二十面体多硼氢阴离子化合物及其在火炸药中的应用研究进展
2017-06-28单自兴绳利丽杨荣杰
单自兴,绳利丽,杨荣杰
(1. 北京理工大学材料学院,北京 100081;2. 武汉大学化学学院,湖北 武汉 430072)
单自兴1,2,绳利丽1,杨荣杰1
(1. 北京理工大学材料学院,北京 100081;2. 武汉大学化学学院,湖北 武汉 430072)


引 言
硼氢化合物作为高能材料,由于其热值高,燃烧产物分子质量低,在大推力火箭的研发中受到高度关注[1-2]。早在20世纪40年代,Leonard[3]和Sawyer[4]就指出,具有适度高体积密度的硼氢化合物是最有效的火箭液体燃料,它们燃烧完全,不发生爆燃,可产生最高的喷气动力。1947年,Rundle[5]基于硼烷化学的结构研究首次提出二电子三中心键新概念,大大促进了硼氢化合物化学的发展。
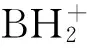
本文主要依据文献资料,系统介绍推进剂及火炸药研究中涉及的十二氢十二硼酸阴离子化合物的制备、热行为、热化学、能量性质以及它们对推进剂及火炸药组分性质的影响,展望此类化合物在推进剂及火炸药中的应用前景,指出未来多硼氢化合物推进剂研究的主要方向。
1 十二氢十二硼酸盐研究概况
1955年,Longuet-Higgins[6]通过分子轨道理论计算,第一次预测到稳定的硼氢化合物B12H12只能以阴离子形式存在。1960年,Hawthorne等[7]在2-碘代十硼烷与三乙胺反应中首次低收率分离出十二氢十二硼酸双三乙胺盐[bis(triethylammonium) dodecahydrododeca-borate],并通过X-射线分析确认了这种硼氢阴离子的二十面体闭笼型结构(见图1)[8]。
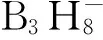
最简单的十二氢十二硼酸化合物是十二氢十二硼酸(H2B12H12)[33],它通常由水溶性十二氢十二硼酸盐通过氢离子柱上交换制备,并常以二水合物(H3O)2B12H12的形式存在。水合十二氢十二硼酸是一种强酸,可与金属、金属氧化物或金属碳酸盐以及氨、肼、链状或杂环有机胺、有机膦、有机硫等Lewis碱反应生成水溶性的或水难溶的、高度热稳定的十二氢十二硼酸盐。十二氢十二硼酸的轻金属盐、氨盐、低级胺盐、杂环富氮盐等具有良好的水溶性,使得通过复分解反应可以方便实现阳离子转换(见图3)。
2 十二氢十二硼酸阴离子的制备
2.1 较低级硼烷在有机碱存在下热解

2.2 较低级离子型硼氢合物热解


2.3 MBH4与卤化四烷基铵混合物热解
2.4 MBH4与Lewis酸混合物热反应

2.5 较低级硼烷与硼烷络合物的热反应


2.6 较低级硼烷与离子型硼氢合物热反应

2.7 甲硼烷络合物与金属硼氢化物热加成反应


2.8 硼合物的高温还原偶联反应

3 十二氢十二硼酸盐的热行为
3.1 十二氢十二硼酸
3.2 十二氢十二硼酸金属盐
十二氢十二硼酸金属盐一般都具有高的热稳定性。轻的碱金属(Li、Na)的十二氢十二硼酸盐常与水、氨和作为溶剂的有机Lewis碱(如THF、diglyme、MeCN等)生成溶剂合物,而重的碱金属(Cs、Rb)的十二氢十二硼酸盐则易从水中得到纯品。碱金属十二氢十二硼酸盐都具有非常高的分解温度,如无水Cs2B12H12在真空管中加热到810℃不发生任何变化;K2B12H12加热到700℃未显示出分解迹象;Na2B12H12·4H2O在140℃和195℃分别失去两分子水得到无水物,直至505℃因发生氧化降解而呈现放热效应(可能生成B12H11OH,在830℃燃烧)。碱金属(Li、Na、K)十二氢十二硼酸盐的高温热分解研究表明,分解过程中包含多个步骤,先生成含有二十面体B12骨架的失氢单体M2B12H12-x,接着生成(M2B12Hz)n聚合物[105]。碱土金属及第三族金属(包括稀土)的十二氢十二硼酸盐通常在水中得到含不同数目水分子的溶剂合物;这些水合物在空气中加热时不同程度失水;有的在惰性气氛中加热时可得到无水物,但有的在高温脱水时会发生分解。如,无水MgB12H12不能通过Mg(H2O)6B12H12·6H2O的简单加热脱水来制备[106]。研究表明,十二氢十二硼酸金属盐在惰性气氛和真空状态下的热分解行为几乎相同;而在空气中加热时分解温度明显降低,意味着氧化剂的存在会加速硼笼化合物的分解。
其他族金属的十二氢十二硼酸盐在较高温度下的去溶剂化或去配体过程中也常常伴随着分解。
3.3 十二氢十二硼酸铵盐
十二氢十二硼酸铵盐的热稳定性与铵阳离子的组成和结构以及热分解的气氛密切相关。(NH4)2B12H12在200~400℃热分解[107]得到H2和NH3的气体混合物;而高于400℃只有H2放出;(NH4)2B12H12在惰性气氛中热解得到单质硼,而在氨中得到六角形氮化硼[108]。质子化的有机铵盐热稳定性较低。质子化富氮杂环铵盐的热分解温度一般低于250℃,有的甚至在100℃以下就开始分解;如3,5-二氨基-1,2,4-三唑盐的分解温度为60~70℃[109]。然而,tran-丁烯-1,4-双(3-甲基-1,3-咪唑)盐[110]的分解温度却在300℃以上。加热介质也对富氮杂环铵盐的热稳定性产生重大影响。Nedel′ko, V. V.等[111]研究了十二氢十二硼酸六次甲基四胺盐的热行为,指出在高于150℃时开始分解出NMe3等气体产物,160~200℃时的热分解不能用简单的动力学方程描述,热解残余物是高孔隙难溶固体。Saldin等[112]研究了(C6O4H9NH3)2B12H12的热转变,发现它在约300℃ 时开始着火,加热到1000℃ 时生成碳、无定型硼或(和)碳化硼的混合物。如果在空气中加热,则碳和氧化硼是主产物,且融化的氧化硼保护了硼或(和)碳化硼。一般说来,由正烷基构成的季铵盐热稳定性较高,分解温度一般在250℃以上;随着烷基碳链的增长,铵盐的分解点或熔点迅速降低;如 (Me4N)2-B12H12约在342℃分解; (Et4N)2B12H12于306℃分解; (n-Bu4N)2B12H12熔点为240~242 ℃;(Hex4N)2B12H12熔点为83~84℃ (离子液体)。十二氢十二硼酸双胍盐在胍中引入一个氨基后,熔点和分解温度明显升高;但引入2个氨基或3个氨基后分解点反而有所降低。
4 十二氢十二硼酸盐的热化学
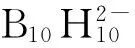
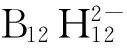
Shubina等[115]在低极性介质中测定了(Bu4N)2B12H12与各种质子给体作用的焓变和熵变,指出由[B12H12]2-到[B10H10]2-BH…HO的键强度增加,并且酸的给质子能力的增加导致生成双叉氢键(bifurcate H-bonds)。
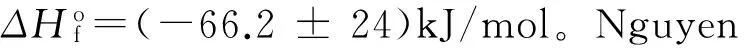

Hanumantha Rao等[109]测定了多种十二氢十二硼酸富氮盐的热化学数据。指出除十二氢十二硼酸羟胺盐(离子液体,具有最低的摩尔生成焓和最高的爆热,800℃燃烧后的残留质量分数近20%)外,其他多氮盐燃烧时残留质量分数均在60%以上;十二氢十二硼酸双三氨基胍盐燃烧后的残留质量分数竟高达78.5%。另一方面,十二氢十二硼酸富氮盐的爆热随着氮含量的增加而降低。但是,这些十二氢十二硼酸富氮盐在纯化、干燥及应用过程中可安全处理而不发生爆炸。
Hanumantha Rao等[119]也测定了一些烷基咪唑盐[1,4-2(3-甲基亚胺-唑)-2-丁炔、2(3-甲基-n-丁基咪唑)、2(3-甲基-n-己基咪唑)、2(3-甲基- n-辛基咪唑)、2(3-甲基-n-十一烷基咪唑)]的标准摩尔燃烧热(-15000~ -28000kJ/mol)、标准摩尔生成焓(-700~ -3300kJ/mol)和爆热(-43300~ -45300kJ/mol),其中,丁炔-2-撑-3-甲基咪唑盐和双3-甲基-丁基咪唑盐的标准摩尔生成焓分别为-811和-745kJ/mol。
5 十二氢十二硼酸盐在推进剂及火炸药中的应用
在火炸药和推进剂研究中涉及的十二氢十二硼酸盐主要是碱金属盐、氮基阳离子盐和少数过渡金属盐(包括Ti、Cu、Fe、Pb等),如表1 所示。

表1 火炸药研究中主要涉及的十二氢十二硼酸盐Table 1 Major salts involved in the study of propellant and explosive
5.1 与推进剂组分的作用及催化硝胺分解的机制
庞维强等[120-121]研究了十二氢十二硼酸四乙铵盐与固体推进剂中一些常见组分的相容性,指出,十二氢十二硼酸四乙铵盐与3-硝基-1,2,4-三唑-5-酮铅(NTO-Pb)、六硝基六氮杂异伍兹烷(CL-20)、环氧乙烷-四氢呋喃共聚醚(PET)、聚乙二醇(PEG)、N-100、端羟基聚丁二烯(HTPB)、CB、A1、Mg、Al2O3, C2、二酸二异辛酯(DOS)和KP组成的二元体系是相容的,对端羟基叠氮聚醚(GAP)和奥克托今(HMX)稍敏感,对己二酸酮(AD-Cu)、β-Cu和φ-Pb敏感,对RDX、3,4-二硝基呋咱基氧化呋咱(DNTF)和GUDN不相容。他们观察发现除2,4-甲苯二异氰酸酯(TDI)外,CL-20、HMX、GAP、RDX、AP、NTO-Pb、HTPB、DOS、IPDI、AD-Cu、Al 粉和 Mg粉对十二氢十二硼酸四乙铵盐的热分解(305.8℃)不产生明显影响。
另一方面,大量的研究[122-126,132-134,137]表明,十二氢十二硼酸盐能改善固体推进剂的燃烧性能,特别是催化硝胺推进剂的分解。当十二氢十二硼酸钾应用于含三氨基胍的推进剂配方中时,推进剂的燃速大幅度提高。20世纪90年代初,Schroeder[122]报道了硼氢化合物加速硝胺推进剂的分解,指出碱金属硼氢化合物可使黑索金(RDX)在其熔点附近开始分解。之后Schroeder[123]又对硼氢酸盐对硝胺推进剂燃烧的促进作用进行了评述。后来Schroeder等[124]研究了K2B12H12对RDX热分解行为时指出K2B12H12能够加速RDX分解,并改变分解产物的分布,证明1,3,5-三嗪、单亚硝基-RDX (MRDX)和相关硝胺是这种热分解的产物,这对于阐明K2B12H12加速RDX热分解的机制非常有意义。关于多硼氢化合物催化硝胺分解的机制,Schroeder[123]认为是硼氢化合物中的B-H键进攻硝胺官能团所致(见图 4)。
5.2 在推进剂及火炸药中的应用
十二氢十二硼酸盐在推进剂中主要用作高能、高密度燃料、燃料添加剂和气体发生剂以及燃速调节剂[125-126]。
早在1963年,Du Pont公司[127]就合成了通式为M(2-n)(B12H12-n-yXy·nSRR′)b(n-2)的十二氢十二硼酸盐,用作彩色火焰的燃料添加剂。

Trofimenko[130]指出,将硫、氟、叠氮基、氰酸基、硫氰酸基等在光照条件下引入全卤代的二十面体硼酸盐(NH4)2B12Br12或H2B12Cl12中,再用四甲基氯化铵水溶液处理得到的四甲基铵盐,如(Me4N)2B12Br11N3,可以用作高能燃料的组分。

Shibata等[132]将K2B12H12用于含三氨基胍的推进剂配方中,当压强为49.02MPa时,燃速为54000mm/s,而在98.04MPa时燃速高达92000mm/s,是M30推进剂燃速的1000倍。Johnson等[133]将K2B12H12用于含硝基胍的推进剂配方中,结果显示组分反应速率加快,且短时间内产生大量气体。
Mangum[134]最近公开了一种无氯的可燃固体推进剂配方,其中,K2B12H12、CS2B12H12和(Bu4N)2B12H12被用作固体氧化剂KIO4、燃料和黏合剂组成的火箭推进剂燃烧的催化剂。实验表明,十二氢十二硼酸盐的加入(质量分数0.5%~5.0%)使推进剂的密度提高0.15~0.51g/cm3, 体积比冲可提高3511~10646N·s/kg,比冲可提高10~50s (见表2)。有趣的是, 当硼氢盐的质量分数为2%、与KIO4的质量比为10∶90时,引入上述3种硼氢盐后推进剂的燃速分别为4089.4、787.4和736.6mm/s;而当CS2B12H12的用量增加时,燃速迅速增加;当CS2B12H12与KIO4的质量比为30∶70时,推进剂的燃速高达25400mm/s。即使以廉价的硝酸钾代替高碘酸钾,在类似情况下,推进剂燃速也能达到17881.6mm/s (见表 3)。 基于表3数据不难推想,用廉价的K2B12H12代替CS2B12H12可能效果更佳。

表2 十二氢十二硼酸盐对固体复合推进剂性能的影响[134]Table 2 Effect of dodecahydrododeca-borate salts on the properties of solid composite propellants
注:黏合剂为多元醇+异氰酸酯+稳定剂+添加剂;十二氢十二硼酸盐的质量分数是固体复合推进剂的2%。

表3 十二氢十二硼酸盐与不同氧化剂混合物的燃速[134]Table 3 Burning rate of the mixture of dodecahydrododeca- borate salts with different oxidizers
Saldin等[94-99]发明了十二氢十二硼酸甲壳素盐[(C6O4H9NH3)2B12H12]的制备方法,观察到这种盐能快速点火并完全燃烧,是一种防爆、无毒和无毒气排放、耐热、耐湿、具有优良力学性能和键合作用的高能物质,适于用作枪粉、烟火、炸药、推进剂的点火剂、起爆药的组分[98-99, 137-141]。如十二氢十二硼酸甲壳素盐本身[140]以及与过渡金属,特别是Cu2+、Co2+、Ni2+、Zn2+或Mn2+[138]以及镁或铝[138]的硝酸盐(或高氯酸盐)组成的复合物,用作烟火药等的能量增强型点火添加剂(energy-intensive igniting additives)。含有有效量铬酸甲壳素盐(氧化剂)的十二氢十二硼酸甲壳素盐用作烟火的点火添加剂,使得烟火药燃烧时不产生有害气体[140]。十二氢十二硼酸甲壳素盐与高氯酸或高氯酸铵或高氯酸甲壳素盐的加合物被用作耐热的和具有良好力学性能的烟火配方中的无毒贮藏稳定剂[141]。
Saldin等也将十二氢十二硼酸乌洛托品盐[142]和超细聚四氟乙烯-氧化石墨水溶胶与十二氢十二硼酸生成的匀胶[143]分别用作烟火的高能点火剂和燃烧稳定剂以及集能系统(condensed energy systems)的高能组分。
Saldin等[144]还将多乙撑亚胺的水溶液与十二氢十二硼酸作用制得的十二氢十二硼酸乙撑亚胺盐[(C2H4NH·0.4H2B12H12]用作烟火药点火添加剂。Saldin等[145]指出,由H2B12H12水溶液和超细PTFE加到含氧化石墨的水溶胶中,制得的均相水溶液可用作火药、烟火药和炸药中的高能组分。
将十二氢十二硼酸富氮盐及杂环铵盐应用于固体火箭推进剂中近年受到较多关注,已有多项专利和研究报告。Hanumantha Rao和Muralidharan[109,119]以及Shackelford[146]等认为,十二氢十二硼酸氮杂环铵盐,包括咪唑盐、1,2,4-三唑盐、1,2,3-三唑盐、四唑盐、吡唑盐、噁唑盐、异噁唑盐、呋咱盐、氧化呋咱盐等高的体积密度以及燃烧产物的低分子量,适于在固体火箭推进剂中用作钝感含能材料和燃烧加速剂。它们有利于提供高密度比冲,增强推进剂的能量,增加导弹有效负荷,改善燃烧性能,进而提高火箭推进剂的比冲。另一方面,这些盐的极低蒸汽压为推进剂的生产、使用以及突发事件的处理提供了方便和健康保证。
6 多氢多硼酸盐离子液体在推进剂中的应用
硼氢离子液体具有蒸汽压低、熔点低、密度高、稳定性高和反应性低的特性,对推进剂及火炸药工作者具有极大吸引力。非取代的十二氢十二硼酸铵盐离子液体,迄今仅有数种[十二氢十二硼酸双四正己铵盐、十二氢十二硼酸双羟胺盐、十二氢十二硼酸双5-(3,5-二硝基甲苯)-1H-四唑-4-铵盐、十二氢十二硼酸双3,5-二氨基-1H-1,2,4-三唑-4-铵盐等]。Gabel和Justus[147-149]以及Kopytin[150]等报道了合成含B12H11- (NR1R2R3)-阴离子的高能离子液体的方法,制备出一系列胺取代的二十面体硼氢阴离子的高能离子液体(优选的阳离子是Li+、R4N、咪唑和吡啶)。2015年,Jenne和Kirsch[151]制备出另一类烷氧基取代的[B12X11OR]2-(X=Cl、Br、R = Pr、octyl、dodecyl)的二十面体硼卤酸盐,其中,十一氯正丙氧基十二硼酸-1-己基-3-甲基咪唑盐熔点为96℃。
7 结束语
[1] Balucani N, Zhang F T, Kaiser R I. Organo-boron molecules in combustion systems[J]. Chemical Reviews, 2010, 110: 5107-5127.
[2] Hansen B R S, Paskevicius M, Li H W, et al. Metal boranes: Progress and applications[J].Coordination Chemistry Reviews, 2016,323:60-70.
[3] Leonard A S.Some possibilities for rocket propellants. III.[J].Journal of American Rocket Society, 1947 (72): 10-23.
[4] Sawyer F G. Rocket chemistry at Inyokern [J]. Chem Eng News, 1949,27: 2067-2069.
[5] Rundle R E. Electron-deficient compounds [J].Journal of American Chemical Society,1947, 69: 1327-1331.
[6] Longuet-Higgins H C, Roberts M de V.The electronic structure of an icosahedron of boron atoms[J].Proceedings of the Royal Society of London, Series A: Mathematics Physics Engineering Science, 1955, 230: 110-119.
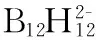
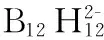
[9] Olid D, Nunez R, Vinas C, et al. Methods to produce B-C,B-P, B-N and B-S bonds in boron clusters[J]. Chemical Society Reviews,2013,42(8): 3318-3336.
[10] Sivaev I B, Bregadze V I, Sjöberg S. Chemistry of closo- dodecaborate anion [B12H12]2-: a review[J]. Coll Czech Chem Commun,2002, 67: 679-727.
[11] SkripovA V, Soloninin A V, Babanova O A,et al. Nuclear magnetic resonance studies of atomic motion inborohydride-based materials: Fast anion reorientations and cation diffusion[J].Journal of Alloys Compound, 2015, 645 (Suppl.1): S428-S433.
[12] Nakamori Y, Orim S.Borohydrides as hydrogen storage materials[J]. Solid-State Hydrogen Storage,2008:420-449.
[13] Hawthorne M F. New discoveries at the interface of boron and carbon chemistries[J].Pure Appllied Chemistry, 2003, 75(9): 1157-1164.
[14] Sivaev I B, Bregadze V I, Kuznetsov N T.Derivatives of the closo-dodecaborate anion and their application in medicine [J]. Khimicheskaya, 2002, 51(8): 1362-1374.
[15] Peymann T, Shelly K, Hawthorne M F. Recent developments in the chemistry of [B12H12]2-[C]∥Proceedings of the International Symposium on Neutron Capture Therapy for Cancer. Los Angeles: CA, 1998 (2001):775-778.
[16] 单自兴. 通过次甲基的自转移反应制备N-甲基乌洛托品盐: CN, 104910166[P]. 2015
[17] 王维伦, 兰燕花, 单自兴,等. 硼氢化合物K2B12H12对HTPB推进剂燃烧性质的影响[C]∥2016年火炸药技术学术研讨会论文集.西安:西安近代化学研究所,2016:497.
[18] 单自兴.一种新的高氮高硼氢含能材料的制备、表征及热性质[C]∥第七届化学推进剂全国学术会议论文集.北京:中国化学会,2015:386.
[19] 单自兴,单燚明,吴冰斌.不带结晶水的高氮高硼氢化合物:1. 十二氢十二硼酸双三氨基胍盐的制备及热性质[C]∥第六届化学推进剂全国学术会议论文集. 北京:中国化学会,2013:343-344.
[20] 单自兴,单燚明,吴冰斌.不带结晶水的高氮高硼氢化合物:1. 十二氢十二硼酸双乌洛托品盐的制备及热性质[C]∥第六届化学推进剂全国学术会议论文集.北京:中国化学会,2013: 345-346.
[21] 单自兴,吴冰斌.二十面体硼氢阴离子胍盐二水合物的晶体结构[C]∥第五届化学推进剂全国学术会议论文集.北京:中国化学会,2011.
[22] 赵德杰, 单自兴, 邓延卓,等. 通过复分解反应制备八三硼酸铵的方法: CN, 85100255[P]. 1985.
[23] 张国敏,单自兴,赵德杰,等. 八氢三硼酸钾及其衍生物的制备:CN, 85100254[P]. 1985.
[24] 邓延卓, 赵德杰, 单自兴,等. 通过离子交换制备八氢三硼酸铵:CN, 85100253[P]. 1985.
[25] 赵德杰, 单自兴, 何运凡,等. H3B-SMe2络合物硼氢化试剂的合成:CN, 85100257[P]. 1985.


[27] 赵德杰, 单自兴, 宋建阳,等. 硼氢阴离子铵盐的合成与性质[J]. 高等学校化学学报, 1983, 4(1): 93-99.
SHAN Zi-xing, ZHAO De-jie, SONG Jian-yang,et al. Studies on boron compounds. (IV).Syntheses and properties of borane anion ammonium salts[J].Chemical Journal of Chinese Universities,1983, 4(1): 93-99.
[28] Uspenskaya S I, Solntsev K A, Kuznetsov N T. The x-ray diffraction study of alkali metal dodecahydro-closo-dodeca- borates[J].Z Strukt Khim 1975, 16(3): 482-484.
[29] Shoham G, Schomburg D, Lipscomb W N.The crystal and molecular structure of [B12H12][NEt3H]2[J]. Cryst Struct Commun,1980, 9(2): 429-434.
[30] Solntsev K A, Kuznetsov N T, Rannev N V. Crystalstructure and some physicochemical properties of barium dodecahydro-closo-dodecaborate hexahydrate[J].Dokl Akad Nauk SSSR, 1975, 221(6): 1378-1380.
[31] Her J H, Zhou W, Stavila V, et al. Role of cation size on the structural behavior of the alkali-metal dodecahydro-closo-dodecaborates[J]. Journal of Physical Chemistry C, 2009, 113(26): 11187-11189.

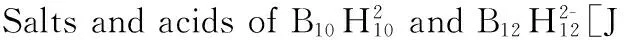
[34] Ionov S P, Kuznetsov N T. Heat of formation of boron hydride B60H60, an analog of fullerene C60. The structural-thermochemical model[J]. Koordinatsionnaya Khimiya, 1995, 21(12): 845-849.
[35] Miller H C, Miller N E, Muetterties E L. Chemistry of boranes. XX. Syntheses of polyhedral boranes[J]. Inorganic Chemistry,1964, 3: 1456.
[36] Agafanov A V, Solntsev K A, Kuznetsov N T. New reaction of boron hydride (BHx), condensation[J]. Koordinatsionnaya Khimiya, 1980, 6(2): 252-254.
[37]Mal′tseva N N, Shishkin Yu L, Golovanova A I, et al. Thermal decomposition of a complex compound of triethylenediamine with borane[J]. Zh Neorg Khim,1999, 44(4): 575-577.
[38] Titov L V, Eremin E R, Rosolovskii V Ya. Synthesis and study of the thermal decomposition of sodium octahydrotriborate-dioxane[J]. Zh Neorg Khim, 1982, 27: 891-895.
[39] Agafonov A V, Solntsev K A, Vinitskii D M, et al.Problem of the synthesis of lower polyhedral hydroborate anions[J].Zh Neorgan Khim,1982, 27(12): 2995-3006.
[40] Levicheva M D, Titov L V. Thermal decomposition of potassium octahydrotriborate[J]. Izv Akad Nauk SSSR Ser Khim,1984, (7): 1629-1632.
[41]Riccarda Caputo, Sebastiano Garroni, David Olid, et al. Can Na2[B12H12] be a decomposition product of NaBH4[J]. Physical Chemistry Chemical Physics,2010, 12: 15093-15100
[42] Huang Z, Eagles Mitch, Porter Spencer, et al., Thermolysis and solid state NMR studies of NaB3H8, NH3B3H7, and NH4B3H8[J]. Dalton Transaction,2013, 42: 701.


[45] Makhlouf J M, Hough W V, Hefferan G T.Practical synthesis for decahydrodecaborates[J]. Inorganic Chemistry, 1967,6(6): 1196-1198.
[46] Mongeot H, Bonnetot B, Atchekzai J, et al.(Et4N)2B10H10and (Et4N)2B12H12: synthesis from Et4NBH4, separation and purification[J].Bull Soc Chim Fr,1986(3): 385-289.
[47]Power D, Spalding T R.Some comments on the mechanism of the preparation of (Et4N)2B10H10by thermolysis of Et4NBH4[J]. Polyhedron, 1985, 4: 1329-1332.
[48] Bonnetot B, Frange B, Mongeot H, et al. Pyrolysis of tetramethyl-, tetrapropyl-, and tetrabutylammonium octahydrotriborates[J].Bull Soc Chim Fr, 1989(3): 632-634.

[50] Safronov A V, Jalisatgi S S,Hawthorne M F. Synthesis of amine boranes and polyhedral boranes: WO, 2015117123[P]. 2015.
[51] Lee M W, Hawthorne M F. Process and divice for the production of polyhedral boranes:US, 2014/378707[P]. 2014.
[52] Lee M W, Hawthorne M F.Process and device for the production of polyhedral boranes:WO, 2013115889[P]. 2013.
[53] Yang Yong, Hu Xiaolun, Wang Jianli, et al.Preparation method of bis(tetraethylammonium) dodecahydrododecaborate, and its application as ultrahigh-burning-rate propel lant:CN, 104557570[P]. 2015.
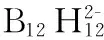
[55] Geis V, Guttsche K, Knapp C, et al.Synthesis and characterization of synthetically useful salts of the weakly-coordinating dianion [B12Cl12]2-[J].Dalton Transaction, 2009(15): 2687-2694.
[56] Miller H C, Miller N E, Muetterties E L. Synthesis of polyhedral boranes[J]. Journal of American Chemical Society, 1964:3885-3886.
[57] Miller N E.Boron amine and process for formation thereof:US, 3265737[P]. 1966.
[58] Miller N E.Polyhydrododecaborates:FR, 1352813[P]. 1964.
[59] E I. du Pont de Nemours & Co.Compounds containing boron and their preparation:BE, 637008[P]. 1963.
[60] Zhigach A F, Svitsyn R A, Sobolev E S, Starostina N A.Triethylammonium dodecahydrododecaborate(2-):SU, 622815[P]. 1978.
[61] Miller H C, Muetterties E L.Borane anions[J].Inorganic Synthesis, 1967, 10: 81-91.
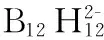
[63] Miller H C, Muetterties E L.Substituted dodecaborates:US, 3551120[P]. 1970.
[64] Miller H C, Muetterties E L.Preparation of polyhydro- polyborates:US, 3328134[P]. 1967.
[65] Miller H C, Muetterties E L. Dodecahydrododecaborate:US, 3169045[P]. 1965.
[66] Miller N E. Polyhydrododecaborates:FR,1352813[P].1964.
[67] Marshall M D, Hunt R M.Boron hydride studies, AEC Access. Nos, (UCRL-13240),[S.l.]:AEC,1996:21.
[68] DrinkardW C, Jr.Dodecahydrododecaborates:FR,1378107[P].1964.
[69] Procedure for the preparation of dodecahydrododecaborates:FR, 1358357[P]. 1964.
[70] E. I. du Pont de Nemours & Co. Polyhydropolyborates:FR, 1355516[P]. 1964.
[71] Knoth Walter H Jr. Derivatives of polyhedral dodeca- boranes:US, 3328422[P]. 1967.
[72]Titov L V, Zhemchugova L V, Petrovskii P V, Kuznetsov N T.A study of the reactions of tetrabutylammonium octahydrotriborate with pentaborane(9) in toluene[J].Zh Neorg Khim,2001, 46(5): 814-817.
[73] Adams R M, Siedle A R, Grant J. Convenient preparation of the dodecahydrododecaborate ion[J]. Inorganic Chemistry, 1964, 3(3): 461.
[74] Kuznetsov I T, Klimchuk G S. Dodecahydro-closo- dodecaborates of alkali metals[J].Zh Neorgan Khim,1971, 16(5): 1218-1223.
[75] Miller H C, Muetterties E L. Dodecahydrododecaborates:US, 3355261[P]. 1967.
[76] Cartolano A R, Teich C I, Ivanov S V, et al. Synthesisof alkali metal dodecaborates:EP, 2206680[P]. 2010.

[78] Volkov V V, Posnaya I S. Synthesis of dodecahydro- closo-dodecaborates(2-) by reaction of alkali metal tetrahydroborates with triethylaminoborane[J].Zh Neorg Khim,1979, 24(10): 2824-2826.
[79] Volkov V V, Posnaya I S. Synthesis of decahydrodeca- borate(2-) and dodecahydrododecaborate(2-) anions by the reaction of alkali metal tetrahydroborates with alkylamineboranes[J].Izv Akad Nauk SSSR Ser Khim,1979(4): 88-92.
[80] Banavali R M, Stephens R W, Yamamoto J H. Preparation of ammonium or metal dodecahydrododecaborates (MnB12H12):US, 20090118526[P]. 2009.
[81] Hough WV, Guibert C R, Hefferan G T. Dodecahydro- dodecaborate (2-) anions:US, 3961017[P]. 1976.
[82] Harzdorf C, Niederpruem H, Odenbach H. Preparation and analysis of icosahedral dodecahydrododecaborates[J].Z Natur, Teil B: Anorg Chem, Org Chem, Biochem, Biophys, Bio,1970, 25(1): 6-10.
[83] Volkov V V, Posnaya I S. Alkali metal dodecahydro- closo-dodecaborates(2-):SU, 588179[P]. 1978.
[84] Gruner B, Prochazka V, Subrt J, et al.Reaction of sodium hydride of high surface area with boron trichloride[J]. European Journal of Solid State Inorganic Chemistry, 1991, 28(3/4): 597-609.
[85] Ivanov S V, Casas B. Production of dodecahydrododecaborates: US, 20060286019[P]. 2006.
[86] Ivanov S V, Casas B. Method for producing dodeca- hydrododecaborates:US, 20060286020[P]. 2006.
[87] Saldin V I, Sukhovei V V, Buznik V M, et al.Synthesis of chemical compounds with dodecahydro-closo-dodecaborate anion:RU, 2378196[P]. 2010.
[88] Saldin V I, Sukhovei V V, Ignat′eva L N, et al. Improved method for separation and purification of dodecahydro-closo-dodecaborate anion[J].Khim Tekhnol, 2009 (1): 1-4.
[89] Saldin V I, Sukhovei V V, Buznik V M, et al.Synthesis of chemical compounds with dodecahydro-closo-dodecaborate anion:RU, 2378196[P]. 2010.
[90] Saldin V I, Sukhovei V V, Savchenko N N, et al. Thermal studies of potassium tetrahydroborate-sodium tetrafluoroborate mixtures[J].Russian Journal of Inorganic Chemistry, 2017, 62(4): 489-497.
[91] Saldin V I, Sukhovey V V, Savchenko N N, et al. Thermal studies of sodium tetrahydroborate-potassium tetrafluoroborate mixtures[J]. Russian Journal of Inorganic Chemistry, 2016, 61(5): 630-637.
[92] Saldin V I, Sukhovei V V, Buznik V M, et al.Method for preparing potassiumdodecahydro-closo-dodecaborate:RU, 2573679[P]. 2016.
[93] Solntsev K A, Saldin V I, Kuznetsov N T. Method for the preparation of potassium dodecahydro-closo-dodecaborate: RU, 1695619[P]. 1997.
[94] Saldin V I, Sukhovey V V, Ignatieva L N, et al.Isolation of the dodecahydro-closo-dodecaborate anion with chitosan from aqueous solutions[J].Theo Found Chem Engin, 2010, 44(4): 467-470.
[95] Saldin V I, Sukhovei VV, Ignat′eva L N, et al.Extraction of dodecahydro-closo-dodecaborate anion from aqueous solutions using chitosan[J]. Khim Tekhnol, 2009 (4): 193-196.
[96] Saldin V I, Buznik V M, Sukhovei V V.Method to manufacture of salts of dodecahydro-closo-dodecaboric acid:RU, 2323879[P]. 2008.
[97] Saldin V I, Ignatieva L N, Nikolenko Yu M.Reactions of dodecahydro-closo-dodecaboric acid with chitosan[J].Journal of Structural Chemistry, 2006, 47(1): 35-40.
[98] Saldin V I, Babii A P.Dodecahydroborate adducts of chitosan as high caloric, rapid burning material: RU, 2172745[P]. 2001.
[99] Saldin V I, Babii A P. Synthesis of polyhedral closo-complexes of hydroborates and chitosan as potential pyrotechnic components:RU, 2158221[P]. 2000.
[100] 单自兴, 杨荣杰, 吴冰斌,等. 一种制备K2B12H12的方法:CN, 201710044483.9[P]. 2017.
[101] Paetzold P, Bettinger H F, Volkov O. The anions [B24H23]3-and [B36H34]4-from the thermal protolysis of [B12H12]2-[J]. Z Anorg Allg Chem,2007, 633(5-6): 846-850.
[102] Bechtold R, Kaczmarczyk A. Coupled products from low temperature decomposition of hydronium dodecahydrododecaborate(2-) [J]. Journal of American Chemical Society, 1974, 96(18): 5953-5954.


[105] He L Q, Li H W, Akiba E. Thermal decomposition of anhydrous alkali metal dodecaborates M2B12H12(M = Li, Na, K)[J].Energies (Basel, Switzerland), 2015, 8(11): 12429-12438.
[106] Chen X N, Lingam H K, Huang Z G,et al. Desolvation and dehydrogenation of solvated magnesium salts of dodecahydrododecaborate: relationshipbetween structure and thermal decomposition [J]. Journal of Physical Chemistry Letter, 2010, 1(1): 201-204.
[107] Yisgedu T B, Huang Z G, Chen X N, et al. The structural characterization of (NH4)2B10H10andthermal decomposition studies of (NH4)2B10H10and(NH4)2B12H12[J].International Journal of Hydrogen Energy 2012, 37(5): 4267-4273.
[108] Ivanov S V, Malinina E A, Solntsev K A,et al.Protolytic conversion of ammonium and alkylammonium salts with polyhedral hydroborate anions [J].Koord Khim, 1992, 18(4): 394-400.

[110] Shackelford Scott A, Belletire John L,et al. Bridged heterocyclium dicationic closo-icosahedral perfluoro- borane, borane, and carborane salts via aqueous, open-Air benchtop synthesis[J].Organic Letter, 2010, 12(12): 2714-2717.
[111] Nedel′ko V V, Mikhailov Yu M, Chukanov N V, et al. The thermal decomposition of hexamethylenetetraammonium dodecahydro-closo-dodecaborate[J].Russian Journal of Physics Chemistry B, 2011, 5(1): 26-32.
[112] Saldin V , Ignat′eva L N, Nikolenko Yu M, et al. Thermal conversions of chitosanium dodecahydro-closo-dodecaborate[J]. Russian Journal of Physics Chemistry B,2010, 55(8): 1221-1227.

[114] Caputo R, Garroni S, Olid D,et al.Can Na2[B12H12] be a decomposition product of NaBH4[J].Physical Chemistry Chemical Physics, 2010, 12(45): 15093-15100.
[115] Shubina E S, Bakhmutova E V, Filin A M,et al.Dihydrogen bonding of decahydro-closo-decaborate(2-)anddodecahydro-closo-dodecaborate(2-) anions with proton donors:experimental and theoretical investigation[J].J. Organomet Chemistry, 2002, 657(1/2): 155-162.
[116] Chulkov A S, Zharkova L A, Ippolitov E G,et al. Determination of the heat of formation of decahydrodecaborate(2-) anddodecahydrododecaborate(2-) in aqueous solutions[J].Z Neorg Khim,1992, 37(9): 2061-2063.

[118] Saldin V I, Buznik V M, Mikhailov Yu M, et al. Thermodynamic properties of chitosan dodecahydro-closo-dodecaborate[J]. Russian Journal of Physics Chemistry A, 2014, 88(3): 377-380.

[120] 庞维强,胥会祥,廖林泉,等. 用DSC法研究高能硼氢燃烧剂与固体推进剂一些常见组分的相容性[J].固体火箭技术,2013, 36(1):67-72, 78.
PANG Wei-qiang, XU Hui-xiang, LIAO Lin-quan,et al. Compatibility of high energetic boron-hydrogenincendiary agent with some common components in solid propellant by means of DSC method[J]. Solid Rocket Technology, 2013, 36(1): 67-72, 78.
[121] 庞维强,薛云娜,樊学忠,等.十氢十硼酸双四乙铵的热行为及其与推进剂主要组分的相容性[J]. 含能材料, 2012,20(3): 280-285.
PANG Wei-qiang, XUE Yun-na, FAN Xue-zhong, et al. thermal behaviorof tetraethylammoniumdodecahydrododecaborates (BHN) and its compatibility with main components of propellant[J]. Energetic Materials, 2012, 20(3): 280-285.
[122] Schroeder M A.Borohydride catalysis of nitramine thermal decomposition and combustion. 3. Literature review and wrap-up discussionof possible chemical mechanisms ,AD-A224918[R]. Springfiled : NTIS,1990:30.
[123] Schroeder M A. Possible chemical mechanisms for boronhydride acceleration of nitramine decomposition: literature review[J].Journal of Propulsion Power, 1998, 14(6): 981- 990.
[124] Schroeder M A, Fifer R A, Kaste P J,et al. Thermal decomposition of RDX in the presence of added K2B12H12[J]. Journal of Propulsion Power, 2001, 17(2): 441-448.
[125] 王为强,薛云娜,杨建明,等.高燃速推进剂用硼氢化物的研究进展[J].含能材料,2012, 20(1): 132-136.
WANG Wei-qiang,XUE Yun-na, YANG Jian-ming, et al. Advancein studies on boron-hydrogen compounds for highburning rate propellant[J]. Energetic Materials, 2012,20 (1): 132-136.
[126] 唐松青,丁宏勋.硼氢化合物作为固体推进剂高燃速调节剂的最新进展[J].推进技术,1983(2):35-51.
TANG Song-qing, DING Hong-xun. New advance in boron-hydrogencompounds as high burning rate regulator for solidpropellant[J]. Journal of Propulsion Technology, 1983(2):35-51.
[127] EIdu Pont de Nemours & Co. Compounds containing boron and their preparation: BE, 637008[P]. 1963.
[128] Paris O E. Polyesters containing boron andnitrogen:US, 34415421[P]. 1969.
[129] Balthis J H, Jr. Polyhydropolyborates useful in blasting Caps: US, 3411890[P]. 1968.
[130] Trofimenko S. Photochemical preparation of substituted polyhedral borane anions:US, 3373098[P]. 1968.
[131] Miller N E. Nitrogen-containing boron compounds:FR, 1394178[P]. 1965.
[132] Shibata A, Miyake A, Ogawa T.Ignition and combustion characteristics of very high burning rate composite propellant charges[J].Kayaku Gakkaishi,2000, 61(2): 85-93.
[133] Johnson S, Barr L H, Smith B E. Nitroguanidine-based propellants and gas generators with potassiumborohydride catalysts for inflation of safety devices:WO, 9916731[P]. 1999.
[134] Mangum M G.Solid combustible propellant composi- tion:US, 20160096781[P]. 2016.
[135] Saldin V I, Tsvetnikov A K.Energetic composition containing boron and fluorine and method of its production:RU, 2610605[P]. 2017.
[136] Saldin V I, Sukhovei V V.Hemihydrate dodecahydro-klose-dodecaborate melamine and its method of preparation :RU, 2617778[P]. 2017.
[137] Saldin V I, Ignat′eva L N, Buznik V M. Preparation of chitosanium perchlorate for energy absorbing compositions with chitosanium dodecahydro-clozododecaborate:RU, 231577[P]. 2008.
[138] Saldin V I, Sukhovei V V. Adducts of chitosan dodecahydro-closo-dodecaborate with nitrates or perchlorates of magnesium or aluminum and method for production thereof:RU, 2596741[P]. 2016.
[139] Saldin V I, Sukhovei V V. Adducts of chitosan dodecahydro-closo-dodecaborate with salts-oxidizers of transition metals and method of their preparation:RU, 2562480[P]. 2015.
[140] Saldin V I, Sukhovei V V. Chitosan chromate synthesis for use as oxidant in energy-intensive orpyrotechnic compositions burningwithout emissions:RU, 2439081[P]. 2012.
[141] Saldin V I, Buznik V M, Mikhailov Yu M,et al. Adducts of chitosan dodecahydro-closo-dodecaborate with perchloric acid or ammonium perchlorate:RU, 2394840[P]. 2010.
[142] Saldin V I, Karpenk, M A. Production of urotropin dodecahydro-closo-dodecaborate:RU, 2282586[P]. 2006.
[143] Saldin V I, Sukhovei V V. Method for producing boron- and fluorine-containing high-energy composition:RU, 2479560[P]. 2013.
[144] Saldin V I, Sukhovei V V, Buznik V M,et al. Polyethyleneimine dodecahydro-closo-dodecaborate and a method of its production:US, 2556930[P]. 2015.
[145] Saldin V I.Preparation of intercalated compounds of graphite oxide with fluorine-substituted derivatives of dodecahydro-closo-dodecaboric acid and their salts:RU, 2371383[P]. 2009.
[146] Shackelford S A. Borane and carborane salts with heterocyclic amine cations for use in propellants, fueladditives, and hydrogenstorage material: US, 7521564[P]. 2009.
[147] Gabel D, Justus E. Low melting point ionic liquids containing dodecaboraneamine anions and process for preparation thereof:WO, 2006108862 [P]. 2006.
[148] Justus E,Rischka K,Wishart J F, et al. Trialkylammo-niododecaborates: anions for ionic liquids with potassium, lithium and protons as cations [J].European Journal of Chemistry,2008, 14: 1918-1923.
[149] Justus E,Vöge A, Gabel D. N-alkylation of ammonio-undecahydro-closo-dodecaborate(1-) for the preparation of anions for ionic liquids [J].European Journal of Inorganic Chemistry,2008:5245-5250.
[150] Kopytin A V, Zhizhin K Yu, Urusov Yu I,et al. Potentiometric sensors with membranes based on ionic liquid tetradecyltriethylammonio-closo-dodecaborate [J].Journal of Analytical Chemistry, 2012, 67(2): 168-171.
[151] Jenne C, Kirsch C. Alkoxy substituted halogenated closo-dodecaborates as anions for ionic liquids[J].Dalton Transaction, 2015, 44(29): 13119-13124.
[152] Li S Q, Gao H X, Shreeve J M. Borohydride ionicliquids and borane/ionic-liquid solutions as hypergolic fuels with superior low ignition-delay times, Angew[J]. Chem Int Ed, 2014, 53: 2969-2972.
[153] Peymann T, Lork E, Schmidt M, et al. N-alkylation of ammine-undecahydro-closo-dodecaborate(1-) [J]. Chem Ber/ Recueil ,1997, 130: 795-799.
[154] McCrary P D, Rogers R D. Hypergolic salts with borane cluster anions:US, 20140373984[P]. 2014.
[155] King R Bruce.Strained configurations in three-dimensional analogues of Kekule-type structures for deltahedral boranes[J].Coll Czech Chem Commun,2002, 67(6): 751-768.
[156] Aihara J.Aromatic character of deltahedral borane dianions revisited[J].Inorganic Chemistry, 2001, 40(19): 5042-5044.
[157] Solntsev K A, Mebel A M, Votinova N A,et al.Dodecahydrododecaborate(2-) polyhedral anion as a spatialaromatic system[J]. Koordinatsionnaya Khimiya, 1992, 18(4): 340-364.
[158] Koblova Elena A, Saldin Vitaly I.Quantum chemical calculations of anion complex [B12Hx(CF3)12-x]2-, x = 9-12(M) [C]∥AIP Conference Proceedings.[S.l.]:AIP,2016:1790.

SHAN Zi-xing1,2, SHENG Li-li1, YANG Rong-jie1
(1. School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081,China;2. College of Chemistry and Molecular Sciences,Wuhan University, Wuhan 430072,China)
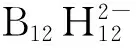
10.14077/j.issn.1007-7812.2017.03.001
2016-10-17;
2017-02-13
单自兴(1945-),男,教授,博导,研究方向:元素有机化学、硼化学与合成化学。E-mail: zxshan@whu.edu.cn
杨荣杰(1963-),男,教授,博导,研究方向:含能材料、阻燃材料、高分子及功能材料。E-mail: yrj@bit.edu.cn
TJ55
A
1007-7812(2017)03-0001-16
