三核钌簇合物[PyCH=C(Ph)O]2Ru3(CO)8的合成、结构及反应性
2017-07-05马志宏刘倩秦玫韩占刚郑学忠林进
马志宏 刘倩 秦玫*, 韩占刚 郑学忠 林进*,
(1河北师范大学化学与材料科学学院,石家庄050024)(2河北医科大学基础医学院,石家庄050017)
三核钌簇合物[PyCH=C(Ph)O]2Ru3(CO)8的合成、结构及反应性
马志宏1,2刘倩1秦玫*,1韩占刚1郑学忠1林进*,1
(1河北师范大学化学与材料科学学院,石家庄050024)
(2河北医科大学基础医学院,石家庄050017)
配体PyCH2COPh与Ru3(CO)12在甲苯中加热回流,得到了标题簇合物[PyCH=C(Ph)O]2Ru3(CO)8(1)。通过红外光谱、核磁共振氢谱和碳谱对1的结构进行了表征,用X射线单晶衍射法测定了1的结构。结果表明:3个钌原子呈等腰三角形分布,其中Ru(2)-Ru(1)和Ru(2)-Ru(1)i的键长均为0.280 nm,Ru(1)-Ru(1)i的键长为0.307 nm。同时研究了簇合物1与环戊二烯及茚的反应,分别得到双核钌羰基配合物[(η5-C5H5)Ru(CO)]2(μ-CO)2(2)和[(η5-C9H7)Ru(CO)]2(μ-CO)2(3)。
合成;表征;金属羰基簇合物;反应性
0 Introduction
Transition metal carbonyl compounds are useful intermediates in the synthesisof important coordination compounds and have applications in homogeneous catalytic reactions such as hydrogenation, hydroformylation and carbonylation[1].The coordination chemistry of numerous nitrogen donor ligands has been extensively studied and theirmetal compounds played an important role in the development of coordination chemistry,resulting in an enormous number of publications,ranging from pure synthetic work to physicochemical[2]and biochemically relevant studies of metal complexes[3-6]and found wide range ofapplications.Furthermore,metal carbonylderivativesof nitrogen donor ligands are important routes to prepare interestingmetal carbonyl complexes[7-8].
Many metal carbonyl cluster compounds have been prepared and their structures have been determined.Triosmium cluster containing phenoxy[9]and triruthenium cluster containingmethoxy[10]have been studied in detail.These clusters maintain a structure based on an equilateral triangle ofmetals and only rarely is an isosceles triangle formed.Our interest in investigation of the reactions ofmetal carbonylswith pyridine derivatives has prompted us to investigate the reaction of Ru3(CO)12with PyCH2COPh.In order to develop a deeper understanding of the structure and reactivity of pyridine-containing alcohol compound, here we report the synthesis,structure and reaction activity ofa new pyridine-alkoxide cluster compound.
1 Experimental
1.1 General considerations
Schlenk and vacuum line techniques were employed for all manipulations.All solvents were distilled from appropriate drying agents under nitrogen atmosphere.1H and13C NMR spectra were recorded on a Bruker AvⅢ-600 instrument in CDCl3.IR spectra were recorded as KBr disks on a Thermo Fisher is 50 spectrometer.Elemental analyses were obtained on a Vario ELⅢanalyzer.The ligand precursor PyCH2COPh was synthesized according to the literatures[11-12].
1.2 Reaction of ligand precursor w ith Ru3(CO)12in xylene
A solution of ligand precursor PyCH2COPh(0.18 g,0.938 mmol)and Ru3(CO)12(0.3 g,0.469 mmol)in 30 mL of toluene was refluxed for 6 h.After removal of solvent under reduced pressure,the residue was chromatographed on an alumina column using petroleum ether/ethyl acetate as eluent.The orangered band was eluted and collected.After vacuum removalof thesolvents from theaboveeluate,the residue was recrystallized from n-hexane/CH2Cl2at room temperature to give the title compound 1 as orange-red crystals(Yield:0.148 g,34.3%).m.p.190.1℃.Anal. Calcd.forC34H20N2O10Ru3(%):C,44.40;H,2.19;N,3.05. Found(%):C,44.32;H,2.12;N,3.00.1H NMR(600 MHz,CDCl3):8.35(d,1H,J=5.0Hz,Py-H),7.81~7.82 (m,1H,Py-H),7.58(s,2H,-CH=C-),7.42~7.51(m,4H, Py-H),7.38(d,2H,J=6.0Hz,Ph-H),7.35(t,3H,J=11.5 Hz,Ph-H),7.20~7.32(m,3H,Ph-H),7.27(s,2H,Ph-H), 7.25(s,2H,Py-H),13CNMR(CDCl3,150MHz):δ49.8, 50.8,100.4,101.3,117.5,117.8,121.5,124.1,124.4, 124.6,124.9,125.8,126.9,127.3,127.5,127.7,128.4, 135.3,136.5,139.9,140.1,146.1,152.7,152.9 153.4, 153.7,153.8,160.2,164.3,166.0,192.5,193.0,203.4, 203.5.IR(cm-1):2 047(s),1967(s),1923(s).
1.3 Crystal structure determ ination
Crystal of the title compound suitable for X-ray diffraction was isolated from the slow evaporation of hexane-dichloromethane solution.Data collection were performed on a Bruker SMART 1000 CCD detector with graphite monochromated Mo Kα(λ=0.071 073 nm)radiation using theφ-ωscan technique.The structures were solved by directmethods and refined by full-matrix least-squares procedures based on F2using the SHELXL-97 program system[13].All non-H atoms were refined with anisotropic displacement parameters.Hydrogen atoms were included in calculated positions riding on the parent atoms and refined with fixed thermal parameters. Crystallographic data and experimental details of the structure determinations are given in Table 1.
CCDC:1437152.
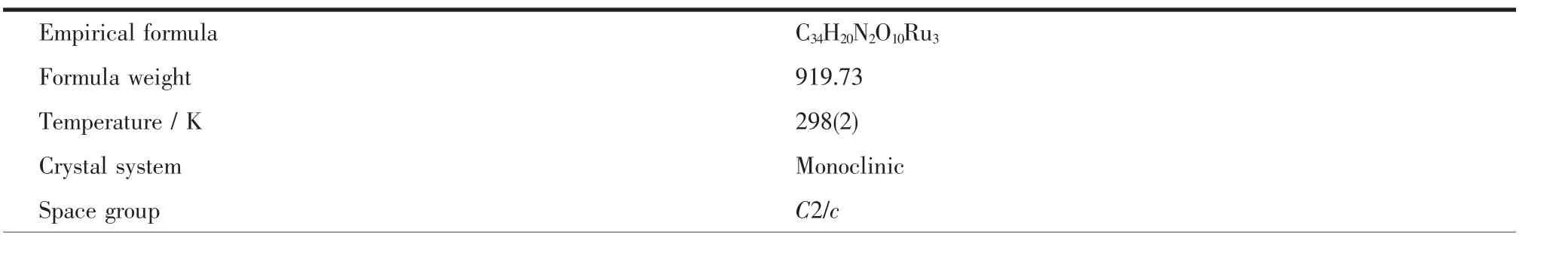
Table 1 Crystal data and structure refinement parameters for title compound
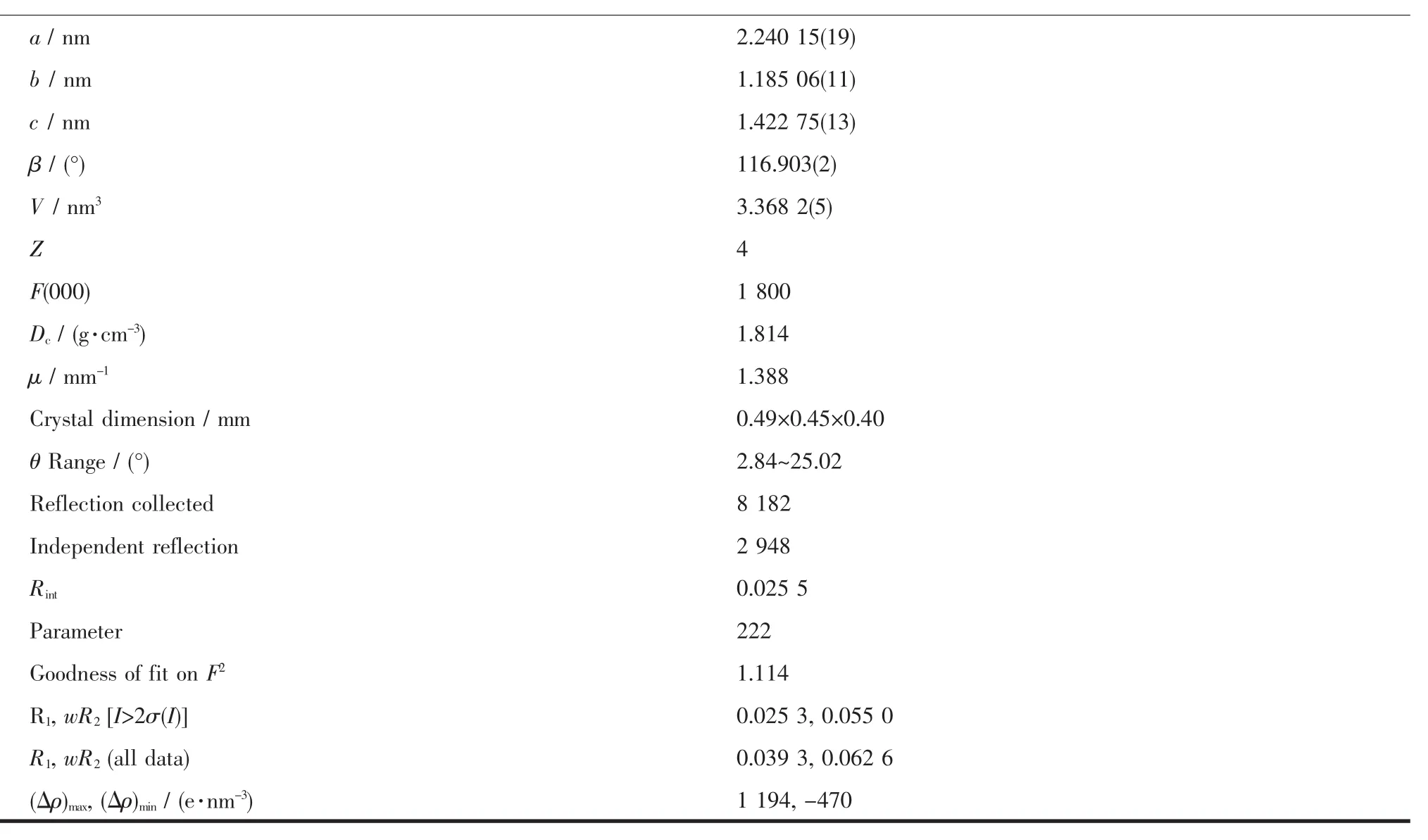
Continued Table 1
1.4 Reactivity of 1 w ith cyclopentadiene in toluene
A solution of 1(0.299 g,0.325 mmol)and cyclopentadiene(0.043 g,0.650 mmol)in 30 mL of toluene was refluxed for 24 h.After removal of solvent under reduced pressure,the residue was chromatographed on an alumina column using petroleum ether/ethyl acetate aseluent.The orange-red band waseluted and collected.After vacuum removalof the solvents from the above eluate,the residue was recrystallized from n-hexane/CH2Cl2at room temperature to give compound 2 asorange-red crystals(Yield:0.06 g,41.7%).1H NMR(600MHz,CDCl3):δ5.28(s,8H, C5H5),3.49(s,2H,C5H5);13CNMR(150MHz,CDCl3):δ 89.3,217.4.
1.5 Reactivity of 1 w ith indene in toluene
Using a procedure similar to that described above,reaction of 1 with indene gave 3 in 32.4% yield.1H NMR(600 MHz,CDCl3):7.22~7.24(m,4H, C9H7),7.13~7.15(m,4H,C9H7),5.56(d,4H,J=2.4 Hz,C9H7),5.50(t,2H,J=2.4 Hz,C9H7);13CNMR(150 MHz,CDCl3):78.6,96.7,109.9,120.0,125.8.
2 Results and discussion
2.1 Synthesisand characterization of com pound 1
Refluxing amixture of Ru3(CO)12and PyCH2COPh in toluene for 6 h resulted in formation of the title cluster compound 1.The equation of the reaction is followed(Scheme 1).
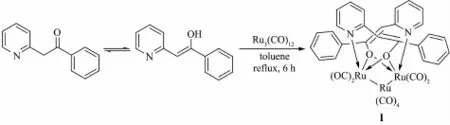
Scheme 1 Synthesis of title compound
The IR spectrum of the title compound showsonly three terminal carbonyl absorptions at 2 047, 1 967 and 1 946 cm-1,indicating the presence of several types of terminal carbonyl ligands.
2.2 X-ray crystal structure
The molecular structure of 1 was confirmed by X-ray diffraction analysis.The ORTEP is shown in Fig.1.The selected bond lengths and bond angles are given in Table 2.The title compound crystallizes in monoclinic space group C2/c with four molecules in the unit cell.As shown in Fig.1,the molecular structure is a triruthenium cluster,two ligands are simultaneously coordinated to two ruthenium atoms with aμ2-O atom behaving as a three-electron donor. The data show that the three ruthenium atoms form an isosceles triangle with two Ru-Ru distances of 0.280 nm and one of 0.307 nm.This symmetric bonding indicates that the oxygens act as three-electron donors,which in terms of the noble gas formalism means that there will be no Ru(1)-Ru(1)ibond,as shown in Fig.1.The elongated Ru(l)-Ru(1)ibond distance of 0.307 nm may well be a result of this.The structure found is very similar to that of Ru3(CO)8[C9H6NO]2[14]or Ru3(CO)8(μ-OC6H4OMe-2)2[9].The Ru-Ru distancesare slightly longer than Ru3(CO)8[C9H6NO]2(0.277 and 0.304 nm)and Ru3(CO)8(μ-OC6H4OMe-2)2(0.273,0.274 and 0.301 nm).Each pyridyl group is coordinated to one ruthenium atom,and the distance between the pyridyl notrogen and the ruthenium atom is 0.217 5(3)nm(Ru(1)-N(1)).The bond distance of the C(6)-C(7)is close to the bond distance of standard C=C(0.133 7 nm).Obviously,C(6)-C(7)is a double bond.The Ru(1)-O-Ru(1)ibond angle is 90.8°,slightly bigger than the Ru-O-Ru bond angles in Ru3(CO)8[C9H6NO]2(89.6°and 89.9°)and Ru3(CO)8(μ-OC6H4OMe -2)2(89.9°and 90.7°).
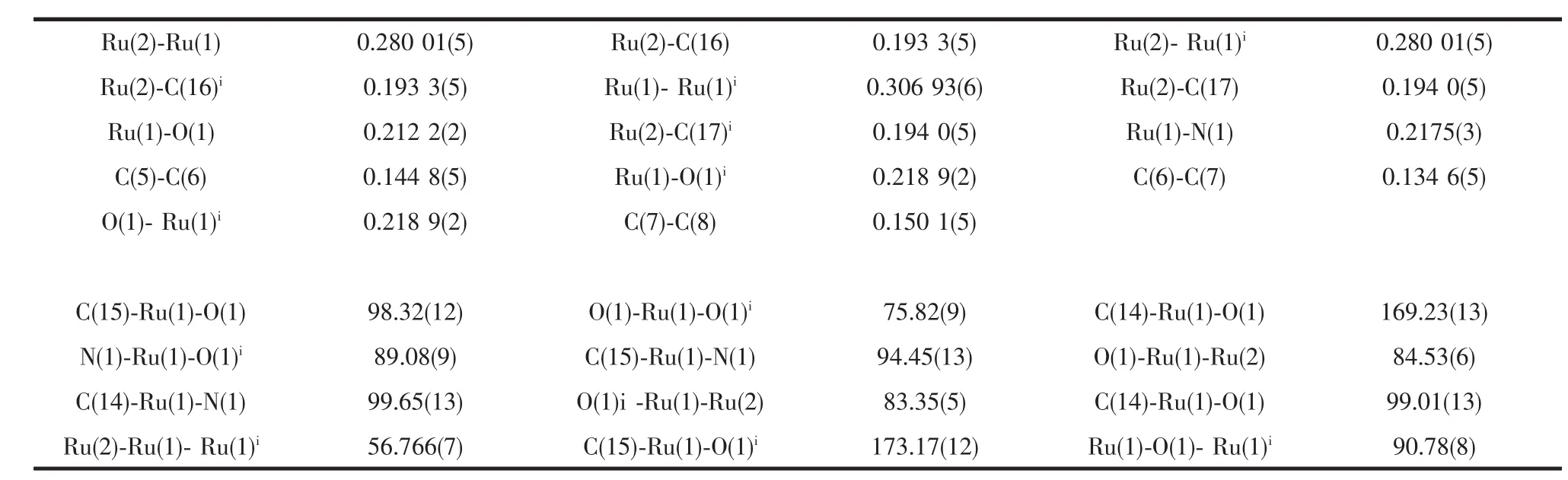
Table 2 Selected bond distances(nm)and angles(°)for title compound
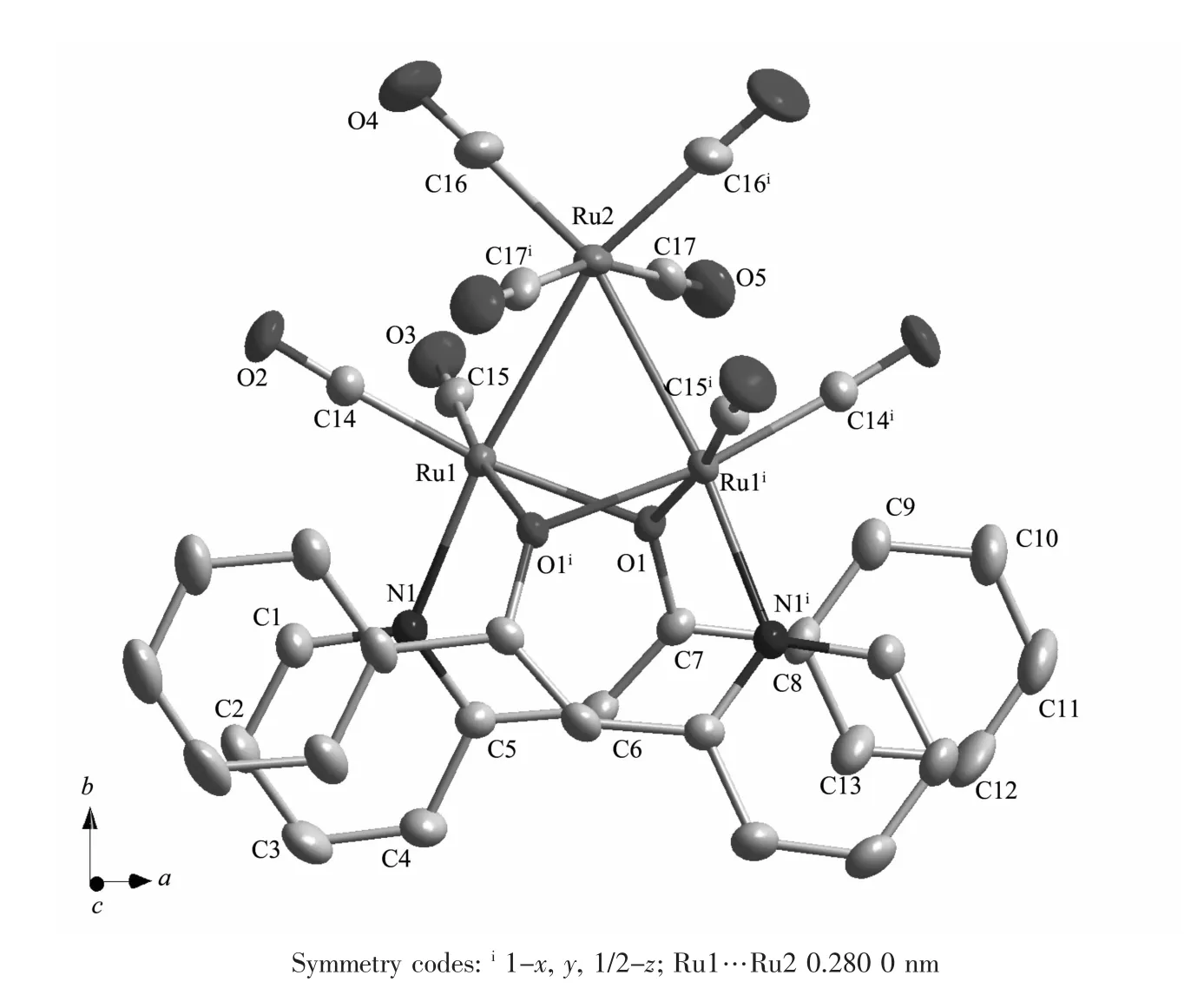
Fig.1 ORTEP view of the title compound 1 with 30%probability level ellipsoids
2.3 Reactionsof com pound 1w ith cyclopentadiene or indene in toluene
When the compound 1 reacted with cyclopentadiene or indene,dinuclear metal carbonyl complexes[(η5-C5H5)Ru(CO)]2(μ-CO)2(2)or[(η5-C9H7) Ru(CO)]2(μ-CO)2(3)were obtained(Schemes 2 and 3). Complexes2 and 3 are known products[15-16].Complexes 2 and 3 were also characterized by single-crystal X-ray diffraction analyses(Supporting Information).A plausiblemechanism for the 2 was proposed.First,the reaction of compound 1 and cyclopentadiene produce the coordinatively unsaturated(η4-cyclopentadiene) dicarbonylruthenium.Then the ruthenium hydride [(η5-C5H5)Ru(CO)2H]is formed by activation of the C-H bond of cyclopentadiene.Finally,[(η5-C5H5)Ru(CO)2H] dimerized to form the ruthenium dimmer 2 by eliminating H2.The generation mechanism of 3 is same as that of 2.The results show that the coordination properties of cyclopentadiene and indene are higher than thatof pyridine alcohol.
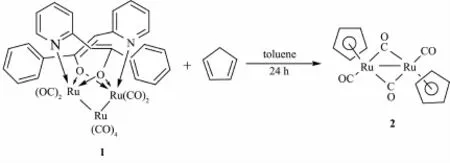
Scheme 2 Reactivity of compound 1 with cyclopentadiene
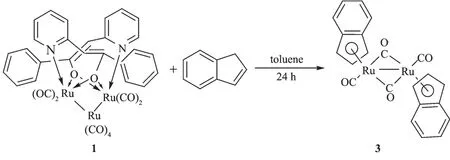
Scheme 3 Reactivity of compound 1 with indene
3 Conclusions
Reaction of PyCH2COPh with Ru3(CO)12in refluxing toluene was investigated.A new triruthenium cluster compound was obtained and determined by single-crystal X-ray diffraction.The reactivity of the title compound reacted with cyclopentadiene or indene was also studied,and the results clearly reveal that the coordination properties of cyclopentadiene and indene are higher than that of pyridine alcohol.
Supporting information isavailableathttp://www.wjhxxb.cn
[1]Kaim L W,Schwederski B.Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life.New York: Wiley,1996.
[2]Luo X F,Hu X,Zhao X Y,et al.Polymer,2003,44:5285-5291
[3]Murthy A SN,Reddy A R.J.Chem.Sci.,1981,90:519-526 [4]Ackermann M N,Kiihne S R,Saunders P A,et al.Inorg. Chim.Acta,2002,334:193-203
[5]Royer R E,Deck LM,Vander Jagt T J,etal.J.Med.Chem., 1995,38:2427-2432
[6]Baumgrass R,Weiwad M,Erdmann F,et al.J.Biol.Chem., 2001,276:47914-47921
[7]Brintzinger H,Bercaw J E.J.Am.Chem.Soc.,1971,93: 2045-2046
[8]Hay R W,Basak A K.J.Chem.Soc.Dalton Trans.,1982, 1819-1823
[9]Bruce M I,Iqbal M Z,Stone FG A.J.Organometal.Chem., 1971,31:275-281
[10]Santini C C,Basset JM,Fontal B,et al.J.Chem.Soc., Chem.Commun.,1987,7:512-513
[11]Wang Y,Zhao W,Liu D T,et al.Organometallics,2012,31: 4182-4190
[12]Koning B,Buter J,Hulst R,et al.Eur.J.Org.Chem., 2000,2735-2743
[13]Sheldrick G M.SHELXL-97,Program for Crystal Structure Renement,University of Göttingen,Germany,1997.
[14]van Doorn J A,van Leeuwen P W N M.J.Organomet. Chem.,1981,222:299-309
[15]Mills O S,Nice N P.J.Organomet.Chem.,1967,9:339-344 [16]Sridevi V S,Leong W K.J.Organomet.Chem.,2007,692: 4909-4916
Synthesis,Structure and Reactivity of a Trinuclear Ruthenium Cluster Compound [PyCH=C(Ph)O]2Ru3(CO)8
MA Zhi-Hong1,2LIU Qian1QIN Mei*,1HAN Zhan-Gang1ZHENG Xue-Zhong1LIN Jin*,1
(1College of Chemistry&Material Science,HebeiNormal University,Shijiazhuang 050024,China)
(2College of Basic Medicine,HebeiMedical University,Shijiazhuang 050017,China)
The title compound,[PyCH=C(Ph)O]2Ru3(CO)8(1),wasobtained from the reaction of PyCH2COPhwith Ru3(CO)12in refluxing toluene.Compound 1 was characterized by IR,1H NMR,and13CNMR spectroscopy aswell as by single-crystal X-ray diffraction analysis.X-ray study of the cluster compound 1 shows that the three ruthenium atoms define an isosceles triangle with two distances of 0.280 nm and one of 0.307 nm.When 1 was treated with cyclopentadiene in refluxing toluene,dinuclear metal carbonyl complexes[(η5-C5H5)Ru(CO)]2(μ-CO)2(2)was obtained.Similar treatmentof1with indenegave the product[(η5-C9H7)Ru(CO)]2(μ-CO)2(3).CCDC:1437152.
synthesis;characterization;metal carbonyl cluster compound;reactivity
O614.82+1
A
1001-4861(2017)07-1293-06
10.11862/CJIC.2017.135
2017-03-16。收修改稿日期:2017-04-25。
国家自然科学基金(No.21372061)、河北省自然科学基金(No.B2017205006)和河北师范大学重点基金(No.L2017Z02)资助项目。*
。E-mail:qinmei2005@126.com;linjin64@126.com;会员登记号:S06N0210M1305。
