia, to determine the mechanism underlying the neuroprotective e ff ect of GC.
Materials and Methods
Animals
Six-week-old adult male specific-pathogen-free Wistar rats (n= 37), weighing 230–270 g, were provided by the Experimental Animal Center, Hubei Province, China (license number: SYXK (E) 2014-0030). The experiment followed the National Guidelines for the Care and Use of Laboratory Animals, and the Consensus Author Guidelines on Animal Ethics and Welfare produced by the International Association of Veterinary Editors.e manuscript was prepared in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Rats were housed in groups of three per cage at 25°C with a 12-hour light/dark cycle and free access to food and water. Rats (n= 37) were randomly assigned to a control group (n= 10), a vascular dementia group (n= 12), and a vascular dementia + GC group (GC-treated,n= 15).
Establishment of rat models of vascular dementia
Under deep anesthesia with 10% chloral hydrate, bilateral common carotid arteries of rats in the vascular dementia and GC-treated groups were carefully separated from the surrounding tissues, then tightly ligated using 10-0 suture thread on both ends, and the common carotid arteries were cut.e rats in the control group were subjected to the same surgical procedure except that the common carotid arteries were exposed but not ligated. The Morris water maze was performed according to previously described methods to verify whether the animal model had been successfully established (Chen et al., 2015).
Pharmacological treatment
Cistanchepowder (Certificate No. Z20050216, Sinphar Tian-Li Pharmaceutical Co., Ltd., Hangzhou, China) was mixed with ultrapure water and extracted by TF-2000C Ultrasonicator (Tuofen, Shanghai, China) (power 100%, 30°C, 40 minutes). GC concentration was measured by assaying phenylethanoid glycosides. After injury, all rats in the GC-treated group received GC (10 mg/kg/day, 1 mL, intraperitoneally, once daily) for 14 consecutive days. Rats in the control and vascular dementia groups received saline (1 mL, intraperitoneally).
Immunohistochemistry
After 14 days of GC (or saline) administration, seven rats from each group were decapitated and the brains extracted. The cerebral cortices were removed and hippocampal tissue was carefully dissected out, and cut into 3-μm thick sections using freezing microtome. Sections of hippocampal tissue were fixed with 4% paraformaldehyde for 30–60 minutes, washed with PBS, and incubated in 3% H2O2for 10 minutes followed by goat serum for 15 minutes, all at room temperature. Sections were then incubated in rabbit anti-rat phosphorylated p-tau primary antibody (1:1,000 in PBS; Bioworld Technology, Inc., TX, USA) and rabbit anti-rat amyloid beta (Aβ) primary antibody (1:1,000; Bioworld Technology, Inc.) in a wet box at 37°C for 2–3 hours, before incubation in the secondary antibody (goat anti-mouse IgG; Bioworld Technology, Inc.) for 15 minutes at room temperature. Specimens were visualized with 3,3′-diaminobenzidine tetrahydrochloride (Dako, Tokyo, Japan). Images were captured using a light microscope (Olympus, Tokyo, Japan) and processed using Photoshop (version 7.0; Adobe, San Jose, CA, USA). Immunopositive particles were brown, so brown staining in the cytoplasm was selected as positive reaction, and semi-quantification was carried out using an HMIAS-2000 image analysis system (Qianpin Co., Wuhan, China). In each specimen, five fields of view were selected at random and fi ve positive cells were selected in each view and the average gray value was measured as a set of relative values.
Two-dimensional gel electrophoresis (2-DE)
After 14 days of GC or saline administration, eight rats from each group were anesthetized and decapitated, and the hippocampal tissues were quickly harvested and immediately frozen in liquid nitrogen. All tissues were homogenized in liquid nitrogen using a mortar and pestle, and collected in lysis bu ff er (7 M urea, 2 M thiourea, 2% CHAPS, 20 mM Tris). Insoluble particles were removed by centrifugation at 13,000 ×gfor 20 minutes at 4°C. Contaminated nucleic acid was disrupted by intermittent sonic oscillation for 5 minutes. Samples were centrifuged again under the same conditions and the supernatants were collected. Protein concentration was measured using the Bradford assay (Beyotime Inc., China) and the samples were store at −80°C until use.
To investigate the protein expression pro fi le in the hippocampus, 2-DE was performed according to the manufacture’s instructions (GE Healthcare, Pittsburgh, PA, USA). The protein solution (120 μg/sample) was adjusted with rehydration bu ff er for a fi nal volume of 350 μL. Isoelectric focusing was performed using IPG strips (pH 4–7, size 22 cm) on an Ettan IPG phor II system (all from GE Ettan IPGphor3, GE Healthcare). Aerwards, the strips were equilibrated for 15 minutes.
2-DE was performed using 12.5% sodium dodecyl sul-phate (SDS) polyacrylamide gels (24 cm × 19.5 cm × 1.0 mm) with 0.5% agarose sealing glue in an Ettan DALTSix electrophoresis system (GE, Ettan DALTSix, GE Healthcare). Electrophoresis was carried out at 2 W for 45 minutes, followed by separation at 17 W for 4 hours until the bromophenol blue had nearly reached the bottom of 2-DE gel.
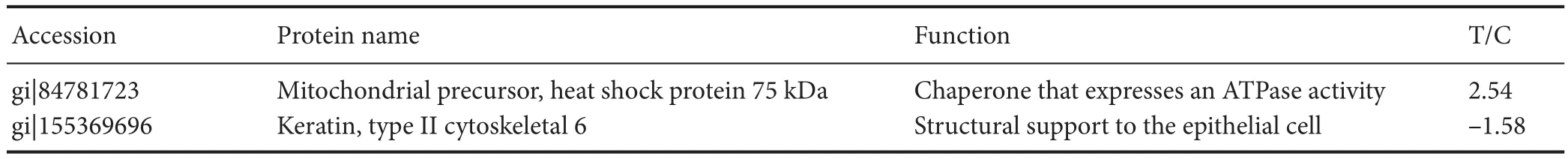
Table 1 Protein information of mitochondrial precursor protein and type II cytoskeletal 6A identi fi ed by time-of- fl ight mass spectrometry
Protein spots were excised from the gel using an automated SpotPicker (GE, Inc.) aer matching and identifying differences in protein points. Subsequently, peptide information was obtained using a 4700 MALDI TOF/TOF mass spectrometer (GE, Inc.).
Matrix-assisted laser desorption/ionization time-of- fl ight mass spectrometry
For peptide mass fingerprinting and subsequent analysis, gels were sliced and subjected to a slightly modified in-gel protocol as described in the manufacturer’s instructions.ere were methanol (Fisher M/4056/17), acetonitrile (Fisher A/0626/17), trypsin (Promega V5280), trypsin resolve solution (Promega V530), ammonium bicarbonate (Sigma A6141), C-18 ZipTip (Millipore ZTC18M096), and tri fl uoroacetic acid (GE HealthCare).
Brie fl y, protein spots were destained with destaining solution (30 mM K3Fe(CN)6:100 mM NaS2O3= 1:1) and dehydrated with 100 mm ammonium bicarbonate and acetonitrile, reduced with trichloroethyl phosphate for 20 minutes at room temperature, and then alkylated in iodoacetic acid for 30 minutes in the dark.e gel was incubated in 50 μL of 12 ng/μL modi fi ed trypsin solution in 25 mM ammonium bicarbonate, pH 8.6, at 37°C overnight. Peptides were extracted from the gel plug with 1% formic acid/2% acetonitrile and concentrated using C-18 Zip-Tips. Aerwards, samples were prepared using a Prespotted AnchorChip (PAC96) target with an alpha-cyano-4-hydroxycinnamic acid matrix for 96 sample spots and 24 calibration spots. Mass spectrum peptide information was obtained using a 4700 MALDI TOF/TOF mass spectrometer. Resulting data were analyzed using a GPS Explorer (Applied Biosystems Inc., NY, USA), which invoked a MASCOT database search (Matrix Science, London, UK) using a mouse subset of the National Center for Biotechnology Information database.
Western blot assay
The hippocampus was prepared and protein concentration was measured as described above. Protein samples were loaded onto an SDS polyacrylamide gel, electrophoresed and transferred to a polyvinylidene di fl uoride membrane. The membrane was washed in Tris-buffered saline and Tween 20, blocked in 10% non-fat milk and 0.05% Tween in phosphate-buffered saline. The membranes were incubated overnight at 4°C with rabbit anti-mitochondrial precursor protein polyclonal antibody (1:2,000; Proteintech Inc., Chicago, IL, USA) and rabbit anti-keratin type II cytoskeletal 6A (KRT6A) polyclonal antibody (1:1,000; Proteintech Inc.) in 10% non-fat milk.e secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit (Google Biotechnology Inc., Wuhan, Hubei Province, China) was diluted (1:2,000) in 10% non-fat milk and incubated at 25°C for 1 hour. Mouse anti-rat β-actin monoclonal antibody (1:2,000; Google Biotechnology Inc.) was used as an internal reference. A western blot scanning system (V300; EPSON, Nagano-ken, Japan), and AlphaEaseFC Adobe PhotoShop (Alpha Innotech, CA, USA) were used to determine mitochondrial precursor protein and KRT6A expression levels expressed as integrated optical density normalized to β-actin.
Statistical analysis
Quantitative data were expressed as the mean ± SD. Image-Master 2D Platinum 5.0 soware (GE, Inc.) was used to scan and analyze the 2-DE information. Two-way analysis of variance was performed using GraphPad Prism 6.0 (GraphPad Soware, Inc., La Jolla, CA, USA).P< 0.05 was considered statistically signi fi cant.
Results
E ff ects of GC on p-tau and Aβ immunoreactivity in the hippocampus of rat models of vascular dementia
Immunohistochemistry showed that p-tau and Aβ expression was greater aer 14 days of treatment with GC than in the control group. However, expression of these proteins in the GC-treated group was signi fi cantly lower than that in the vascular dementia group (P< 0.05; Figure 1).
GC regulated expression of mitochondrial precursor protein and KRT6A in the hippocampus of rat models of vascular dementia
Expression of two proteins was significantly different in the GC-treated group than in the vascular dementia group: mitochondrial precursor protein (also known as heat shock protein (HSP) 75 kDa) was upregulated, and KRT6A was downregulated (Figures 2, 3). Protein information is listed in Table 1.
Validation of proteomic data by western blot assay
Western blot results confirmed the data obtained by proteomics: mitochondrial precursor protein expression was greater, and KRT6A expression was lower, in the GC-treated group than in the vascular dementia group (Figure 4).
Discussion
Vascular dementia is a syndrome characterized by impairments in memory, behavior and cognition, and is mainly caused by cerebrovascular diseases (Barker et al., 2014; Burke et al., 2014; Oliveira et al., 2014; Szalárdy et al., 2015).e pathology common to all types of dementia, and responsible for its progression, is neurodegeneration (Foster et al., 2014; Fischer and Maier, 2015; Kalaria et al., 2015; Chen et al., 2016; Pérez-Hernández et al., 2016). Many plant extracts have therapeutic properties. The bioactivity of these compounds against neurodegeneration is mainly due to their antioxidant and anti-amyloidogenic e ff ects (Fischer and Maier, 2015; You et al., 2015; Pérez-Hernández et al., 2016).
Cistancheis a Chinese herb that is used as a nootropic in traditional Chinese medicine. Emerging research indicates thatCistancheis beneficial in amnesia, specifically in the inhibition of memory loss progression (Procaccio et al., 2014; You et al., 2015; Pérez-Hernández et al., 2016).ere is considerable evidence that GC can promote axonal regeneration and act as a nerve growth modulator (Talarowska et al., 2014; Gu et al., 2016; Xu et al., 2016). In our previous work using the Morris water maze, we found that the escape latency of rat models of vascular dementia decreased after treatment with GC, indicating that GC improves spatial learning ability. Although GC has been used extensively for its neuroprotective e ff ect (Chen et al., 2015), the mechanism underlying this e ff ect remains unclear.
It is well known that Aβ peptides and p-tau are highly expressed in senile plaques and neurofibrillary tangles. Aβ and tau proteins play key roles in the morphogenesis of neurons, but in certain pathological situations, they generate aberrant aggregates that are neurotoxic (Love et al., 2015; Sadigh-Eteghad et al., 2015; Sinclair et al., 2015;omas et al., 2015; Wang et al., 2015; Reijmer et al., 2016).e present immunohistochemistry results indicate that p-tau and Aβ protein expression in GC-treated rat models of vascular dementia was signi fi cantly greater than that in control rats, but lower than that in untreated model rats.erefore, the neuroprotective e ff ect of GC may occur by reducing the toxicity of Aβ and p-tau.
Our proteomic study in rat hippocampus revealed large differences in the expression of mitochondrial precursor protein, keratin, and KRT6A. In terms of function, we speculate that the neuroprotective e ff ect of GC may be associated with the remodeling of dendritic spine structure.
Mitochondrial precursor protein belongs to the HSP70 family (Rao et al., 2014; Gutiérrez-Aguilar and Baines, 2015; McGeer and McGeer, 2015). HSP75 is involved in molecular chaperone activation and maturation, and maintains the stability and normal function of cells (Booth et al., 2014, 2016; Tavallai et al., 2015). Mitochondrial precursor protein is thought to play an important role in the transduction of some signaling pathways (Reid et al., 2014; Booth et al., 2015a, b). Recent studies have revealed that the HSP90 family regulates the operating environment of mitochondrial protein folding (An et al., 2014; Liu and Landgraf, 2015; Roberts et al., 2015; Stary and Gi ff ard, 2015). HSP75 is localized in mitochondria and inhibits the generation of oxygen free radicals (Radu et al., 2014; Holloway et al., 2016). Our results show that the expression of HSP75 in the GC-treated group was increased 2.54-fold, suggesting that GC plays an antioxidant role, improves cellular oxidative balance, and delays apoptosis.is fi nding provides interesting clues about the interactions of the mitochondrial chaperone protein with other proteins.
Another differently expressed protein was KRT6A. The keratins, also called cytokeratins, are intermediate fi lament proteins that create an insoluble dense meshwork through the cytoplasm, giving structural support to the epithelial cell (Haricharan et al., 2014; Rorke et al., 2015; Szymanski et al., 2015). However, cytokeratins also play an active role in various cellular survival processes (proliferation and apoptosis) (Zhu et al., 2013; Schwingshackl et al., 2015; Popov and Komianos, 2016). These proteins may undergo phosphorylation and are also part of the bridging contact between the epithelial cell and its microenvironment (Lessard et al., 2013; Gil Lorenzo et al., 2014; Zhou et al., 2014; Chakrabarti et al., 2015). CK6a is the dominant isoform in the mammary gland (Bramanti et al., 2015a, b, 2016) and is upregulated in healing wound edges of the skin, indicating that cytokeratin is involved in cellular proliferation and migration. However, we are the first to show downregulation of KRT6A in the hippocampus; KRT6A expression was decreased 1.58-fold in the GC-treated group compared with the vascular dementia group. Therefore, the neuroprotective effect of GC may be associated with inhibition of neuroglial hyperplasia during cerebrovascular ischemia.
Together, the results of our study suggest that GC exerts its learning and memory-promoting effects in rat models of vascular dementia by reducing the expression of Aβ and p-tau, thus weakening the accumulation of these two toxic proteins and, in turn, reducing neuronal toxicity. Furthermore, GC might regulate the expression of mitochondrial precursor protein and KRT6A, which are associated with synaptic cytoskeleton morphogenesis and mitochondrial energy metabolism.erefore, our data provide new insight into the underlying mechanisms of GCs, highlighting the possibility of GCs having multiple targets, which offers a promising novel therapeutic option for the treatment of vascular dementia.
Author contributions:YMZ and JY conceived and designed the experiments. YMZ, WM, WW, and FW performed the experiments. YMZ and WW analyzed the data. WM provided reagents/materials/analysis tools. YMZ and FW wrote the paper. All authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
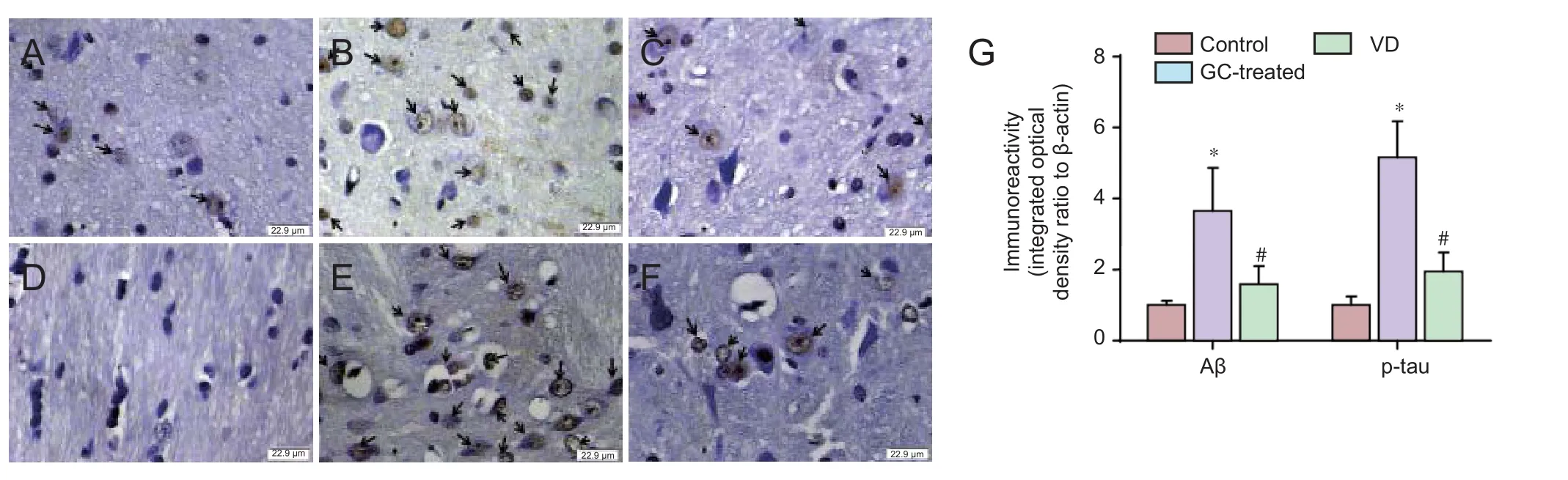
Figure 1 E ff ect of GCs on p-tau and Aβ immunoreactivity in the hippocampus of a rat model of vascular dementia.
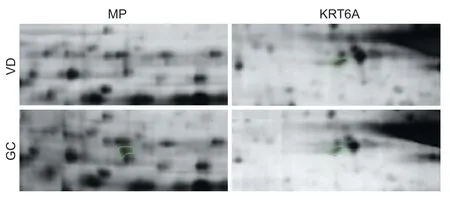
Figure 2 Two-dimensional gel electrophoresis pro fi les of mitochondrial precursor protein and KRT6A expression in rat models of vascular dementia with or without GC treatment
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
An J, Haile WB, Wu F, Torre E, Yepes M (2014) Tissue-type plasminogen activator mediates neuroglial coupling in the central nervous system. Neuroscience 257:41-48.
Barker R, Ashby EL, Wellington D, Barrow VM, Palmer JC, Kehoe PG, Esiri MM, Love S (2014) Pathophysiology of white matter perfusion in Alzheimer’s disease and vascular dementia. Brain 137:1524-1532.
Booth L, Roberts JL, Cruickshanks N, Grant S, Poklepovic A, Dent P (2014) Regulation of OSU-03012 toxicity by ER stress proteins and ER stress-inducing drugs. Mol Cancerer 13:2384-2398.
Booth L, Roberts JL, Tavallai M, Nourbakhsh A, Chuckalovcak J, Carter J, Poklepovic A, Dent P (2015a) OSU-03012 and Viagra treatment inhibits the activity of multiple chaperone proteins and disrupts the blood-brain barrier: implications for anti-cancer therapies. J Cell Physiol 230:1982-1998.
Booth L, Roberts JL, Cash DR, Tavallai S, Jean S, Fidanza A, Cruz-Luna T, Siembiba P, Cycon KA, Cornelissen CN, Dent P (2015b) GRP78/ BiP/HSPA5/Dna K is a universal therapeutic target for human disease. J Cell Physiol 230:1661-1676.
Booth L, Shuch B, Albers T, Roberts JL, Tavallai M, Proniuk S, Zukiwski A, Wang D, Chen CS, Bottaro D, Ecroyd H, Lebedyeva IO, Dent P (2016) Multi-kinase inhibitors can associate with heat shock proteins through their NH2-termini by which they suppress chaperone function. Oncotarget 7:12975-12996.
Bramanti V, Grasso S, Tibullo D, Giallongo C, Raciti G, Viola M, Avola R (2015a) Modulation of extracellular signal-related kinase, cyclin D1, glial fi brillary acidic protein, and vimentin expression in estradiol-pretreated astrocyte cultures treated with competence and progression growth factors. J Neurosci Res 93:1378-1387.
Bramanti V, Grasso S, Tomassoni D, Traini E, Raciti G, Viola M, Li Volti G, Campisi A, Amenta F, Avola R (2015b) E ff ect of growth factors and steroid hormones on heme oxygenase and cyclin D1 expression in primary astroglial cell cultures. J Neurosci Res 93:521-529.
Bramanti V, Grasso S, Tibullo D, Giallongo C, Pappa R, Brundo MV, Tomassoni D, Viola M, Amenta F, Avola R (2016) Neuroactive molecules and growth factors modulate cytoskeletal protein expression during astroglial cell proliferation and differentiation in culture. J Neurosci Res 94:90-98.
Brandenburg S, Muller A, Turkowski K, Radev YT, Rot S, Schmidt C, Bungert AD, Acker G, Schorr A, Hippe A, Miller K, Heppner FL, Homey B, Vajkoczy P (2016) Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol 131:365-378.
Burke MJ, Nelson L, Slade JY, Oakley AE, Khundakar AA, Kalaria RN (2014) Morphometry of the hippocampal microvasculature in poststroke and age-related dementias. Neuropathol Appl Neurobiol 40:284-295.
Chakrabarti KR, Whipple RA, Boggs AE, Hessler LK, Bhandary L, Vitolo MI,ompson K, Martin SS (2015) Pharmacologic regulation of AMPK in breast cancer affects cytoskeletal properties involved with microtentacle formation and re-attachment. Oncotarget 6:36292-36307.
Figure 3 Peptide mass fi ngerprinting.

Figure 4 Western blot assay of mitochondrial precursor protein and KRT6A in the hippocampus of rat models of vascular dementia.
Chen A, Akinyemi RO, Hase Y, Firbank MJ, Ndung’u MN, Foster V, Craggs LJ, Washida K, Okamoto Y,omas AJ, Polvikoski TM, Allan LM, Oakley AE, O’Brien JT, Horsburgh K, Ihara M, Kalaria RN (2016) Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post-stroke dementia. Brain 139:242-258.
Chen J, Zhou SN, Zhang YM, Feng YL, Wang S (2015) Glycosides of cistanche improve learning and memory in the rat model of vascular dementia. Eur Rev Med Pharmacol Sci 19:1234-1240.
Chen S, Yin ZJ, Jiang C, Ma ZQ, Fu Q, Qu R, Ma SP (2014) Asiaticoside attenuates memory impairment induced by transient cerebral ischemia-reperfusion in mice through anti-inflammatory mechanism. Pharmacol Biochem Behav 122:7-15.
Fischer R, Maier O (2015) Interrelation of oxidative stress and in fl ammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev 2015:610813.
Foster V, Oakley AE, Slade JY, Hall R, Polvikoski TM, Burke M,omas AJ, Khundakar A, Allan LM, Kalaria RN (2014) Pyramidal neurons of the prefrontal cortex in post-stroke, vascular and other ageing-related dementias. Brain 137:2509-2521.
Gil Lorenzo AF, Bocanegra V, Benardon ME, Cacciamani V, Valles PG (2014) Hsp70 regulation on Nox4/p22phox and cytoskeletal integrity as an e ff ect of losartan in vascular smooth muscle cells. Cell Stress Chaperones 19:115-134.
Gu C, Yang X, Huang L (2016) Cistanches herba: a neuropharmacology review. Front Pharmacol 7:289.
Gutiérrez-Aguilar M, Baines CP (2015) Structural mechanisms of cyclophilin D-dependent control of the mitochondrial permeability transition pore. Biochim Biophys Acta 1850:2041-2047.
Haricharan S, Hein SM, Dong J, Tone ff MJ, Aina OH, Rao PH, Cardi ff RD, Li Y (2014) Contribution of an alveolar cell of origin to the highgrade malignant phenotype of pregnancy-associated breast cancer. Oncogene 33:5729-5739.
Holloway KR, Sinha VC, Bu W, Tone ff M, Dong J, Peng Y, Li Y (2016) Targeting oncogenes into a de fi ned subset of mammary cells demonstrates that the initiating oncogenic mutation defines the resulting tumor phenotype. Int J Biol Sci 12:381-388.
Kalaria RN, Ferrer I, Love S (2015) Vascular disease, hypoxia and related conditions. In: Green fi eld’s Neuropathology (Love S, Perry A, Ironside J, Budka H, eds). London: CRC.
Lessard JC, Pina-Paz S, Rotty JD, Hickerson RP, Kaspar RL, Balmain A, Coulombe PA (2013) Keratin 16 regulates innate immunity in response to epidermal barrier breach. Proc Natl Acad Sci U S A 110:19537-19542.
Liu W, Landgraf R (2015) Phosphorylated and unphosphorylated serine 13 of CDC37 stabilize distinct interactions between its client and HSP90 binding domains. Biochemistry 54:1493-1504.
Love S, Chalmers K, Ince P, Esiri M, Attems J, Kalaria R, Jellinger K, Yamada M, McCarron M, Minett T, Matthews F, Greenberg S, Mann D, Kehoe PG (2015) Erratum: Development, appraisal, validation and implementation of a consensus protocol for the assessment of cerebral amyloid angiopathy in post-mortem brain tissue. Am J Neurodegener Dis 4:49.
McGeer PL, McGeer EG (2015) Targeting microglia for the treatment of Alzheimer’s disease. Expert Opiner Targets 19:497-506.
Neltner JH, Abner EL, Baker S, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Hammack E, Kukull WA, Brenowitz WD, Van Eldik LJ, Nelson PT (2014) Arteriolosclerosis that a ff ects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain 137:255-267.
Oliveira NR, Marques SO, Luciano TF, Pauli JR, Moura LP, Caperuto E, Pieri BL, Engelmann J, Scaini G, Streck EL, Lira FS, Pinho RA, Ropelle ER, Silva AS, De Souza CT (2014) Treadmill training increases SIRT-1 and PGC-1 alpha protein levels and AMPK phosphorylation in quadriceps of middle-aged rats in an intensity-dependent manner. Mediators In fl amm 2014:987017.
Pérez-Hernández J, Zaldívar-Machorro VJ, Villanueva-Porras D, Vega-Ávila E, Chavarría A (2016) A potential alternative against neurodegenerative diseases: phytodrugs. Oxid Med Cell Longev 2016:8378613.
Popov K, Komianos J (2016) MEDYAN: mechanochemical simulations of contraction and polarity alignment in actomyosin networks. PLoS Comput Biol 12:e1004877.
Procaccio V, Bris C, Chao de la Barca JM, Oca F, Chevrollier A, Amati-Bonneau P, Bonneau D, Reynier P (2014) Perspectives of drugbased neuroprotection targeting mitochondria. Rev Neurol (Paris) 170:390-400.
Radu M, Semenova G, Kosoff R, Chernoff J (2014) PAK signalling during the development and progression of cancer. Nat Rev Cancer 14:13-25.
Rao VK, Carlson EA, Yan SS (2014) Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochim Biophys Acta 1842:1267-1272.
Reid SP, Shurtle ff AC, Costantino JA, Tritsch SR, Retterer C, Spurgers KB, Bavari S (2014) HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 109:171-174.
Reijmer YD, van Veluw SJ, Greenberg SM (2016) Ischemic brain injury in cerebral amyloid angiopathy. J Cereb Blood Flow Metab 36:40-54.
Roberts JL, Tavallai M, Nourbakhsh A, Fidanza A, Cruz-Luna T, Smith E, Siembida P, Plamondon P, Cycon KA, Doern CD, Booth L, Dent P (2015) GRP78/Dna K is a target for nexavar/stivarga/votrient in the treatment of human malignancies, viral infections and bacterial diseases. J Cell Physiol 230:2552-2578.
Rorke EA, Adhikary G, Young CA, Rice RH, Elias PM, Crumrine D, Meyer J, Blumenberg M, Eckert RL (2015) Structural and biochemical changes underlying a keratoderma-like phenotype in mice lacking suprabasal AP1 transcription factor function. Cell Death Dis 6:e1647.
Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE, Blacker D, Blazer DG, Chen C, Chui H, Ganguli M, Jellinger K, Jeste DV, Pasquier F, Paulsen J, Prins N, Rockwood K, Roman G, Scheltens P, Internationlal Society for Vascular Behavioral and Cognitive Disorders (2014) Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 28:206-218.
Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J (2015) Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Princ Pract 24:1-10.
Schwingshackl A, Roan E, Teng B, Waters CM (2015) TREK-1 regulates cytokine secretion from cultured human alveolar epithelial cells independently of cytoskeletal rearrangements. PLoS One 10:e0126781.
Sinclair LI, Tayler HM, Love S (2015) Synaptic protein levels altered in vascular dementia. Neuropathol Appl Neurobiol 41:533-543.
Stary CM, Gi ff ard RG (2015) Advances in astrocyte-targeted approaches for stroke therapy: an emerging role for mitochondria and microRNAS. Neurochem Res 40:301-307.
Szalárdy L, Zádori D, Klivényi P, Toldi J, Vécsei L (2015) Electron transport disturbances and neurodegeneration: from Albert Szent-Gyorgyi’s Concept (Szeged) till novel approaches to boost mitochondrial bioenergetics. Oxid Med Cell Longev 2015:498401.
Szymanski WG, Zauber H, Erban A, Gorka M, Wu XN, Schulze WX (2015) Cytoskeletal components define protein location to membrane microdomains. Mol Cell Proteomics 14:2493-2509.
Talarowska M, Bobinska K, Zajaczkowska M, Su KP, Maes M, Galecki P (2014) Impact of oxidative/nitrosative stress and in fl ammation on cognitive functions in patients with recurrent depressive disorders. Med Sci Monit 20:110-115.
Tatlisumak T, Putaala J, Debette S (2014) Less common causes of stroke: diagnosis and management. In: Oxford Textbook of Stroke and Cerebrovasular Disease (Norrving B, ed), pp 153-162. Oxford: Oxford University Press.
Tavallai M, Hamed HA, Roberts JL, Cruickshanks N, Chuckalovcak J, Poklepovic A, Booth L, Dent P (2015) Nexavar/Stivarga and viagra interact to kill tumor cells. J Cell Physiol 230:2281-2298.
Thomas T, Miners S, Love S (2015) Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer’s disease and vascular dementia. Brain 138:1059-1069.
Wang Y, Lin J, Chen QZ, Zhu N, Jiang DQ, Li MX, Wang Y (2015) Overexpression of mitochondrial Hsp75 protects neural stem cells against microglia-derived soluble factor-induced neurotoxicity by regulating mitochondrial permeability transition pore opening in vitro. Int J Mol Med 36:1487-1496.
Xu Q, Fan W, Ye SF, Cong YB, Qin W, Chen SY, Cai J (2016) Cistanche tubulosa Protects Dopaminergic Neurons through Regulation of Apoptosis and Glial Cell-Derived Neurotrophic Factor: in vivo and in vitro. Front Aging Neurosci 8:295.
You SP, Zhao J, Ma L, Tudimat M, Zhang SL, Liu T (2015) Preventive e ff ects of phenylethanol glycosides from Cistanche tubulosa on bovine serum albumin-induced hepatic fi brosis in rats. Daru 23:52.
Zhou Q, Anderson C, Zhang H, Li X, Inglis F, Jayagopal A, Wang S (2014) Repression of choroidal neovascularization through actin cytoskeleton pathways by microRNA-24. Moler 22:378-389.
Zhu M, Lu C, Li W (2013) Transient exposure to echinacoside is su ffi -cient to activate Trk signaling and protect neuronal cells from rotenone. J Neurochem 124:571-580.
Copyedited by Slone-Murphy J, Frenchman B, Yu J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211196
saline. Western blot assay con fi rmed these fi ndings. Our results suggest that the neuroprotective e ff ect of GC in vascular dementia occursviathe promotion of neuronal cytoskeleton regeneration.
Accepted: 2017-06-19
*Correspondence to: Jun Yuan, zhangyanmei2277@sina.com.
杂志排行
中国神经再生研究(英文版)的其它文章
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Recovery of sympathetic nerve function after lumbar sympathectomy is slower in the hind limbs than in the torso
- Short-term observations of the regenerative potential of injured proximal sensory nerves crossed with distal motor nerves
- Aldehyde dehydrogenase 2 overexpression inhibits neuronal apoptosis after spinal cord ischemia/ reperfusion injury
- Long-term acupuncture treatment has a multitargeting regulation on multiple brain regions in rats with Alzheimer’s disease: a positron emission tomography study
- How does conserved dopamine neurotrophic factor protect against and rescue neurodegeneration of PC12 cells?
