Syntheses,Crystal Structures and Catalytic Activity of Rhenium Carbonyl Complexes Containing Aryl-Substituted Tetramethylcyclopentadienyl Ligands
2017-07-05MAZhiHongLIZhanWeiQINMeiLISuZhenHANZhanGangZHENGXueZhongLINJin
MA Zhi-HongLI Zhan-WeiQIN Mei*,LI Su-ZhenHAN Zhan-GangZHENG Xue-ZhongLIN Jin*,
(1College of Chemistry&Material Science,Hebei Normal University,Shijiazhuang 050024,China) (2College of Basic Medicine,Hebei Medical University,Shijiazhuang 050017,China) (3Hebei College of Industry and Technology,Shijiazhuang 050091,China)
Syntheses,Crystal Structures and Catalytic Activity of Rhenium Carbonyl Complexes Containing Aryl-Substituted Tetramethylcyclopentadienyl Ligands
MA Zhi-Hong1,2LI Zhan-Wei1QIN Mei*,1LI Su-Zhen3HAN Zhan-Gang1ZHENG Xue-Zhong1LIN Jin*,1
(1College of Chemistry&Material Science,Hebei Normal University,Shijiazhuang 050024,China) (2College of Basic Medicine,Hebei Medical University,Shijiazhuang 050017,China) (3Hebei College of Industry and Technology,Shijiazhuang 050091,China)
Cyclopentadienes C5HMe4Ar(Ar=Ph,4-CH3Ph,4-OCH3Ph,4-ClPh,4-BrPh)reacted with Re2(CO)10in refluxing xylene to give new aryl-substituted tetramethylcyclopentadienyl mononuclear metal carbonyl complexes [(η5-C5Me4Ar)Re(CO)3](Ar=Ph(1),4-CH3Ph(2),4-OCH3Ph(3),4-ClPh(4),4-BrPh(5)),respectively.The five new complexes were characterized by elemental analysis,IR,1H NMR and13C NMR spectroscopy.The crystal structures of complexes 1~5 were determined by X-ray crystal diffraction analysis.All five of these complexes have significant catalytic activity in Friedel-Crafts reactions of aromatic compounds with alkylation reagents. CCDC:1463217,1;1506704,2;1484954,3;1484955,4;1506705,5.
synthesis;mononuclear rhenium carbonyl complex;Friedel-Crafts alkylation reaction;catalysis
0 Introduction
Cyclopentadienylligandshavebeenstudied intensively as the most important ligands in organometallicchemistrybecauseoftheircapacityfor binding to hard and soft metal centers in a hemilabile manner,giving the complexes distinctive chemical and physical properties.Substituents on such ligands mayincludephosphines[1-2],amines[3-4],ethers[5-8], sulfids[9-11]and alkenes[12-16],which have been widely studied.These types of complexes have been significantly applied in catalysis and in the construction of molecular materials.Despite these notable contributions,thedevelopmentoffunctionalizedligands bearing other substituents remains a worthwhile task. Ourgrouphasreportedaseriesofsubstituted cyclopentadienyl metal carbonyl complexes,and the electronic and steric effects of the substituents on the final structures and properties of the complexes were discussed[17-19].We have also reported catalytic reactivity of mononuclear substituted tetramethylcyclopentadienyl molybdenum carbonyl complexes in Friedel-Crafts alkylation of aromatic compounds[20].However, few half-sandwich complexes of this type are known for rhenium[21-23].On the other hand,to the best of our knowledge,only a few examples of Friedel-Crafts alkylation reactions catalyzed by rhenium carbonyl complexes have been reported to date[24-25].To develop a deeper understanding of the structures and catalytic activityofsubstitutedcyclopentadienylrhenium carbonyl complexes,herein we report the syntheses, structures and catalytic activity of a series of arylsubstitutedtetramethylcyclopentadienylrhenium carbonyl complexes.
1 Experimental
1.1 General considerations
Schlenkandvacuumlinetechniqueswere employed for all manipulations.All solvents were distilled from appropriate drying agents under nitrogen atmosphere.1H and13C NMR spectra were recorded on a Bruker AvⅢ-500 instrument in CDCl3.IR spectra were recorded as KBr disks on a Thermo Fisher is 50 spectrometer.Agilent 6820 gas chromatograms were used for analysis of samples.Elemental analyses were obtained on a Vario ELⅢanalyzer.The ligand precursors C5HMe4Ar(Ar=Ph,4-CH3Ph,4-OCH3Ph,4-ClPh,4-BrPh)were synthesized according to the literature[26-27].Treatment of ligand precursors(C5HMe4Ar) with Re2(CO)10afforded the corresponding complexes [(η5-C5Me4Ar)Re(CO)3](Ar=Ph(1),4-CH3Ph(2),4-OCH3Ph(3),4-ClPh(4),4-BrPh(5))(Scheme 1).

Scheme 1 Syntheses of complexes 1~5
1.2 Synthesis of complex 1
A solution of the ligand(C5HMe4Ph)(0.12 g,0.6 mmol)and Re2(CO)10(0.2 g,0.3 mmol)in xylene(15 mL)was refluxed for 48 h.After removal of solvent the residue was loaded onto an alumina column. Elution with petroleum ether developed a colorless band,which was collected and concentrated to afford (η5-C5Me4Ph)Re(CO)3(1)as colorless crystals,yield: 0.19 g(67.4%).m.p.128.3~128.9℃;Anal.Calcd.for C18H17O3Re(%):C,46.24;H,3.66.Found(%):C,45.87; H,3.87;1H NMR(CDCl3,500 MHz):δ 2.11(s,6H, C5Me2),2.24(s,6H,C5Me2),7.29~7.38(m,5H,C6H5);13C NMR(CDCl3,125 MHz):δ 10.85,11.24,97.53, 102.11,105.03,127.75,128.43,132.11,132.64, 197.45;IR(KBr,cm-1):2 006(s),1 931(s),1 900(s).
1.3 Synthesis of complex 2
Using a procedure similar to that described above,C5HMe4(4-CH3Ph)was reacted with Re2(CO)10in refluxing xylene for 48 h.After chromatography and elution with petroleum ether,[η5-C5Me4(4-CH3Ph)] Re(CO)3(2)was obtained(0.23 g,76.7%yield)as colorless crystals.m.p.127.0~127.5℃;Anal.Calcd. for C19H19O3Re(%):C,47.39;H,3.98.Found(%):C, 47.58;H,3.81;1H NMR(CDCl3,500 MHz):δ 2.11(s, 6H,C5Me2),2.23(s,6H,C5Me2),2.38(s,3H,CH3),7.18 (s,4H,C6H4);13C NMR(CDCl3,125 MHz):δ 10.85, 11.25,21.14,97.49,102.01,105.01,129.01,129.12, 132.46,137.55,197.55.IR(KBr,cm-1):2 004(s),1 923 (s),1 899(s).
1.4 Synthesis of complex 3
Using a procedure similar to that described above,C5HMe4(4-OCH3Ph)was reacted with Re2(CO)10in refluxing xylene for 48 h.After chromatography and elution with petroleum ether,[η5-C5Me4(4-OCH3Ph)] Re(CO)3(3)was obtained(0.19 g,61.9%yield)as colorless crystals.m.p.119.8~120.6℃;Anal.Calcd. for C19H19O4Re(%):C,45.87;H,3.85.Found(%):C, 45.54;H,3.74;1H NMR(CDCl3,500 MHz):δ 2.10(s, 6H,C5Me2),2.23(s,6H,C5Me2),3.84(s,3H,CH3), 6.89(d,J=8.5 Hz,2H,C6H2),7.21(d,J=8.0 Hz,2H, C6H2);13C NMR(CDCl3,125 MHz):δ 10.86,11.25, 55.31,97.43,102.16,104.81,113.77,124.10,133.70, 159.12 197.60.IR(KBr,cm-1):2 005(s),1 918(s), 1 903(s).
1.5 Synthesis of complex 4
Using a procedure similar to that described above,C5HMe4(4-ClPh)was reacted with Re2(CO)10in refluxing xylene for 48 h.After chromatography and elution with petroleum ether,[η5-C5Me4(4-ClPh)]Re (CO)3(4)was obtained(0.23 g,73.5%yield)as colorless crystals.m.p.137.8~138.0℃;Anal.Calcd.for C18H16ClO3Re(%):C,43.07;H,3.21.Found(%):C, 43.38;H,3.02;1H NMR(CDCl3,500 MHz):δ 2.10(s, 6H,C5Me2),2.23(s,6H,C5Me2),7.24(d,J=8.0 Hz,2H, C6H2),7.34(d,J=8.5 Hz,2H,C6H2);13C NMR(CDCl3, 125 MHz):δ 10.85,11.24,97.54,102.11,105.04, 127.75,128.43,132.12,132.54,197.46.IR(KBr,cm-1): 2 005(s),1 922(s),1 897(s).
1.6 Synthesis of complex 5
Using a procedure similar to that described above,C5HMe4(4-BrPh)was reacted with Re2(CO)10in refluxing xylene for 48 h.After chromatography and elution with petroleum ether,[η5-C5Me4(4-BrPh)]Re (CO)3(5)was obtained(0.21 g,63.6%yield)as colorless crystals.m.p.115.0~116.0℃;Anal.Calcd.for C18H16BrO3Re(%):C,39.56;H,2.95.Found(%):C,39.93; H,3.12;1H NMR(CDCl3,500 MHz):δ 2.10(s,6H, C5Me2),2.23(s,6H,C5Me2),7.17(d,J=8.0Hz,2H,C6H2), 7.50(d,J=8.5 Hz,2H,C6H2).13C NMR(CDCl3,125 MHz):δ 10.83,11.21,97.79,102.01,103.50,121.96, 131.25,131.62,134.18,197.10.IR(KBr,cm-1):2 005 (s),1 920(s),1 900(s).
1.7 Crystallographic characterization
Single crystals of complexes 1~5 suitable for X-raydiffractionwereobtainedfromtheslow evaporation of hexane-dichloromethane solutions.All X-ray crystallographic data were collected on a Bruker AXS SMART 1000 CCD diffractometer with graphite monochromated Mo Kα(λ=0.071 073 nm)radiation using the φ-ω scan technique.The structures were solved by direct methods and refined by full-matrix least-squares procedures based on F2using the SHELXL -97 program system[28].Hydrogen atoms were included in calculated positions riding on the parent atoms and refined with fixed thermal parameters.The crystal data and summary ofX-raydatacollectionare presented in Table 1.
CCDC:1463217,1;1506704,2;1484954,3; 1484955,4;1506705,5.
1.8 General procedure for catalytic tests
The catalytic reactions were carried out under anargon atmosphere with magnetic stirring.The required rhenium carbonyl complex(0.04 mmol)was mixed with 1,2-dichloroethane(3.5 mL)in a 25 mL roundbottom flask at room temperature.Aromatic compounds (2 mmol)and tert-butyl halides(4 mmol)were added by syringe.The reaction mixture was stirred at 80℃for 18 h.After cooling to room temperature,the solid catalyst was separated from the reaction mixture by filtration.The filtrate was concentrated by rotary evaporation,and the residue was purified by Al2O3column chromatography,eluting with petroleum ether to give a colorless liquid.The course of the reaction was monitored using an Agilent 6820 gas chromatograph.

Table 1Crystal data and structure refinement parameters for complexes 1~5

Continued Table 1
2 Results and discussion
2.1 Crystal structures
The selected bond distances and angles for complexes 1~5 are presented in Table 2 and complex 1 is depicted in Fig.1.The four remaining complexes [(η5-C5Me4Ar)Re(CO)3](Ar=4-CH3Ph(2),4-OCH3Ph (3),4-ClPh(4),4-BrPh(5))are shown in Fig.S1~S4 (Supporting Information).
Complexes 1~5 are mononuclear substituted tetramethylcyclopentadienyl rhenium carbonyl complexes and have similar structures.Similar to the CpRe(CO)3type(Cp=substituted cyclopentadienyl ligand),all five structuresexhibittypicalthree-leggedpiano-stool structures,in which the rhenium atom is coordinated by a η5-cyclopentadienyl,plus three terminal CO ligands.The Re-CEN(CEN:centroid of the cyclopentadienyl ring)distances are 0.195 1 nm for 1,0.196 2 nm for 2,0.203 8 nm for 3,0.194 2 nm for 4,and 0.195 3 nm for 5,which are correlated with the steric effects of the different cyclopentadienyl substituents. The(O)C-Re-C(O)angle in all of these Re tricarbonyl complexes investigated is very close to 90°,whichmay simply be a consequence of the reduction in nonbondedrepulsionsbetweencarbonylgroups.The dihedral angles between the cyclopentadienyl and phenyl ring planes in these complexes are between 56.03°and 61.86°,to further decrease the intramolecular non-bonding interaction.On the other hand,the average Re-C(O)distances and the Re-C-O angles of thefivecomplexesareconcordantwithrelatedtricarbonyl cyclopentadienyl rhenium(Ⅱ)complexes[29-30].
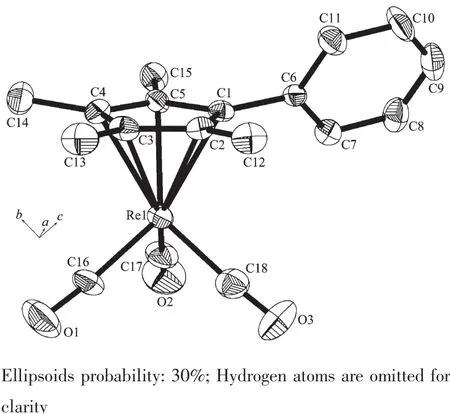
Fig.1Molecular structure of complex 1
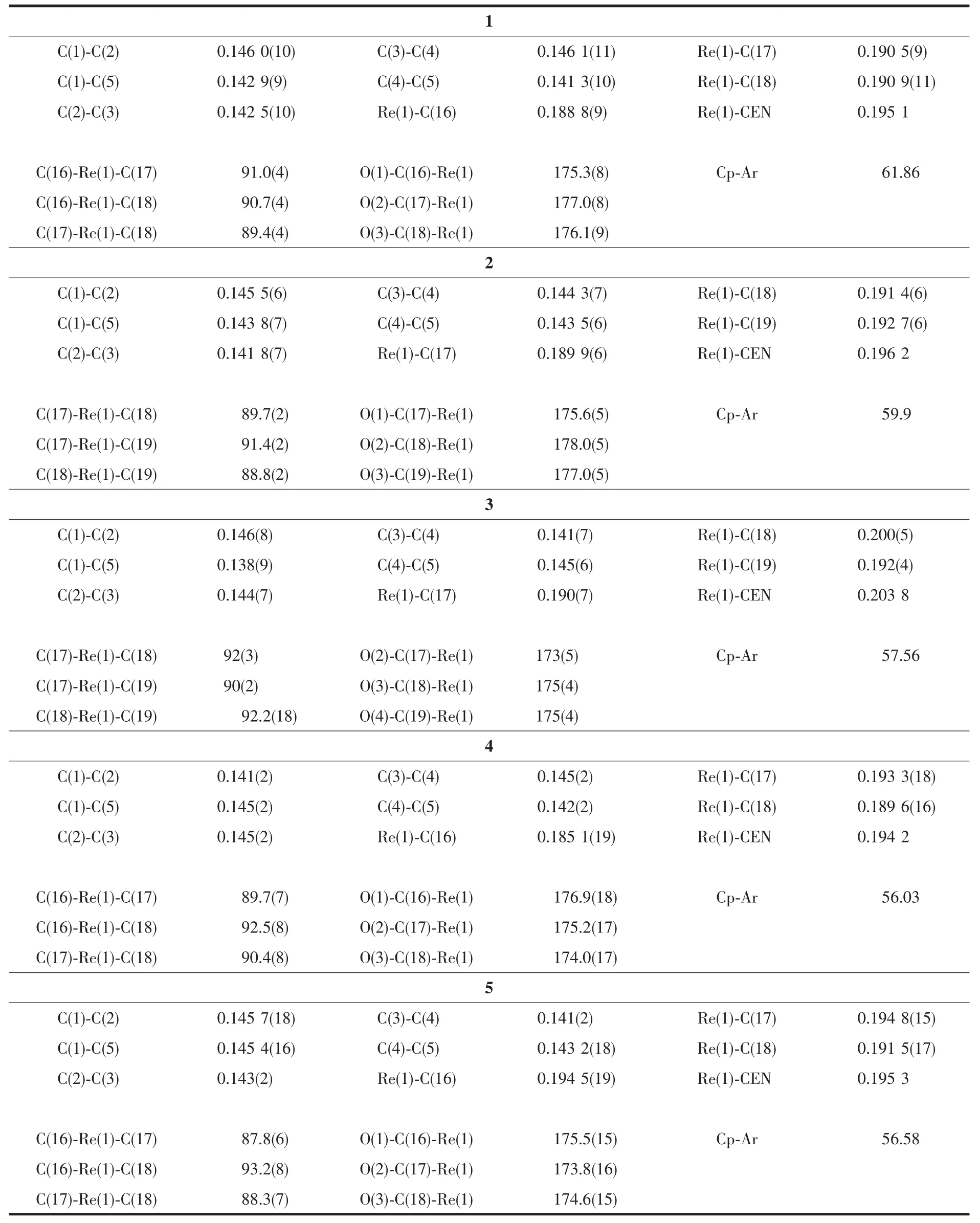
Table 2Selected bond lengths(nm)and angles(°)for complexes 1~5
2.2 Catalytic studies
Inordertotestthecatalyticcapabilityin Friedel-Crafts alkylation reactions(Scheme 2)catalyzed by these complexes,the effects of the reaction time, yield,economic considerations etc.were considered. The experimental conditions were chosen for catalytic work:1,2-dichloroethane as solvent;the molar ratio of aromatic substrates and alkylation reagents was 1∶2;the amount of catalyst was 2%(molar percentage,substrate asreference);refluxingtemperature;reactiontime:18h.
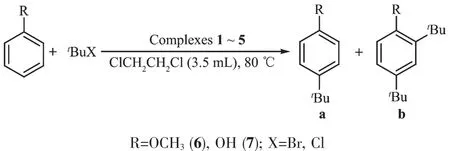
Scheme 2[(η5-C5Me4Ar)Re(CO)3]catalyzed Friedel-Crafts alkylation reaction of anisole/phenol with tertbutyl bromide/chloride
Complexes 1~5 were examined under the experimental conditions,with the results shown in Table 3. Using refluxing 1,2-dichloroethane,mixtures of the corresponding mono-and di-substituted products were obtained.All five complexes proved to be capable of catalyzing Friedel-Crafts alkylation reactions,moreover, the product yields were found to vary with the different catalysts used.In no case there was any detectable alkylation product in the absence of the rhenium complexes.The obvious influence of the different substituents on the catalytic behavior may be due to their modest variations in steric and electronic properties.The higher product yields obtained for thealkylation of anisole and phenol with t-butyl bromide than with t-butyl chloride is expected,since bromide is a better leaving group.
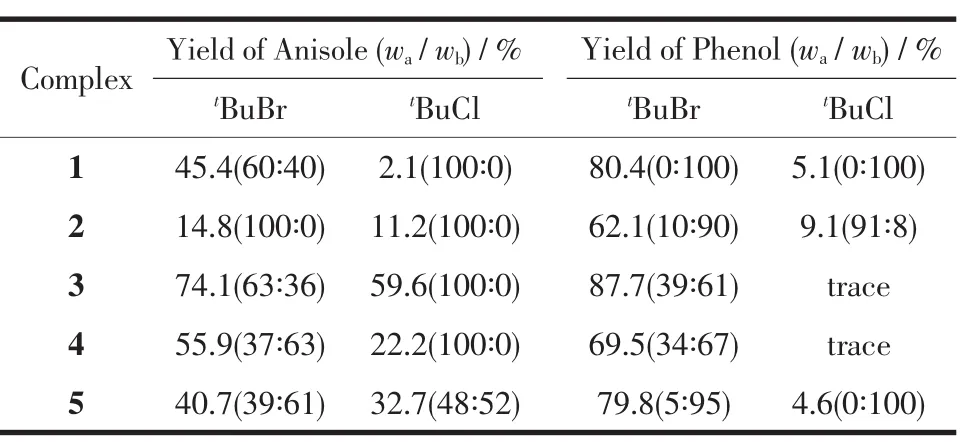
Table 3Complexes catalyzed reaction of aromatic substrates with different alkylation reagents
3 Conclusions
Reactions of aryl-substituted tetramethylcyclopentadienyl ligands C5HMe4Ar(Ar=Ph,4-CH3Ph,4-OCH3Ph,4-ClPh,4-BrPh)with Re2(CO)10in refluxing xylenefurnishedfivenewmononuclearrhenium carbonylcomplexes.Friedel-Craftsreactionsof aromatic substrates with tert-butyl halides catalyzed by these complexes showed that they have obvious catalytic activity.tert-Butyl halides could be used as alkylation reagents in these reactions.Compared with traditional catalysts,these complexes have some significant practical advantages,namely lower amounts of catalyst,mildreactionconditions,andmore environmentally friendly chemistry.Further studies to elucidate the reaction mechanism and expand the synthetic utility of these catalysts are in progress.
Supporting information is available at http://www.wjhxxb.cn
[1]Ishiyama T,Miyoshi K,Nakazawa H.J.Mol.Catal.A:Chem., 2004,221:41-45
[2]Krutko D P,Borzov M V,Veksler E N,et al.Eur.J.Inorg. Chem.,1999,11:1973-1979
[3]Jutzi P,Redeker T.Eur.J.Inorg.Chem.,1998,6:663-674
[4]Shapiro P J,Bunel E,Schaefer W P,et al.Organometallics, 1990,9:867-869
[5]Hou X F,Cheng Y Q,Wang X,et al.J.Organomet.Chem., 2005,690:1855-1860
[6]Yeh P H,Pang Z,Johnston R F.J.Organomet.Chem.,1996, 509:123-139
[7]Dou Y Y,Xie Y F,Tang L F.Appl.Organomet.Chem.,2008, 22:25-29
[8]Pang Z,Burkey T J.Organometallics,1997,16:120-123
[9]Huang J,Wu T,Qian Y.Chem.Commun.,2003:2816-2817
[10]Daugulis O,Brookhart M.Organometallics,2003,22:4699-4704
[11]Draganjac M,Ruffing C J,Rauchfuss T B.Organometallics, 1985,4:1909-1911
[12]Schumanna H,Heima A,Schuttea S,et al.Z.Anorg.Allg.Chem.,2006,632:1939-1942
[13]Erker G,Kehr G,Fröhlich R.J.Organomet.Chem.,2004, 689:1402-1412
[14]Lukešová L,Stepnicka P,Fejfarová K,et al.Organometallics, 2002,21:2639-2653
[15]Horácek M,Stepnicka P,Gyepes R,et al.Chem.Eur.J., 2000,6:2397-2408
[16]Castro A,Turner M L,Maitlis P M.J.Organomet.Chem., 2003,674:45-49
[17]Ma Z H,Zhao M X,Li F,et al.Transition Met.Chem., 2010,35:387-391
[18]Ma Z H,Wang N,Guo K M,et al.Inorg.Chim.Acta,2013, 399:126-130
[19]Ma Z H,Guo K M,Wang N,et al.J.Coord.Chem.,2014, 67:64-71
[20]Ma Z H,Lü L Q,Wang H,et al.Transition Met.Chem., 2016,41:225-233
[21]Godoy F,Klahn A H,Lahoz F J,et al.Organometallics, 2003,22:4861-4868
[22]Godoy F,Klahn A H,Oelckers B,et al.Dalton Trans., 2009:3044-3051
[23]Klahn A H,Oelckers B,Godoy F,et al.J.Chem.Soc. Dalton Trans.,1998:3079-3086
[24]Nishiyama Y,Kakushou F,Sonoda N.Bull.Chem.Soc.Jpn., 2000,73:2779-2782
[25]Kuninobu Y,Matsuki T,Takai K.J.Am.Chem.Soc.,2009, 131:9914-9915
[26]Bensley D M.J.Org.Chem.,1988,53:4417-4419
[27]Enders M,Ludwig G,Pritzkow H.Organometallics,2001,20: 827-833
[28]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of Göttingen,Germany,1997.
[29]Fitzpatrick P J,Le Page Y,Butler I A.Acta Crystallogr. Sect.B,1981,37:1052-1058
[30]Arancibia R,Godoy F,Buono-Core G E,et al.Polyhedron, 2008,27:2421-2425
芳基取代四甲基环戊二烯基铼羰基化合物的合成、晶体结构及催化性能
马志宏1,2李战伟1秦玫*,1李素贞3韩占刚1郑学忠1林进*,1
(1河北师范大学化学与材料科学学院,石家庄050024) (2河北医科大学基础医学院,石家庄050017) (3河北工业职业技术学院,石家庄050091)
芳基取代的四甲基环戊二烯C5HMe4Ar(Ar=Ph,4-CH3Ph,4-OCH3Ph,4-ClPh,4-BrPh)分别与Re2(CO)10在二甲苯中加热回流,得到了5个单核配合物[(η5-C5Me4Ar)Re(CO)3](Ar=Ph(1),4-CH3Ph(2),4-OCH3Ph(3),4-ClPh(4),4-BrPh(5))。通过元素分析、红外光谱、核磁共振氢谱对配合物1~5的结构进行了表征,用X射线单晶衍射法测定了配合物的结构。同时,研究了这五种配合物在芳香族化合物Friedel-Crafts烷基化反应中的催化活性。
合成;单核铼羰基配合物;Friedel-Crafts烷基化反应;催化
O614.71+3
A
1001-4861(2017)06-1074-07
2017-02-10。收修改稿日期:2017-03-22。
10.11862/CJIC.2017.117
国家自然科学基金(No.21372061)、河北省自然科学基金(No.B2013205025,B2014205018)和河北师范大学重点基金(No.L2012Z02)资助项目。
*通信联系人。E-mail:qinmei2005@126.com,linjin64@126.com;会员登记号:S06N0210M1305。
猜你喜欢
杂志排行
无机化学学报的其它文章
- CoAl2O4/蜂窝陶瓷催化剂的制备及其催化臭氧化性能
- Photocatalytic Hydrogen Production Based on Cobalt-Thiosemicarbazone Complex with the Xanthene Dye Moiety
- 两种金属-有机钙钛矿材料的负热膨胀性质
- Pyrazolate-Based Dipalladium(Ⅱ,Ⅱ)Complexes:Syntheses,Characterization and Catalytical Performance in Suzuki-Coupling Reaction
- 以滤纸为模板合成新型介孔生物活性玻璃微管材料
- Br-掺杂Bi2WO6的水热法合成及其可见光催化性能
