Syntheses,Structures and Magnetic Analysis of Co(Ⅰ),Ni(Ⅱ)Coordination Polymers Based on Pyridine-2,4,6-tricarboxylic Acid
2017-07-05YINXiuJuLIAOBeiLingWUHanMinPANGYiLinLIShiXiong
YIN Xiu-JuLIAO Bei-LingWU Han-MinPANG Yi-LinLI Shi-Xiong*,,2
(1College of Chemistry and Biological Engineering,Hechi University,Yizhou,Guangxi 546300,China) (2School of Environment and Energy,South China University of Technology,Guangzhou 510006,China)
Syntheses,Structures and Magnetic Analysis of Co(Ⅰ),Ni(Ⅱ)Coordination Polymers Based on Pyridine-2,4,6-tricarboxylic Acid
YIN Xiu-Ju1LIAO Bei-Ling1WU Han-Min1PANG Yi-Lin1LI Shi-Xiong*,1,2
(1College of Chemistry and Biological Engineering,Hechi University,Yizhou,Guangxi 546300,China) (2School of Environment and Energy,South China University of Technology,Guangzhou 510006,China)
The title coordination polymers of{[M3(pyta)2(H2O)8]·4H2O}n(M=Co(1),Ni(2))based on H3pyta(H3pyta= pyridine-2,4,6-tricarboxylic acid)had been synthesized under hydrothermal synthesis conditions with same temperature,molar ratio and solvent,but different metal salts.X-ray diffraction analysis shows that these two polymers are hetero-isomorphic and belong to the monoclinic system,P21/c space group.The magnetic investigation shows that polymers 1 and 2 exhibit a ferromagnetic coupling between M(Ⅱ)ions.CCDC:1524314,1;1000880,2.
coordination polymer;pyridine-2,4,6-tricarboxylic acid;magnetic
0 Introduction
Since“single-molecule magnets”(SMMs)were discovered,design and synthesis of the paramagnetic transitionpolymetallicclusterareattractiveto researchers[1-5].Recently many different directions have being pursued in the research field of SMMs[6-11],in which an important direction has been found that the intermolecular interaction with different correlation in the whole molecular arrangement,though very weak, can perturb the intrinsic properties of individual SMMs[12].In our previous work[13-14],we have reported and studied antiferromagnetic(AF)coupling interaction betweenmagneticcenters.Ligands3-/4-pyridinecarboxylateandpyridinecarboxylatehave recently been found to act as excellent buildingblocks with charge and multi-connecting ability in the construction of functional coordination polymers with porosity,photolumineseent or magnetic properties[15-16]. Comparedwiththepreviouslyinvestigated pyridinecarboxylateligands,pyridine-2,4,6-tricarboxylic acid(H3pyta)have the advantages of multiple bridging moieties,which leads to a variety of connection modes with transition metal centers and provides abundant structural motifs.It can act not only as N-donors but also as Ocarboxylate-donors to chelate or bridge metal ions to form coordination polymers[17], and some complexes can act as SMMs[18].In this paper,we report Co(Ⅰ)and Ni(Ⅱ)polymers based on pyridine-2,4,6-tricarboxylic acid(H3pyta):{[M3(pyta)2(H2O)8]·4H2O}n,and analyze their thermogravimetric and magnetic properties.
1 Experimental
All solvents and chemicals were commercial reagentsandusedwithoutfurtherpurification. pyridine-2,4,6-tricarboxylicacidwassynthesized according to the reference[17].Elemental analyses (carbon,hydrogen,and nitrogen)were performed with a Perkin-Elmer 240 elemental analyzer.IR spectra were measured from KBr pellets on a Nicolet 5DX FTIR spectrometer.XRD was performed using Rigaku D/max 2500 X-ray diffractometer(Cu Kα radiation, λ=0.156 04 nm,U=40 kV,I=150 mA,2θ=5°~65°). The TGA was determined by Perkin Elmer Pyris Diamond TG-DTA.The magnetic measurements were carried out with a Quantum Design MPMS-XL7 and a PPMS-9 ACMS magnetometer.
1.1 Synthesis of{[Co3(pyta)2(H2O)8]·4H2O}n(1)
CoCl2·6H2O(0.072 4 g,3 mmol),H3ptc(0.021 1 g, 1 mmol)were mixed in 7 mL distilled H2O water and 3 mL ethanol.The pH value of the solution was adjusted to 7 with 1 mol·L-1NaOH,and then sealed in a 23 mL Teflon-lined stainless steel autoclave.The mixture was heated in an oven at 100℃for two days, and cooled to room temperature at a rate of 10℃·h-1. Red massive crystals of 1 were obtained.Yield:95% (based on H3ptc).Anal.Calcd.for C16H28Co3N2O24(%): C,23.74;H,3.46;N,3.46.Found(%):C,23.77;H, 3.40;N,3.48.IR(cm-1):3 448s,1622s,1 509w,1 444w, 1 378s,1 312w,1 256w,1 114w,926w,747m,512m.
1.2 Synthesis of{[Ni3(pyta)2(H2O)8]·4H2O}n(2)
The procedures were similar to the synthesis of 1 except that the metal salts was NiCl2·6H2O.Green massive crystals of 2 were obtained.Yield:86%. (based on H3ptc).Anal.Calcd.for C16H28Ni3N2O24(%): C,23.75;H,3.46;N,3.46.Found(%):C,23.81;H, 3.41;N,3.52.IR(cm-1):3 853m,3 749m,3 438s, 2 348w,1 622s,1 430w,1 378s,1 289w,1 113w, 926w,747m,512m.
1.3 X-ray diffraction
Single-crystal X-ray diffraction data were collected on a Agilent supernova diffractometer equipped with graphite-monochromated Mo radiation with radiation wavelength 0.071073 nm,by usingthex-scan technique.For 1 and 2,the structures were solved by direct methods using the SHELXS-2013[19],and refined by full-matrix least-squares on F2using the Olex2program[20].All non-hydrogen atoms were refined anistropically.Hydrogenatomsweregenerated geometrically and refined isotropically with the riding mode.Crystallographiccrystaldataandstructure processing parameters for polymers 1~2 are summarized in Table 1.Selected bond lengths and bond angles for polymers 1~2 are listed in Table 2,and hydrogen bonds for polymers 1~2 are listed in Table 3.
CCDC:1524314,1;1000880,2.
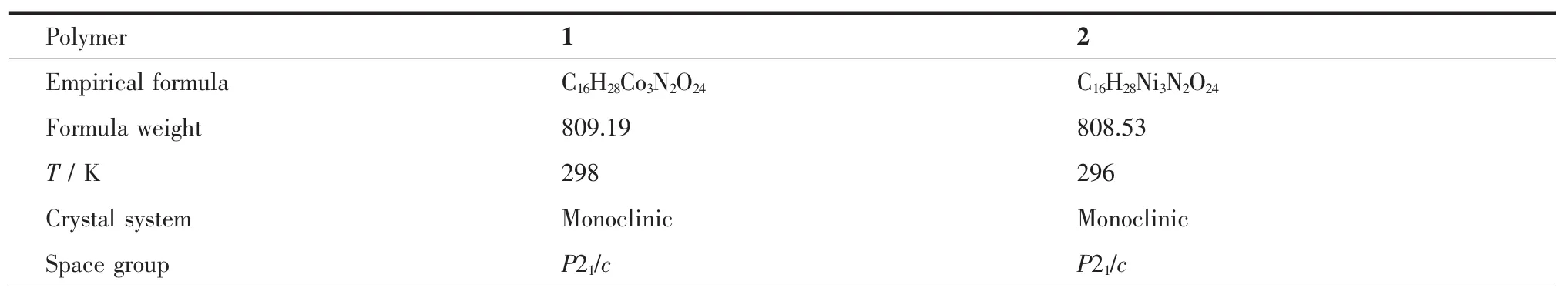
Table 1Crystal data and structure parameters for polymers 1~2
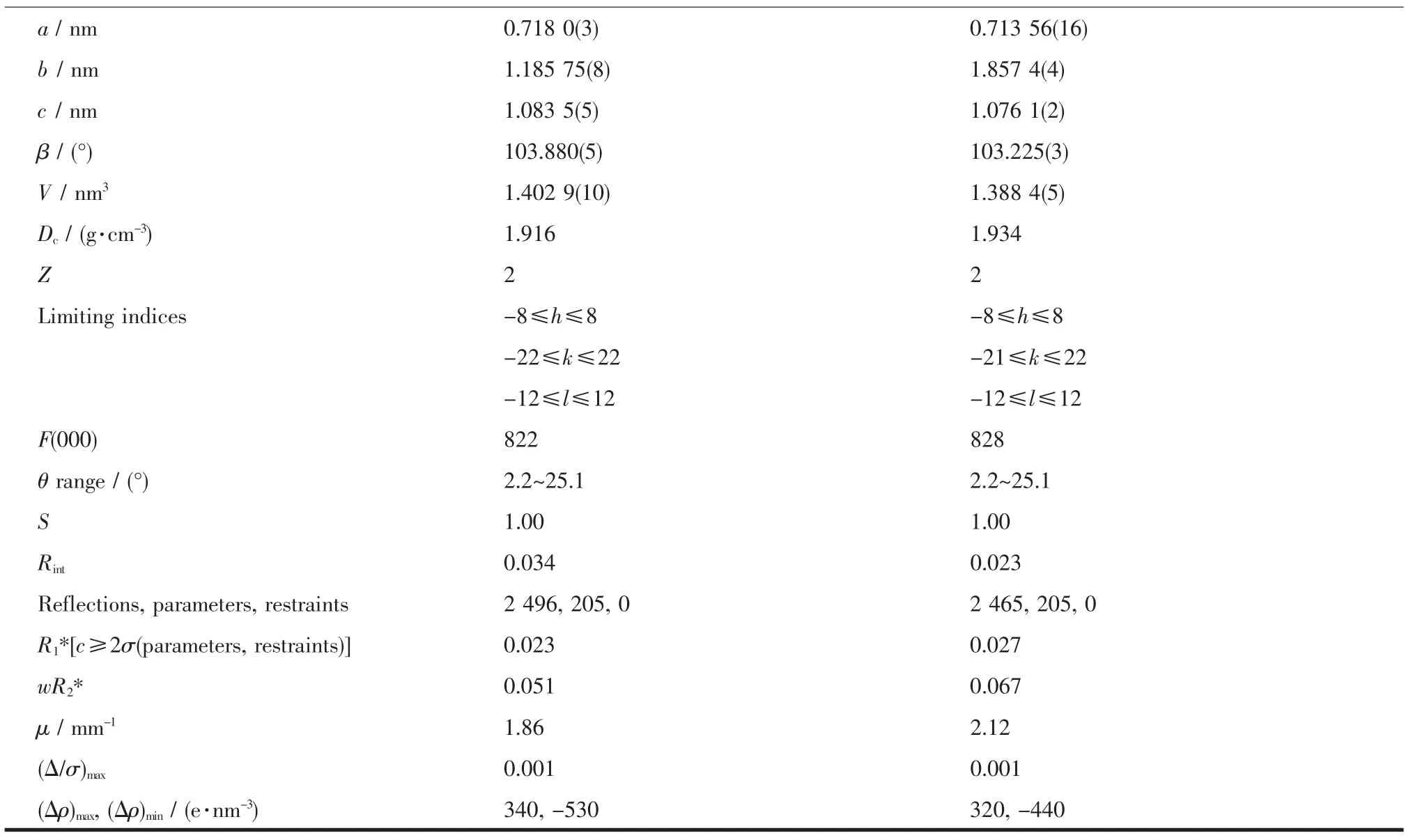
Continued Table 1
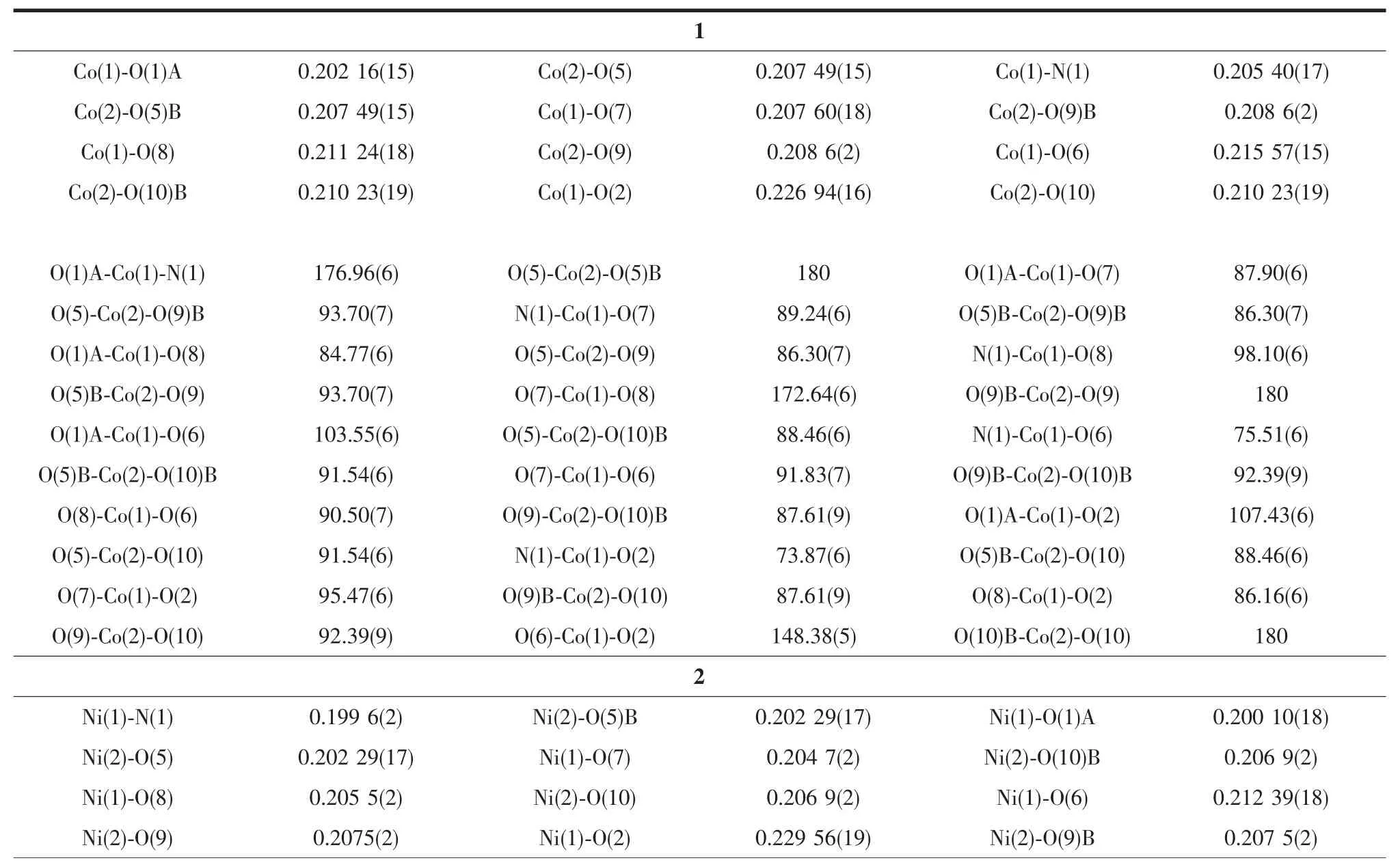
Table 2Selected bond lengths(nm)and bond angles(°)for polymers 1~2
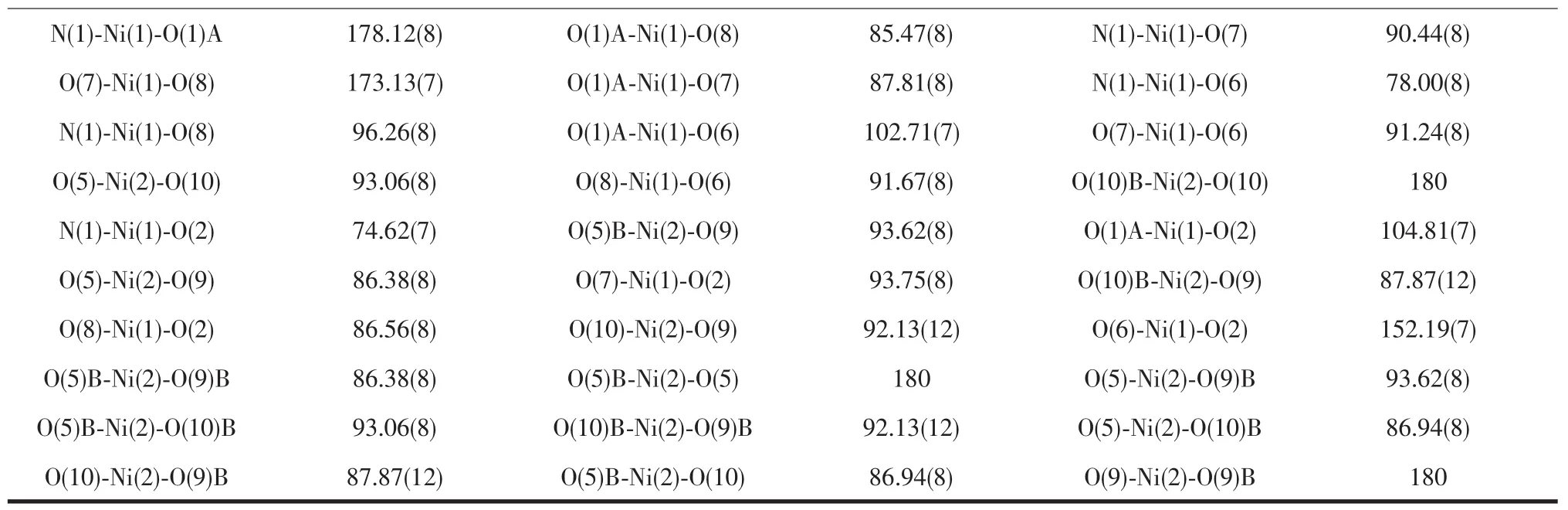
Continued Table 2
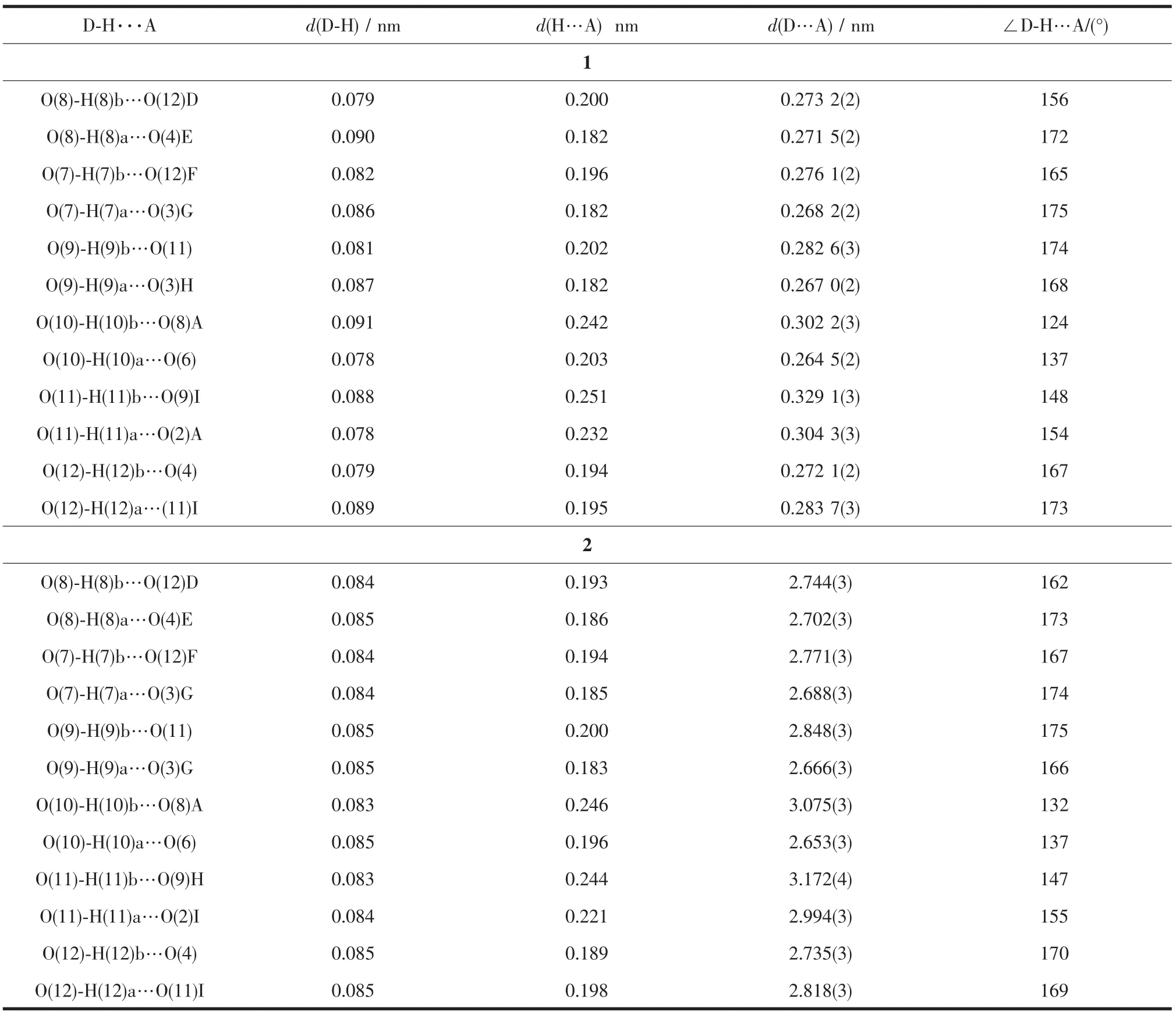
Table 3Hydrogen bonds parameters for polymers 1 and 2
2 Results and discussion
2.1 Crystal structures of 1 and 2
X-ray single-crystal diffraction analysis reveals that polymers 1 and 2 crystallizes in monoclinic system,space group P21/c,they are hetero-isomorphic andtwo-dimensionalstructurepolymers.The coordination environment of M(Ⅱ)in 1 and 2 is shown in Fig.1a.The asymmetric unit consists of three M(Ⅱ)ions,two pyta3-ligand,eight coordinated water molecules,and four free water molecules.
There are three separate M(Ⅱ)ions and two coordinationmodesinthepolymers.Thefirst coordination mode is that the M(Ⅱ)ion is coordinated by one nitrogen atom(N1)and three oxygen atoms(O1, O2,O6)from pyta3-ligand and two coordinated water molecules(O7,O8).The other one is that the M(Ⅱ)ion is coordinated by six oxygen atoms(O5,O5B from two different pyta3-ligand and O9,O9A,O10,O10A from four coordinated water molecules).These two different M(Ⅱ)ions in 1 and 2 both are six-coordinated.For polymer 1,the Co-O distances fall in the range of 0.202 16(15)~0.226 94(16)nm.The Co-N distance is 0.205 40(17)nm.The bond angles of N1-Co1-O2 and O10-Co2-O10B are 73.87(6)°and 180.0°,respectively. For polymer 2,the Ni-O distances fall in the range of 0.200 10(18)~0.229 56(19)nm.The Ni-N distance is 0.199 6(2)nm.The bond angles of N1-Ni1-O2 and O9B-Ni2-O9 are 74.62(7)°and 180.0°,respectively. These bond angles and bond distances all fall in the normal ranges[17,21].
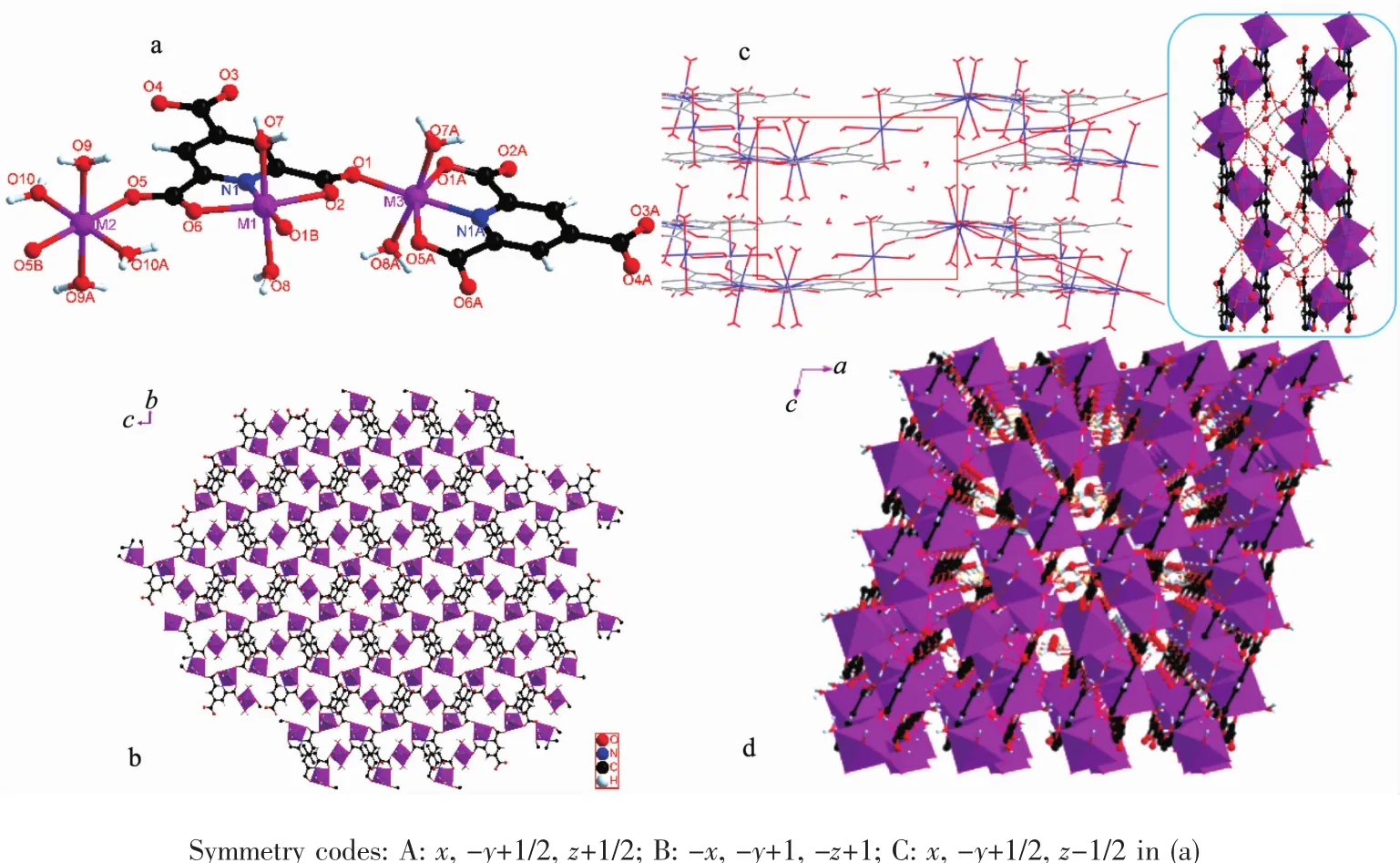
Fig.1(a)Coordination environment of M(Ⅱ)(M=Co,Ni)ions in 1~2;(b)2-D network structure of polymers 1~2 viewed along a axis; (c)Hydrogen bonds in 1~2;(d)3D structure of 1~2 formed by hydrogen bonds viewed along b axis
In polymers 1 and 2,the five coordination sites on the ligand all coordinate with metal ions.So,the pyta3-ligand adopts a μ5-η1:η2:η2bridging style to coordinate with M(Ⅱ)ions.Two O atoms from carboxyl of ligand coordinate with two M(Ⅱ)ions,so carboxyl adopts a μ2-η1:η1bridging style to coordinate with M(Ⅱ)ions.As the ligand adopts a μ5-η1:η2:η2bridging style and carboxyl takes a μ2-η1:η1bridging style,the polymers1and2exhibitaveryspecialtwodimensional network structure along a axis(Fig.1b).
Owing to the introduction of water molecules,the crystals of polymers 1 and 2 have a large number of hydrogen bonds.The O atoms in carbonyl group on the ligand form O-H…O intramolecular hydrogen bonds with coordinated water molecules.The free water molecules in polymers 1 and 2 form O-H…O intermolecular hydrogen bonds with coordinated water molecules(Fig.1c).These hydrogen bonds finallygenerate a three-dimensional network structure by bridgingthetwo-dimensionalplaneswith intermolecular hydrogen bonds(Fig.1d).
2.2 PXRD and thermal gravimetric analysis
In order to check the purity of polymers 1 and 2, powder X-ray diffraction of the as-synthesized samples was measured at room temperature.The peak positions of experimental patterns are in good agreement with the simulated ones,which clearly indicates good purity of the polymers 1 and 2(Fig.2).
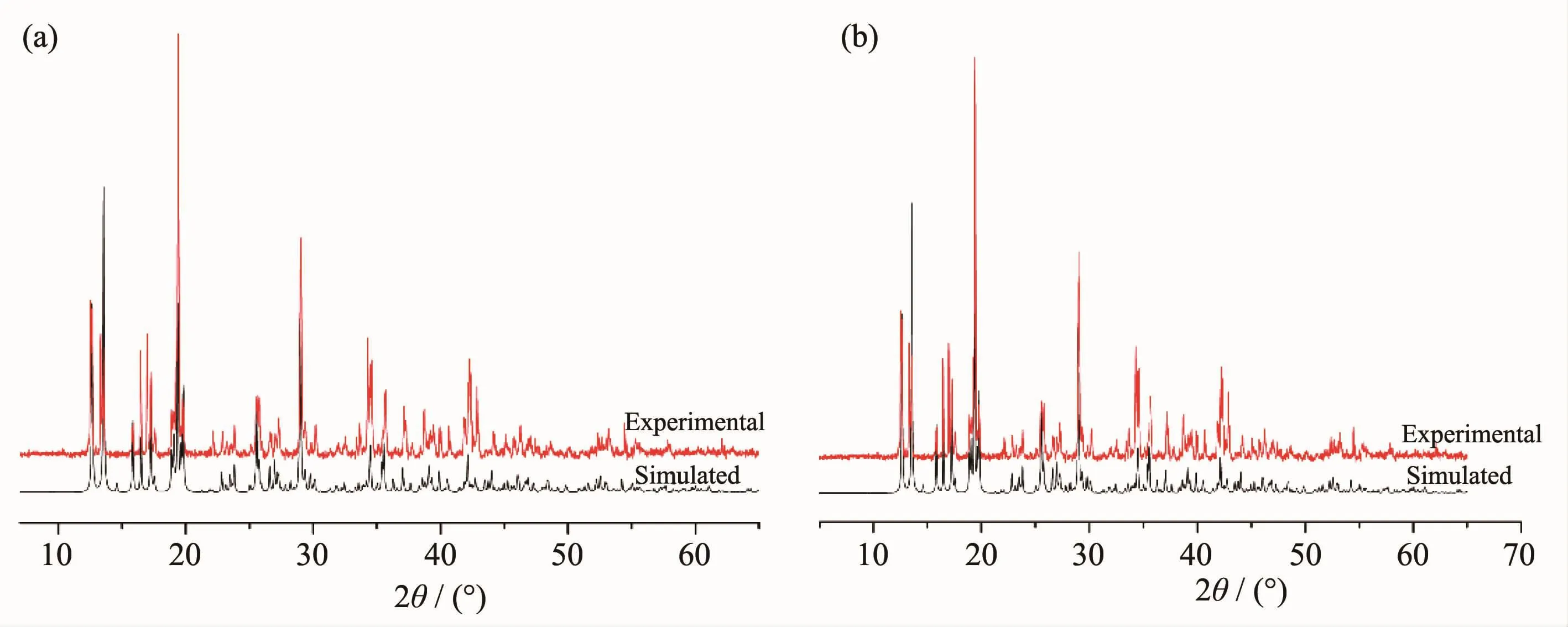
Fig.2PXRD patterns for polymers 1(a)and 2(b)
The thermal stabilities of polymers 1 and 2 were tested in the range of 45~1 000℃under a nitrogen atmosphere at a heating rate of 5℃·min-1and TGA curves of polymers 1 and 2 were shown in Fig.3.The TGA curve of 1 show that polymer 1 first loses twelve water molecules(Obsd.27.59%,Calcd.26.69%)in the range of 45~236℃.The second weight loss is responsibleforthedecompositionofallorganic components in the range of 236~556℃.The residue withweightof31.72%mightbeCo2O3(Calcd. 30.75%).The TGA curve of 2 show that polymer 2 first loses twelve water molecules(Obsd.23.52%,Calcd. 23.75%)in the range of 45~123℃.Further weight loss is responsible for the decomposition of all organic components in the range of 123~503℃.The residue with weight of 28.79%might be NiO(Calcd.27.71%).
2.4 Magnetic analysis for polymers 1~2
Co(Ⅰ)(d7)of polymer 1 and Ni(Ⅱ)(d8)of polymer 2haveunpairedelectrons,sotheirmagnetic properties are studied.The magnetic susceptibilities, χmof 1 and 2 were measured in the 2~300 K temperature range,and shown as plots of χmandχmT versus T in Fig.4a and Fig.4b,respectively.As shown in Fig.4a,the molar magnetic susceptibility χmof polymer 1 increases gradually as the temperature lowers,and more rapidly increases below 25 K,then reaches a maximum value of 2.24 cm3·mol-1at 2 K.It can be seen from the χmT curve that the χmT value is 9.56 cm3·mol-1·K at 300 K,which is significantly higher than the theoretical value 5.64 cm3·mol-1·K of the high-spin triplet Co2+,indicating a great spin-orbit coupling contribution.As the decrease of temperature,the χmT begin to decrease slowly,and the decrease in the range of 300~25 K could be attributed to the single ion behavior of Co2+.But the χmT more rapidly reduces below 25 K,then reaches a minimum value of 4.46 cm3·mol-1·K at 2 K.Combined with the decrease in the χmT value when cooling,this result indicates the presence of weak antiferromagnetic interactions in polymer 1[22].At 300K,the magnetic moment(μeff)of cobalt(Ⅱ),which is determined by the equation μeff=2.828(χmT)1/2,reaches the peak value of 8.74μB.This value is slightly higher than that expected for an isolated divalent high-spin Co(Ⅰ)system with μeff= 3.87μB.
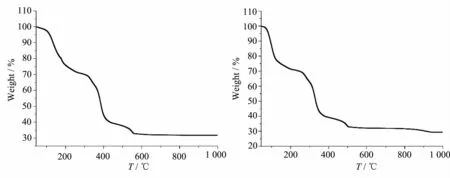
Fig.3TGA curves of polymers 1(a)and 2(b)
AsshowninFig.4b,themolarmagnetic susceptibility χmof polymer 2 increases gradually as the temperature lowers,and more rapidly increases below 25 K,then reaches a maximum value of 0.48 cm3·mol-1at 2 K.It can be seen from the χmT curve that the χmT value is 3.76 cm3·mol-1·K at 300 K, which is equal to the theoretical value of the highspin binuclear Ni2+.As the decrease of temperature, the χmT decrease slowly,and the decrease in the range of 300~25 K could be attributed to the single ion behavior of Ni2+.But there is more rapidly reduction for χmT below 25 K,then it reaches a minimum value of 0.96 cm3·mol-1·K at 2 K.At 300 K,the magnetic moment(μeff)of nickel(Ⅱ)reaches the peak value of 5.48μB.This value is slightl yhigher than that expected for an isolated divalent high-spin Ni(Ⅱ)system with μeff=2.83μB.From magnetic data of the polymers,it is clear that AF coupling mainly occurs between intrachain metal ions.
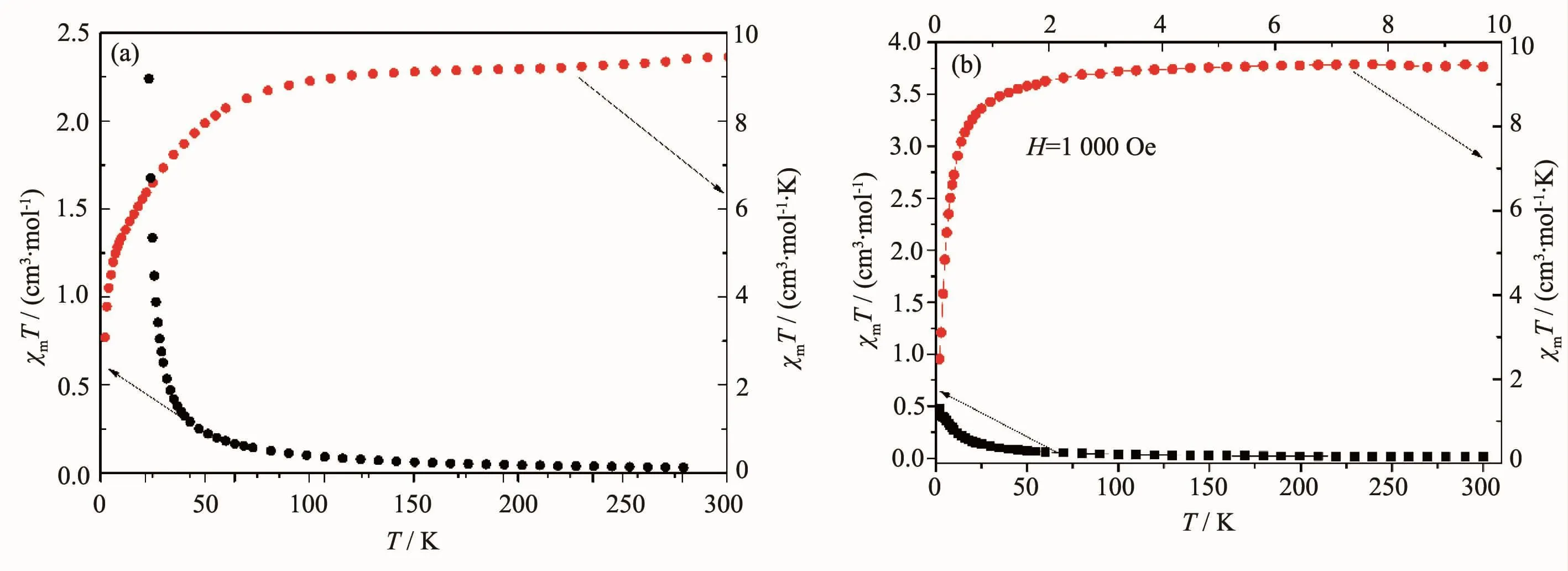
Fig.4Plots of χmand χmT vs T of polymers 1(a)and 2(b)
3 Conclusions
In summary,two coordination polymers based on pyridine-2,4,6-tricarboxylic acid had been synthesized and characterized.X-ray diffraction study reveals that the polymers 1~2 are isostructural and have twodimensionalnetworkstructure.Themagnetic measurement reveals the pyridine-2,4,6-tricarboxylic acidasbridgeligandcanmediatethe antiferromagnetic(AF)coupling interaction between magnetic centers.
[1]Rumberger E M,Shah S J,Beedle C C,et al.Inorg.Chem., 2005,44:2742-2752
[2]Rinehart J D,Long J R.Chem.Sci.,2011,2:2078-2085
[3]Meihaus K R,Long J R.Dalton Trans.,2015,44:2517-2528
[4]Zhang S H,Zhang Y D,Zou H H,et al.Inorg.Chim.Acta, 2013,396:119-125
[5]Zhang S H,Huang Q P,Zhang H Y,et al.J.Coord.Chem., 2014,69:3155-3166
[6]Wang J H,Zhang S H,Wang W,et al.J.Cluster Sci.,2015, 26:1129-1142.
[7]Huang X R,Yang L,Zhou Y J,et al.J.Cluster Sci.,2015, 26:2033-2042
[8]Limas N G,Manz T A.RSC Adv.,2016,6:45727-45747
[9]Liddle S T,van Slageren J.Chem.Soc.Rev.,2015,44:6655-6669
[10]Aubin S M J,Wemple M W,Adams D M,et al.J.Am. Chem.Soc.,1996,118:7746-7754
[11]LI Huan(李欢),CHEN Yun-Zhou(陈云舟),WANG Yan-Jun (王艳君),et al.Chinese J.Inorg.Chem.(无机化学学报), 2016,32:2198-2204
[12]Miyasaka H,Yamashita M.Dalton Trans.,2007,36:399-406
[13]Liao B L,Li S X,Guo J J,et al.Russ.J.Coord.Chem., 2016,42:285-291
[14]LIAO Bei-Ling(廖蓓玲),LI Shi-Xiong(李石雄),YIN Xiu-Ju (银秀菊),et al.Chinese J.Inorg.Chem.(无机化学学报), 2016,32:1255-1260
[15]Lu J Y.Coord.Chem.Rev.,2003,246:327-347
[16]Zhang L Y,Zhang J P,Lin Y Y,et al.Cryst.Growth Des., 2006,6:1684-1689
[17]Gao H L,Yi L,Zhao B,et al.Inorg.Chem.,2006,45:481-483
[18]Li C J,Peng M X,Leng J D,et al.CrystEngComm,2008, 10:1645-1652
[19]Sheldrick G M.Acta Crystallogr.Sect.A,2008,A64:112-122
[20]Bourhis L J,Dolomanov O V,Gildea R J,et al.Acta Crystallogr.Sect.A,2015,A71:59-75
[21]LI Shi-Xiong(李石雄),LIAO Bei-Ling(廖蓓玲),LUO Pei (罗培),et al.Chinese J.Inorg.Chem.(无机化学学报), 2015,31:291-296
[22]Jia L H,Li R Y,Duan J M,et al.Inorg.Chem.,2011,50: 144-154
基于2,4,6-吡啶三酸配体的Co(Ⅰ)、Ni(Ⅱ)配位聚合物的合成、结构和磁性分析
银秀菊1廖蓓玲1吴汉民1庞毅林1李石雄*,1,2
(1河池学院化学与生物工程学院,宜州546300) (2华南理工大学环境与能源学院,广州510006)
以2,4,6-吡啶三酸为配体,采取水热合成的方法,在相同温度、物质的量之比、溶剂,不同金属盐条件下合成了{[M3(pyta)2(H2O)8]·4H2O}n(M=Co(1),Ni(2),H3pyta=2,4,6-吡啶三酸)配位聚合物。X射线单晶衍射测试分析表明2个配合物是异质同晶结构,属于单斜晶系,P21/c空间群。磁性测试表明在配合物的中心离子M(Ⅱ)之间存在反铁磁耦合作用。
配位聚合物;2,4,6-吡啶三酸;磁性
O614.81+2;O614.81+3
A
1001-4861(2017)06-1043-08
2016-12-28。收修改稿日期:2017-04-15。
10.11862/CJIC.2017.116
广西高校科研项目(No.YB2014331)、广西教育厅基础研究(No.200807MS090)、广西教育科学“十二五”规划课题(No.2015C408)、国家自然科学基金(No.21563010)和广西自然科学基金(No.2014GXNSFBA118045)资助项目。
*通信联系人。E-mail:lsx1324@163.com
猜你喜欢
杂志排行
无机化学学报的其它文章
- CoAl2O4/蜂窝陶瓷催化剂的制备及其催化臭氧化性能
- Photocatalytic Hydrogen Production Based on Cobalt-Thiosemicarbazone Complex with the Xanthene Dye Moiety
- 两种金属-有机钙钛矿材料的负热膨胀性质
- Pyrazolate-Based Dipalladium(Ⅱ,Ⅱ)Complexes:Syntheses,Characterization and Catalytical Performance in Suzuki-Coupling Reaction
- 以滤纸为模板合成新型介孔生物活性玻璃微管材料
- Br-掺杂Bi2WO6的水热法合成及其可见光催化性能
