磺化聚苯乙烯/聚苯胺/纳米银复合微球的制备及其催化性能研究
2017-07-07廖光福张力曾维国张淑来张博晓喻春函徐祖顺
廖光福,张力,曾维国,张淑来,张博晓,喻春函,徐祖顺
(有机功能分子合成与应用教育部重点实验室(湖北大学),有机化工新材料湖北省协同创新中心(湖北大学),湖北 武汉430062)
磺化聚苯乙烯/聚苯胺/纳米银复合微球的制备及其催化性能研究
廖光福,张力,曾维国,张淑来,张博晓,喻春函,徐祖顺
(有机功能分子合成与应用教育部重点实验室(湖北大学),有机化工新材料湖北省协同创新中心(湖北大学),湖北 武汉430062)

聚苯乙烯;磺化聚苯乙烯;聚苯胺;纳米银;复合微球;催化性能
0 引言
近年来,纳米结构材料因其化学、物理、生物特性以及在许多领域中的潜在应用而引起人们的广泛关注[1-2]. 特别是金属纳米粒子以及对应的金属氧化物,如Ag[3]、Au[4]、Cu[5]、TiO2[6]、ZnO[7]等,它们在催化剂领域已经得到了广泛的应用. 其中,Ag作为一种催化效率非常高的催化材料[8-10],在污水处理、光催化降解和光催化制氢等方面都有非常重要的应用[11]. 然而,单一的纳米银作为催化材料具有易被氧化、易团聚和高成本等一系列缺点. 为了解决这些问题,科研人员已经尝试了很多方法,其中最重要的方法就是掺Ag至各种基材表面[12-15].选用聚合物微球作为基材,形成聚合物-纳米银核壳结构复合微球可以很好的解决这些问题.
聚合物-金属核壳结构复合微球是由2种或2种以上的物质(包括聚合物和金属纳米粒子)分别形成内核和外壳,通过物理或者化学作用相互连接形成的复合材料[16]. 其中,壳层粒子不仅可以调整表面性质,改变表面电荷密度、生物相容性、稳定性及其分散性,而且还可以通过特殊梯度结构将外壳粒子特有的电学性能、光学性能和催化活性等特性赋予整个微球[17]; 内核微粒可以作为模板,使外壳金属纳米粒子吸附或者接枝在其表面,从而提高复合微球的稳定性和单分散性[18]. 因此,聚合物-金属复合微球集有机、无机纳米粒子等诸多性质于一体,能够实现电学、光学、磁学以及催化等综合性能的调控,在生物医药、组装材料和催化领域具有广阔的应用前景[19-20].
本文中选用SPS/PANI复合微球作为基材,在此基材表面沉积纳米银,制备了一种新型的SPS/PANI/Ag复合微球. 选用SPS/PANI复合微球作为基材不仅可以很好的解决纳米银面临的这些问题,而且SPS/PANI复合微球与纳米银具有很大的接触面积,可以有效的提高纳米银的催化效率. 我们首先用分散聚合制备了PS微球;然后用浓硫酸对PS微球进行磺化形成了SPS微球;随后以SPS微球为模板,并在其表面发生苯胺的氧化聚合形成SPS/PANI复合;最后,Ag纳米粒子被沉积到SPS/PANI微球表面形成SPS/PANI/Ag复合微球. 我们通过SPS/PANI/Ag复合微球催化NaBH4还原MB的模型来研究其催化性能. 结果表明,SPS/PANI/Ag复合微球在NaBH4还原MB的模型中表现出较高的催化活性和较高的重复利用率.
1 实验部分
1.1 实验试剂 苯乙烯(St),苯胺(An) ,分析纯,上海阿拉丁生化科技股份有限公司,减压蒸馏后使用;偶氮二异丁腈(AIBN)、聚乙烯吡咯烷酮(PVP)、无水乙醇、硝酸银(AgNO3)、浓硫酸(H2SO4)、浓盐酸(HCl)、浓氨水(NH3·H2O)、过硫酸铵(APS),分析纯,国药集团化学试剂有限公司.
1.2 实验方法
1.2.1 单分散PS微球的制备 采用分散聚合制备单分散PS微球. 15 g St、3 g PVP、0.2 g AIBN、95 g 无水乙醇和5 g 去离子水加入到250 mL 四口瓶中,通氮气保护,在70 ℃恒温水浴中反应24 h. 停止加热,离心并抽滤,用去离子水和无水乙醇洗涤多次,真空干燥得到PS微球.
1.2.2 SPS微球的制备 将3.4 g PS粉末和120 mL 浓硫酸分散于250 mL 三口瓶中,超声30 min. 随后,在40 ℃恒温水浴中反应12 h. 停止加热,离心并抽滤,用去离子水和无水乙醇洗涤多次,真空干燥得到SPS微球.
1.2.3 SPS/PANI复合微球的制备 将0.3 g SPS 粉末、0.05 g An、40 mL 去离子水和2 mL HCl(2 mol/L)混合均匀,超声30 min. 随后,将混合物至于冰浴中搅拌5 h,然后缓慢滴加0.12 g APS(溶解于2 mL去离子水中),滴加完毕后在冰浴下反应24 h. 停止加热,离心并抽滤,用去离子水和无水乙醇洗涤多次,真空干燥得到SPS/PANI复合微球.
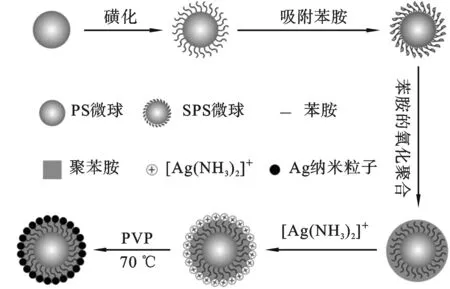
图1 SPS/PANI/Ag复合微球的制备过
1.2.4 SPS/PANI/Ag复合微球的制备 将0.13 g SPS/PANI粉末、1 g PVP 和50 mL 去离子水混合均匀,超声30 min. 随后,10 mL 现配的银氨溶液(0.2 mol/L)迅速加入以上混合物中,在室温下搅拌1h. 最后,在70 ℃和通氮气条件下反应7 h. 停止加热,离心并抽滤,用去离子水和无水乙醇洗涤多次,真空干燥得到SPS/PANI/Ag复合微球. 制备过程如图1所示.

2 结果与讨论

2.2 XRD分析 图3为SPS/PANI/Ag复合微球的XRD谱图. 对照PDF卡片可以看出,在2θ值约为38.0°、44.1°、64.3°、77.2°和 81.5°出现尖锐峰,与纯银纳米粒子衍射峰完全一致,反映了银纳米粒子面心立方结构(111)、(200)、(220)、(311)和(222)的5个晶面[21],这说明SPS/PANI/Ag复合微球中的Ag纳米粒子为结晶态. 样品的衍射峰较尖锐,说明复合微球中生成的银纳米粒子的晶型较好.
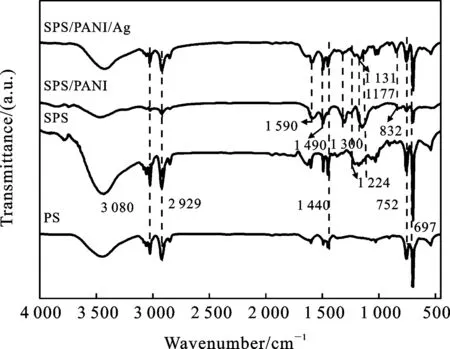
图2 PS微球、SPS微球、SPS/PANI复合微球和SPS/PANI/Ag复合微球的红外图

图3 SPS/PANI/Ag复合微球的XRD图谱

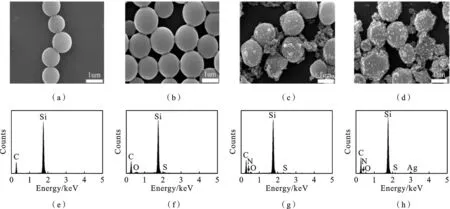
图4 PS微球(a)、SPS微球(b)、SPS/PANI复合微球(c)、SPS/PANI/Ag复合微球(d)的FESEM图和PS微球(e)、SPS微球(f)、SPS/PANI复合微球(g)、SPS/PANI/Ag复合微球(h)的EDX谱图
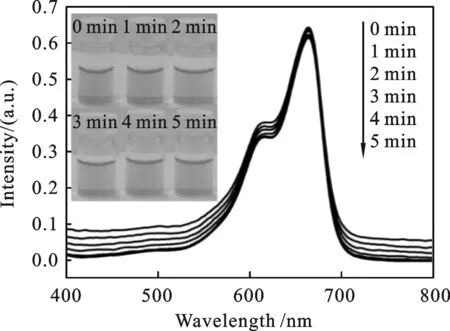
图5 3 mL MB(10 mg/mL)溶液和0.5 mLNaBH4 (10 mg/mL)的混合液在不同时间的紫外图谱(插 图显示相应的MB溶液的颜色)



图6 1 mL SPS/PANI/Ag(0.03 mg/mL)溶液加至3 mL MB(10 mg/mL)溶液和0.5 mL NaBH4(10 mg/mL)的混合液中在不同时间的紫外图谱(插图显示相应的MB溶液的颜色)
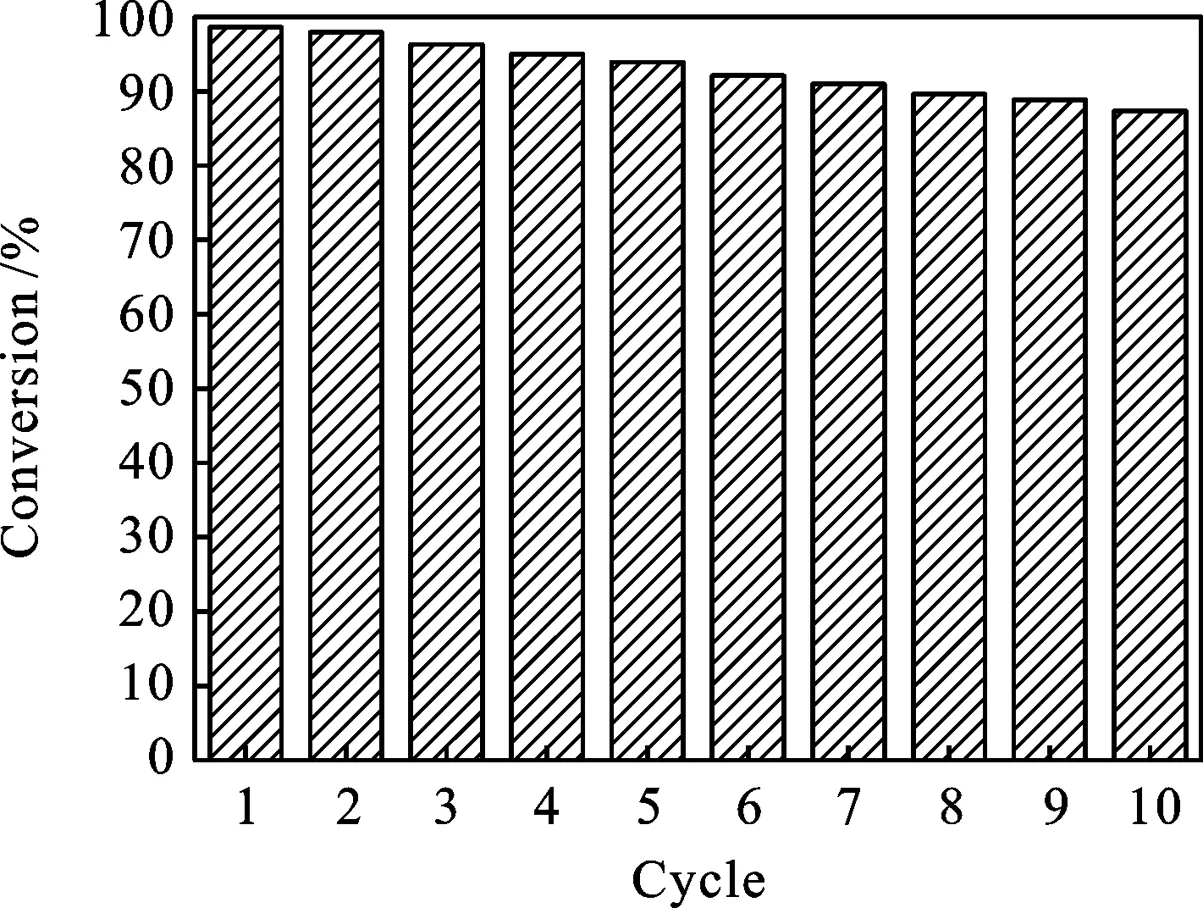
图7 SPS/PANI/Ag催化材料的回收利用图
3 结论

[1] Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles[J]. Science, 2002, 298(5601): 2176-2179.
[2] Park J, Joo J, Kwon S G, et al. Synthesis of monodisperse spherical nanocrystals[J]. Angew Chem Int Ed, 2007, 46(25): 4630-4660.
[3] Zhang X, Su Z. Polyelectrolyte-multilayer-supported Au@Ag core-shell nanoparticles with high catalytic activity[J]. Adv Mater, 2012, 24(33): 4574-4577.
[4] Zeng Z, Greeley J. Theoretical study of CO adsorption on Au catalysts under environmental catalytic conditions[J]. Catal Commun, 2014, 52: 78-83.
[5] Reske R, Mistry H, Bahafarid F, et al. Particle size effects in the catalytic electroreduction of CO2on Cu nanoparticles[J]. J Am Chem Soc, 2014, 136(19): 6978-6986.
[6] Heinlaan M, Ivask A, Blinova I, et al. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus[J]. Chemosphere, 2008, 71(7): 1308-1316.
[7] 毛蜜,周建刚,何瑜,等. G/TiO2/CoFe2O4磁性复合光催化材料的制备及光催化降解亚甲基蓝[J]. 湖北大学学报(自然科学版), 2016, 38(3): 195-200.
[8] Zhang P, Shao C, Zhang Z, et al. In situ assembly of well-dispersed Agnanoparticles(AgNPs) on electrospun carbon nanofibers(CNFs) for catalytic reduction of 4-nitrophenol[J]. Nanoscale, 2011, 3: 3357-3363.
[9] Ji T, Chen L, Schmitz M, et al. Hierarchical macrotube/mesopore carbon decorated with mono-dispersed Ag nanoparticles as a highly active catalyst[J]. Green Chem, 2015, 17: 2515-2523.
[10] Li Y, Cao Y, Xie J, et al. Facile solid-state synthesis of Ag/graphene oxide nanocomposites as highly active and stable catalyst for the reduction of 4-nitrophenol[J]. Catal Commun, 2015, 58: 21-25.
[11] Kuai L, Geng B, Chen X, et al. Facile subsequently light-induced route to highly efficient and stable sunlight-driven Ag-AgBr plasmonic photocatalyst[J]. Langmuir, 2010, 26(24): 18723-18727.
[12] Sureshkumar M, SiswantoI D Y, Lee C K. Magnetic antimicrobial nanocomposite based on bacterial cellulose and silver nanoparticles[J]. J Mater Chem, 2010, 20(33): 6948-6955.
[13] Dallas P, Tucek J, Jancik D, et al. Magnetically controllable silver nanocomposite with multifunctional phosphotriazine matrix and high antimicrobial activity[J]. Adv Funct Mater, 2010, 20(14): 2347-2354.
[14] Chen Z M, Gang T, Yan X, et al. Ordered silica microspheres unsymmetrically coated with Ag nanoparticles, and Ag-nanoparticle-doped polymer voids fabricated by microcontact printing and chemical reduction[J]. Adv Mater, 2006, 18(7): 924-929.
[15] Tang S, Vongehr S, Meng X. Controllable incorporation of Ag and Ag-Au nanoparticles in carbon spheres for tunable optical and catalytic properties[J]. J Mater Chem, 2010, 20(26): 5436-5445.
[16] Zhang K, Chen H, Chen X, et al. Monodisperse silica-polymer core-shell microspheres via surface grafting and emulsion polymerization[J]. Macromol Mater Eng, 2003, 288(4): 380-385.
[17] Shao M, Ning F, Zhao J, et al. Preparation of Fe3O4@SiO2@layered double hydroxide core-shell microspheres for magnetic separation of proteins[J]. J Am Chem Soc, 2012, 134(2): 1071-1077.
[18] Ka F, Qiu L, Yuan Y, et al. Fe3O4@MOF core-shell magnetic microspheres with a designable metal-organic framework shell[J]. J Mater Chem, 2012, 22: 9497-9500.
[19] 陈苗,敖卫,王如意,等. 氮掺杂TiO2中空复合微球的制备及可见光光催化性能[J]. 复合材料学报, 2015, 32(3): 918-923.
[20] Gong X, Peng S, Wen W, et al. Design and fabrication of magnetically functionalized core/shell microspheres for smart drug delivery[J]. Adv Funct Mater, 2008, 19(2): 292-297.
[21] Bedre M D, Basavaraja S, Salwe B D, et al. Preparation and characterization of Pani and Pani-Ag nanocomposites via interfacial polymerization[J]. Polym Composite, 2009, 30(11): 1668-1677.
[22] Yao T, Cui T, Fang X, et al. Preparation of yolk/shell Fe3O4@polypyrrole composites and their applications as catalyst supports[J].Chem Eng J, 2013, 225: 230-236.
[23] Hareesh K, Joshi R P, Dahiwale S S, et al. Synthesis of Ag-reduced graphene oxide nanocomposite by gamma radiation assisted method and its photocatalytic activity[J].Vacuum, 2016, 124: 40-45.
(责任编辑 胡小洋)
Preparation and catalytic properties of sulfonated polystyrene/polyaniline/silver composite microspheres
LIAO Guangfu, ZHANG Li, ZENG Weiguo, ZHANG Shulai, ZHANG Boxiao, YU Chunhan, XU Zushun
(Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules(Hubei University),Hubei Collaborative Innovation Center for Advanced Organic Chemical Materials(Hubei University), Wuhan 430062,China)
In this work, new sulfonated polystryrene/polyaniline/silver(SPS/PANI/Ag) composite microspheres were prepared by using multifunctional sulfonated polystryrene/polyaniline(SPS/PANI) composite microspheres as substrates and utilizing polyvinylpyrrolidone(PVP) as reducing agent and stabilizing agent. The morphology, compenent, structure and catalytic properties of the composite microspheres were characterized and investigated by Fourier transform infrared spectrum(FT IR), field emission scanning electron microscopy(FESEM), energy disperse spectroscopy(EDX), Powder X-ray diffraction(XRD) and UV-visible spectroscopic techniques(UV). The results confirmed the formation of PS microspheres, SPS microspheres, SPS/PANI composite microspheres, and SPS/PANI/Ag composite microspheres. Ag nanoparticles and PANI was uniformly distributed on the surface of SPS microspheres with its size ranging from 1.2 to 1.3 μm. And the SPS/PANI/Ag composite microspheres shown high catalytic activity and high recycling rate in the model with methylene blue(MB) dye was reduced by sodium hydride(NaBH4).
polystyrene;sulfonated polystyrene;polyaniline;silver nanoparticles;composite microspheres;catalytic property
2016-08-24
武汉市高新技术成果转让及专业化项目(2014010303010159)资助
廖光福(1992-),男,硕士生;徐祖顺,通信作者,教授,E-mail:zushunxu@hubu.edu.cn
1000-2375(2017)04-0400-05
O643.3
A
10.3969/j.issn.1000-2375.2017.04.012
