儿童寡肌病性皮肌炎合并肺间质病变2例报告
2017-06-24唐韩云李晓忠
江 璐 唐韩云 闵 月 李晓忠
苏州大学附属儿童医院(江苏苏州 215000)
儿童寡肌病性皮肌炎合并肺间质病变2例报告
江 璐 唐韩云 闵 月 李晓忠
苏州大学附属儿童医院(江苏苏州 215000)
目的探讨托珠单抗对儿童寡肌病性皮肌炎合并肺间质病变的疗效。方法回顾分析2例寡肌病性皮肌炎合并肺间质病变患儿的临床特点及治疗与预后,并复习相关文献。结果男女各1例,女性患儿10岁11个月、男性患儿8岁5个月,起病时均有气促,但无肌肉损害的临床表现;均有典型皮疹,但肌力及肌张力正常。实验室检查以血清铁蛋白、乳酸脱氢酶、谷氨酸氨基转移酶及天冬氨酸氨基转移酶升高为主,肌酸肌酶除首次入院时稍高,复查始终在正常范围。高分辨CT示肺间质病变。临床诊断为寡肌病性皮肌炎合并肺间质病变。女性患儿经大剂量激素、环磷酰胺、环孢素、吡菲尼酮及丙种球蛋白等治疗无效死亡。男性患儿在常规激素治疗的基础上,加用托珠单抗(240 mg/次,2次),病情稳定,随访复查各指标均在正常范围内。结论儿童寡肌病性皮肌炎临床表现以及实验室检查结果不典型,死亡发生率高。联合托珠单抗治疗有效。
皮肌炎; 寡肌病性; 肺间质病变; 托珠单抗
儿童寡肌病性皮肌炎(hypomyopathic dermatomyositis,HDM)是少见的自身免疫性疾病,合并肺间质病变(interstitial lung disease,ILD)死亡率高。现报告2例儿童HDM并发肺间质病变病例,并回顾相关文献,以期加深对此病的认识。
1 临床资料
例1,女,10岁11个月,因“发现面部红斑1个月”入院。入院体格检查:神志清,颜面部紫红水肿、色素性融合性红斑疹,双手指关节、肘部可见紫红色鳞屑性丘疹(Gottron征),肌力Ⅴ级、肌张力正常,心、肺、腹未见异常。实验室检查:谷氨酸氨基转移酶(ALT)448.3 U/L、天冬氨酸氨基转移酶(AST)629.9 U/L、肌酸激酶(CK)229.2 U/L、乳酸脱氢酶(LDH)697 U/ L;血清铁蛋白(SF)1 142.5 ng/mL;肌酸激酶同工酶(CK-MB)正常;抗核抗体阴性,抗增殖细胞核抗原(PCNA)可疑,抗Ro-52阳性,抗干燥综合征相关抗原A(SSA)可疑,其余均为阴性。骨髓穿刺示轻度感染表现。肺高分辨CT(HRCT)示两肺多发斑片、条索状高密度影,部分呈间质性改变。肺功能检查示限制性通气功能障碍。膝关节磁共振成像(MRI)示双侧股骨后方软组织少许条状短时间反转恢复(short time inversion recovery,STIR)序列高信号。肌电图可见数处正锐波及纤颤波出现,运动单位电位时限明显缩短,提示为肌源性损害。根据患儿典型皮疹但无肌肉损害的临床表现,入院时CK轻度增高,结合肌电图、MRI及肺HRCT,诊断为HDM合并ILD。入院后予环磷酰胺8~12 mg/(kg·d),丙种球蛋白1 g/(kg·d)×2 d,甲基泼尼松龙2 mg/(kg·d)及护肝等治疗,1周后复查SF、ALT、AST、LDH较前降低,CK正常。第3周复查肺HRCT示两肺多发斑片状、条索状高密度影较前无明显缓解,予甲基泼尼松龙15~30 mg/(kg·d)冲击治疗3 d,后改泼尼松口服。第4周患儿气促加重,肺部闻及湿啰音,第2次给予丙种球蛋白支持及抗感染治疗,同时口服环孢素、吡非尼酮、乙酰半胱氨酸等治疗,疗效欠佳。第5周患儿突发呼吸困难,肺HRCT示两侧胸膜腔、纵隔、颈部皮下气肿,胸片示左侧胸腔积液,复查SF、ALT及AST较前升高,行胸腔闭式引流排气排液,两周后复查胸部HRCT示纵隔、肺间质、 颈胸部皮下气肿,左侧病灶较前进展,右侧病灶同前, 左肺膨胀不全,左侧少量气胸可疑。第7周行皮下气肿切开排气,继续口服泼尼松、环孢素、吡菲尼酮,间断鼻导管低流量吸氧等治疗。第12周患儿病情无改善,家属要求自动出院。出院1周后电话回访,患儿死亡。
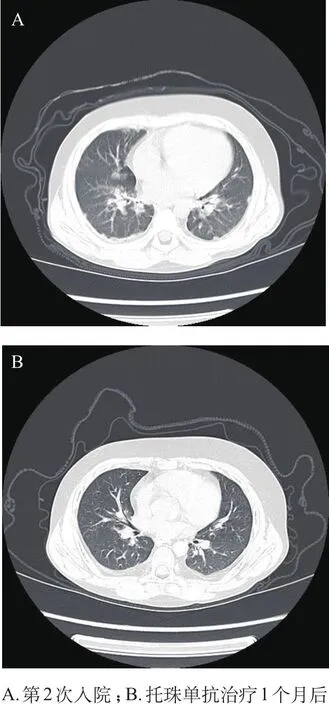
图1 例2患儿肺部HRCT
例2,男,8岁5个月,因“发热,眶周水肿皮疹伴关节肿2周”第一次入院。入院体格检查:眶周水肿伴颜面色素性融合性红斑疹,指关节见Gottron征,伴双手指及膝关节肿胀,无疼痛,无明显活动受限,肌力Ⅴ级,肌张力正常,心、肺、腹未见异常。入院时查高敏C反应蛋白(CRP)77.06 mg/L、CK 233.1 U/L、ALT正常、AST 75.1 U/L、LDH 811 U/L,SF 461.5 ng/mL,自身抗体筛查均阴性。骨髓穿刺示呈感染表现。左腿腘窝皮肤活检病理示真皮小血管周围散在单个核细胞浸润,提示真皮小血管周围炎,免疫荧光标记检查未见明显阳性荧光沉积。上肢MRI示左侧尺桡骨、左肩皮下、双侧股骨内侧脂肪层短时间反转恢复序列(STIR)信号不均匀。肺HRCT示肺纹理增生。肌电图及肺功能未做。根据患儿典型皮疹,肌力、肌张力正常,实验室检查以SF、LDH升高为主,CK入院时稍高,结合皮肤活检结果诊断为HDM。予常规抗炎抗免疫药物治疗后,病情稳定后出院。5个月后,患儿因发热、气促再次入院,入院时精神差,颜面部及指关节皮疹较前消褪,皮肤粗糙,肌力及肌张力正常。实验室检查:CRP 53.63 mg/L;SF>1 650 ng/mL,AST 116.8 U/ L,LDH 1 131.5 U/L;CK、CK-MB、肌钙蛋白均正常,肌红蛋白稍低。肺HRCT示两肺散在小结节、条索影,两侧胸膜增厚(图1A),提示ILD 。临床诊断为HDM合并ILD。入院后予甲基泼尼松龙2 mg/(kg· d)、丙种球蛋白1 g/(kg·d)×2 d以及抗感染、护肝治疗,并口服羟氯喹、吗替麦考酚酯、他克莫司、乙酰半胱氨酸,后激素改甲基泼尼松龙片口服。患儿发热、气急等症状缓解,复查SF> 1 650 ng/mL,考虑疾病仍处于活动状态,加用托珠单抗240 mg,3天后复查SF 800.6 ng/ mL,病情稳定后出院。两周后,患儿入院行第2次托珠单抗治疗,复查SF 179.8 ng/mL、肝功能正常,肺HRCT较前好转明显(图1B),无不适出院。现患儿定期门诊随访,SF等各项指标均在正常范围内,病情得到较好地控制。
2 讨论
幼年特发性炎性肌病( juvenile idiopathic inflammatory myopathies,JIIM)是一类以骨骼肌慢性炎症、皮疹及其他系统表现为特征的全身性自身免疫性疾病[1]。根据临床特点[2]分为幼年皮肌炎(juvenile dermatomyositis,JDM)、幼年多发性肌炎(juvenile polymyositis,JPM)以及寡肌病性皮肌炎(hypomyopathic dermatomyositis,HDM)和无肌病性皮肌炎(amyopathic dermatomyositis,ADM)。Sontheimer[3]提出,临床无肌病性皮肌炎(clinically amyopathic dermatomyositis,CADM)是ADM和HDM的总称。本组2例患儿都有典型皮肌炎皮疹,但肌力、肌张力均正常,实验室检查指标以SF、LDH、ALT及AST升高为主,CK除首次入院时稍高,复查始终在正常范围,此外例1患儿肌电图结果、例2患儿皮肤活检结果也都支持CADM诊断。有文献报道,CADM和DM患者的皮肤病理学结果相同,与两者相关的系统性疾病包括恶性肿瘤和肺部疾病[4]。
由血清学结果发现,在肌炎特异性自身抗体(myositis-specific-autoantibodies,MSAs)中,抗p155/140抗体、抗MJ抗体与JDM相关,前者与光敏性皮疹有关,后者与肌肉、关节病变有关[5];抗Jo-1抗体与肺间质病变、关节炎、发热以及雷诺现象密切相关[5];抗Mi-2抗体与JDM以及典型皮疹特征相关;在CADM合并急进性ILD患者中,抗CADM-140抗体/抗MAD5抗体阳性率高[6,7]。而肌炎相关自身抗体(myositisassociated-autoantibodies,MAAs)如抗U1RNP、抗Ro、抗PM-Scl及抗Ku抗体在15%的JIIM患者中发现[8],在其他自身免疫疾病患者中也能检测到。
CADM患者较DM患者易合并ILD[9]。ILD 是影响预后的重要因素,也是引起死亡的首位原因,合并快速进展型ILD的患者有60%在2个月内死亡[10]。肺HRCT的检查结果与开胸肺活检的组织学基本一致[11],肺HRCT诊断的特异性高达90%[12]。CRP及SF水平与外周血白介素6(IL-6)水平呈正相关,且与疾病活动性评估指标的一般情况及肺部表现相关[13]。Gono等[14]报道,并发急性或亚急性间质性肺病的患者有着非常高的SF水平,与无间质性肺病的患者相比有显著性差异。因此肺HRCT及SF对诊断及监测病情有重要的临床价值。
治疗上,主要采用激素和免疫抑制剂,对于危重症患儿可予大剂量激素冲击联合丙种球蛋白支持治疗,但当合并ILD时,激素冲击治疗疗效欠佳,有加重感染的可能。例2患儿急性发作被控制后,因SF一直较高,使用托珠单抗将SF水平降低,后期随访中各指标均在正常范围内。托珠单抗作为IL-6受体拮抗剂是一种新型生物制剂。有文献报道,IL-6在DM及PM患者中均升高,且在DM患者中更高[15]。DM患者局部炎症部位的组织可检测到升高的IL-6 mRNA,当疾病活动时IL-6也在这些部位聚集[16,17],经托珠单抗治疗后,DM患者的血清IL-6下降,较PM患者明显[15]。 Hiroyuki等[18]认为,可以用IL-6和IL-18水平来预测JDM相关性嗜血细胞综合征(JDM-associated macrophage activation syndrome,JDM-MAS)和ILD的疾病活动性。国外有报道称,托珠单抗治疗PM小鼠[19]和治疗成人难治性PM[20]有效。Masahiro等[21]也报道过1例应用托珠单抗治疗重叠综合征患儿,认为托珠单抗对难治性DM有效。目前托珠单抗已经进入我国市场,但治疗HDM属于超说明书用药。通过分析这2例病例,认为也许可以通过托珠单抗联合常规抗炎抗免疫药物来治疗此类患儿,但由于其长期的临床疗效和不良反应有待进一步观察,未来需要设计更多合理的临床对照试验来加以验证。
[1]Feldman BM, Rider LG, Reed AM, et al. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood [J]. Lancet, 2008, 371(9631): 2201-2212.
[2]Pagnini I, Vitale A, Selmi C, et al. Idiopathic inflammatory myopathies: an update on classification and treatment with special focus on Juvenile forms [J]. Clin Rev Allergy Immunol, 2015, 52(1): 34-44.
[3]Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis as a distinctive subset within the idiopathic in fl ammatory dermatomyopathies spectrum of clinical illness [J]. J Am Acad Dermatol, 2002, 46(4): 626-636.
[4]Ghazi E, Sontheimer RD, Werth VP. The importance of including amyopathic dermatomyositis in the idiopathic inflammatory myositis spectrum [J]. Clin Exp Rheumatol, 2013, 31(1): 128-134.
[5]Rider LG, Shah M, Mamyrova G, et al. The myositis autoantibody phenotypes of the Juvenile idiopathic inflammatory myopathies [J]. Medicine, 2013, 92(4): 223-243.
[6]Sakurai N, Nagai K, Tsutsumi H, et al. Anti-CADM-140antibody-positive juvenile dermatomyositis with rapidly progressive interstitial lung disease and cardiac involvement [J]. J Rheumatol, 2011, 38(5): 963-964.
[7]Chen Z, Cao M, Plana MN, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis [J]. Arthritis Care Res (Hoboken), 2013, 65(8): 1316-1324.
[8]Wedderburn LR, McHugh NJ, Chinoy H, et al. HLA class II haplotype and autoantibody associations in children with juvenile dermatomyositis and juvenile dermatomyositisscleroderma overlap [J]. Rheumatology, 2007, 46(12): 1786-1791.
[9]Bailey EE, Fiorentino DF. Amyopathic dermatomyositis: de fi nitions, diagnosis, and management [J]. Curr Rheumatol Rep, 2014, 16(12): 465.
[10]Ji SY, Zeng FQ, Guo Q, et al. Predictive factors and unfavourable prognostic factors of interstitial lung disease in patients with polymyositis or dermatomyositis: a retrospective study [J]. Chin Med J (Engl), 2010, 123(5): 517-522.
[11]Cortet B, Flipo RM, Remy Jardin M, et al. Use of high resolution computed tomography of the lungs in patients with rheumatoid arthritis. [J]. Ann Rheum Dis, 1995, 54(10): 815-819.
[12]Raghu G, Mageto YN, Lockhart D, et al. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fi brosis and other interstitial lung disease: A prospective study [J]. Chest, 1999, 116(5): 1168-1174.
[13]杨闵, 梁燕, 蔡娅菲, 等. 皮肌炎患者外周血急性时相反应物水平与IL-6、疾病活动度的相关性分析 [J]. 四川大学学报(医学版), 2013, 44 (5): 814-817.
[14]Gono T, Kawaguchi Y, Hara M, et al. Increased ferritin predicts development and severity of acute interstitial lung disease as a complication of dermatomyositis [J]. Rheumatology (Oxford), 2010, 49(7): 1354-1360.
[15]Shimojima Y, Ishii W, Matsuda M, et al. Phenotypes of peripheral blood lymphocytes and cytokine expression in polymyositis and dermatomyositis before treatment and after clinical remission [J]. Clin Med Insights Arthritis Musculoskelet Disord, 2012, 5: 77-87.
[16]Lepidi H, Frances V, Figarella-Branger D, et al. Local expression of cytokines in idiopathic inflammatory myopathies [J]. Neuropathol Appl Neurobiol, 1998, 24(1): 73-79.
[17]Bilgic H, Ytterberg SR, Amin S, et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis [J]. Arthritis Rheum, 2009, 60(11): 3436-3446.
[18]Wakiguchi H, Hasegawa S, Hirano R, et al. Successful control of juvenile dermatomyositis associated macrophage activation syndrome and interstitial pneumonia: distinct kinetics of interleukin-6 and -18 levels [J]. Pediatr Rheumatol Online J, 2015, 13: 49.
[19]Okiyama N, Sugihara T, Iwakura Y, et al. Therapeutic effects of interleukin-6 blockade in a murine model of polymyositis that does not require interleukin-17A [J]. Arthritis Rheum, 2009, 60(8): 2505-2512.
[20]Narazaki M, Hagihara K, Shima Y, et al. Therapeutic effect of tocilizumab on two patients with polymyositis [J]. Rheumatology (Oxford), 2011, 50(7): 1344-1346.
[21]Kondo M, Murakawa Y, Matsumura T, et al. A case of overlap syndrome successfully treated with tocilizumab: a hopeful treatment strategy for refractory dermatomyositis? [J]. Rheumatology (Oxford), 2014, 53(10): 1907-1908.
(本文编辑:邹 强)
《临床儿科杂志》启用科技期刊学术不端文献检测系统
学术不端行为是指违反学术规范、学术道德的行为,国际上一般用来指捏造数据(fabrication)、篡改数据(falsi fi cation)和剽窃(plagiarism)3种行为。为了提高来稿质量,防止抄袭、伪造、剽窃、一稿多投等学术不端行为的发生,本刊已启用“科技期刊学术不端文献检测系统”,对检测出有严重不端行为的稿件,编辑部将一律退稿。
该系统由中国知识资源总库所收录的数千万条中文文献、数百万条英文文献支持。系统将检测的文章与数据库内的文献进行比对,不仅可以检测文献总的文字复制比例,还可详细列出检测文献中每一段雷同文字的详细出处,并准确定位每一段文字的具体位置,能够给出一个完整的比对报告。因此,希望广大作者在撰写论文时,一定要本着实事求是的科学精神,自觉抵制学术不端行为,引用他人的研究成果务必标引参考文献。本刊希望借助此工具,与广大专家、读者、作者一起,共同遏制学术不端之风,构建公平公正的学术交流平台,营造健康的学术环境。
Childhood hypomyopathic dermatomyositis combined with interstitial lung disease: two cases report
JIANG Lu, TANG Hanyun, MIN Yue, LI Xiaozhong
(Department of Nephrology and Rheumatology, Children’s Hospital of Soochow University, Suzhou 215000, Jiangsu, China)
ObjectiveTo discusses the effectiveness of tocilizumab in the treatment of hypomyopathic dermatomysositis (HDM) combined with interstitial lung disease (ILD) in children.MethodsThe clinical characteristic, treatment, and prognosis of HDM combined with ILD were analyzed in 2 patients. The related literatures were reviewed.ResultsBoth ten-year-old girl and 8-year-old boy had shortness of breath after activities, but had no clinical manifestations of muscle damage; both of them had typical rash, but had nornal muscle strength and muscular tension. Laboratory tests showed the elevation of serum ferritin, lactate dehydrogenase, glutamate aminotransferase, and aspartate aminotransferase. Creatine kinase slightly increased in the initial test, and then was in the normal range in the following tests. The high resolution computed tomography showed that pulmonary interstitial lesions. HDM combined ILD was diagnosed clinically. The girl died after treatment with high-dose hormones, cyclophosphamide, cyclosporine, pirfenidone, and gamma globulin failed. The boy was stabled after conventional hormone treatment plus tocilizumab (240 mg twice). His laboratory indicators were in the normal range in the follow-up.ConclusionsThe clinical manifestations and laboratory indicators aren't typical in childhood HDM. The mortality is high. Combined with tocilizumab treatment is effective in one case.
dermatomyositis; hypomyopathic; interstitiallung lung disease; tocilizumab
10.3969/j.issn.1000-3606.2017.06.013
2016-11-28)
国家自然科学基金资助项目(No.81370787);江苏省临床医学科技专项——新型临床诊疗技术攻关(No.SBL2014030237)
李晓忠 电子信箱:xiaozhonglicn@yeah.net
