Ionic liquid based ultrasonication-assisted extraction of essential oil from the leaves of Persicariaminor and conductor-like screening model for realistic solvents study
2017-06-15HabibULLAHCeciliaWILFREDMaizatulSHAHARUN
Habib ULLAH, Cecilia D WILFRED, Maizatul S SHAHARUN
(1. Center of Research in Ionic Liquids Universiti Teknologi PETRONAS, Seri Iskandar 32610, Malaysia; 2. Fundamental and Applied Science Department, Universiti Teknologi PETRONAS, Seri Iskandar 32610, Malaysia)
Article
Ionic liquid based ultrasonication-assisted extraction of essential oil from the leaves ofPersicariaminorand conductor-like screening model for realistic solvents study
Habib ULLAH1*, Cecilia D WILFRED1*, Maizatul S SHAHARUN2
(1.CenterofResearchinIonicLiquidsUniversitiTeknologiPETRONAS,SeriIskandar32610,Malaysia; 2.FundamentalandAppliedScienceDepartment,UniversitiTeknologiPETRONAS,SeriIskandar32610,Malaysia)
Ionic liquids (ILs) based ultrasonic-assisted extract has been applied for the extraction of essential oil fromPersicariaminorleaves. The effects of temperature, sonication time, and particle size of the plant material on the yield of essential oil were investigated. Among the different ILs employed, 1-ethyl-3-methylimidazolium acetate was the most effective, providing a 9.55% yield of the essential oil under optimum conditions (70 ℃, 25 min, IL∶hexane ratio of 7∶10 (v/v), particle size 60-80 mesh). The performance of 1-ethyl-3-methylimidazolium acetate in the extraction was attributed to its low viscosity and ability to disintegrate the structural matrix of the plant material. The ability of 1-ethyl-3-methylimidazolium acetate was also confirmed using the conductor like-screening model for realistic solvents. This research proves that ILs can be used to extract essential oils from lignocellulosic biomass.
gas chromatography mass spectrometry (GC-MS); ionic liquid (IL); ultrasonication; optimization;Persicariaminor; essential oil
The extraction of essential oils from aromatic plants provides a unique source of bioactive compounds, which can be used in antibacterial, antifungal, antiviral, and antiparasitic applications [1,2]. Essential oils are also used in the pharmaceutical, sanitary, cosmetic, food and agricultu-ral industries as fragrances. Therefore, effective extraction of essential oils from plant materials is important [3,4].
Extraction of essential oils from plants has been performed by traditional hydrodistillation, heat reflux, and Soxhlet extraction [5,6]. However, these methods have some limitations such as low extraction capacities, long extraction times, and the need for high temperatures. Moreover, during hydrodistillation, hydrolytic degradation of thermosensitive compounds in the essential oil can occur [7]. Consequently, scientists are searching for an alternative extraction method to greatly enhance the extraction efficiency and also retain the bioactivity of the extract. Recently, ultrasonication has emerged as a suitable energy source for various pre-treatment processes [8]. Ultrasonication generates cavitation, and provides agitation and heating, which are all beneficial for obtained high extraction efficiency.
Various organic solvents can be used for extraction. However, some solvents have low extraction capacities, are volatile, and cause environmental pollution. As an alternative to traditional solvents, ionic liquids (ILs) have emerged as economical and environment-friendly solvents for the dissolution of different types of biomass, including cellulose [9-11], chitin [12], starch [13], and protein [14]. ILs can be used as solvents for the separation and extraction of essential oils from plant materials. ILs has high extraction capability in liquid-liquid extraction and liquid-solid extraction increase the extraction yield of compounds from plant materials in comparison with common solvents such as water, acetone, and hexane have limited ability in this regard [15]. ILs has the ability to absorb ultrasonic energy and not degrade [16]. Therefore, they could be applied to the ultrasonic-assisted extraction of compounds from solid samples.
Persicariaminor(P.minor, Malaysian name kesum) belongs to family polygonaceae and is an important aromatic plant in Malaysia. It is widely used in flavoring agent, food stuff and traditionally used for body aches, digestive disorders, dandruff and perfume [17].
In this work, essential oil from the leaves ofP.minorwas obtained using ultrasonication and IL treatment followed by solvent extraction. The effect of the IL on disintegration of the three-dimensional structure of the plant material, and the effectiveness of extraction of the essential oil from the plant matrix were evaluated. Three ILs, 1-butyl-3-methylimidazolium bromide ([C4MIM][Br]), 1-butyl-3-methylimidazolium hydrogen sulfate ([C4MIM][HSO4]), and 1-ethyl-3-methylimidazolium acetate ([C2MIM][Ac]), were used along with a co-solvent (water or hexane) for the extraction. The process was optimized with respect to the type of IL, extraction tempe-rature, pretreatment time, particle size of the plant material, and concentration of the IL. The kinetics for the extraction process was evaluated. The essential oil was analyzed by Fourier transform infrared (FT-IR) spectroscopy and gas chromatography mass spectrometry (GC-MS). A conductor-like screening model for realistic solvents (combination of quantum chemistry and statistical thermodynamics, COSMO-RS) simulation was performed to correlate the experimental results with the extraction capabilities of the ILs.
1 Experimental
1.1 Materials and methods
P.minorwas purchased from a local market in Tronoh, Malaysia. The leaves were separated from the plant, cleaned with water, and dried in an oven at 45 ℃ for 72 h. Then, the leaves were ground to a powder and sieved to a particle size range of 20-100 mesh. The ILs [C4MIM][HSO4], [C4MIM][Br], and [C2MIM][Ac],n-alkanes (C9-C24) and GC-MS standards were purchased from MerckTM(Darmstadt, Germany). Ultrapure water prepared using a purifier from Merck Millipore (Billerica, MA) was used in this study.
1.2 Extraction procedures
Five different extraction methods, hydrodistillation, ultrasonication-assisted aqueous IL extraction, stirring-assisted hexane extraction, ultrasonication-assisted hexane extraction, and ultrasonication-assisted IL pretreatment followed by hexane extraction, were used in this study and described below.
1.2.1 Hydrodistillation
The sample (4 g) was mixed with deionized water (100 mL) for pretreatment and refluxed at 100 ℃ for 100 min, and the condensate was collected in a clevenger apparatus. The oil layer was separated from the aqueous layer and dry CaCl2was added to remove any traces of water from the essential oil mixture.
1.2.2 Ultrasonication-assisted aqueous IL extraction
The sample (4 g) was mixed with 3 mL of deionized water and 7 mL of one of the ILs in a flask connected to a clevenger apparatus, and sonicated in an ultrasonic bath for 30, 40, 50, or 60 min at 100 ℃. The oil layer was separated from the aqueous IL solution in the clevenger apparatus, and then dried over CaCl2.
1.2.3 Stirring-assisted hexane extraction
The sample (4 g) was mixed with 30 mL of hexane, stirred, and refluxed at 63 ℃ for 100 min. The undissolved plant material was removed by filtration through a filter paper (Whatman No 0.45 mm, GE Healthcare, USA). Hexane was removed from the filtrate using a rotary evaporator, and the essential oil was retained.
1.2.4 Ultrasonication-assisted hexane extraction
The sample (4 g) was mixed with hexane in a flask connected to a clevenger apparatus. The flask was placed in an ultrasonic bath and heated at 63 ℃ until no more essential oil was obtained in the clevenger apparatus. Hexane was removed from the essential oil using a rotary evaporator.
1.2.5 Ultrasonication-assisted IL pretreatment followed by hexane extraction
This extraction procedure consisted of two steps. In the first step, the sample (4 g) and an IL (7 mL) were mixed in a flask (250 mL) connected to a clevenger apparatus and condenser. The flask was sonicated in an ultrasonic bath (Model D-78224 Singen/HTW, Elma Transonic Digital, yokohama, Japan) for 10 to 40 min at a set temperature between 50-100 ℃. In the second step, the dark slurry produced from the first step was mixed with 10 mL of hexane (co-solvent) in the reaction flask with the clevenger apparatus attached. The flask was ultrasonicated again at a set temperature until no more essential oil was obtained. The hexane was removed using a rotary evaporator. The essential oil was collected in vials, stored at 4 ℃ and characterized using FT-IR spectroscopy and GC-MS. The setup for extraction of the essential oil is shown in Fig. 1.
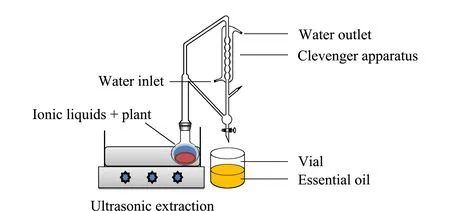
Fig. 1 Setup for extraction of essential oil
To recover the IL, 200 mL of methanol was added to dissolve the IL and precipitate the residue. After stirring, the solution was filtered through a nylon membrane (0.02 mm), and then the methanol was evaporated from this solution by rotary evaporator and ILs is recovered.
After extraction, the essential oil was collected and the yield was calculated using the following equation.
(1)
wheremoilandmsamplerepresent the mass of essential oil and sample, respectively.
1.3 Characterization
Ultrasonication-assisted IL pretreatment followed by hexane extraction was performed in the ultrasonic bath (Model D-78224 singen/HTW) with a frequency of 40 kHz. The power, time, and temperature could all be controlled. FT-IR spectra were recorded between 400-4 000 cm-1using a Perkin Elmer Frontier spectrometer. GC-MS was performed with a GC-MS Clarus 600 (Perkin Elmer, USA) equipped with a DB-5MS non-polar column (30 m×0.25 mm i. d., 0.25 μm film thickness). The injection temperature was 220 ℃, and the detector temperature was 280 ℃. The column temperature was held at 50 ℃ for 3 min, increased to 100 ℃ at 20 ℃/min and held for 3 min, helium as carrier gas at constant flow (1.5 mL/min) the injection volume was 1 μL, and the sample was injected with a split ratio of 1∶40. The system was equipped with a quadrupole MS operated in scan mode. Total ion current chroma-tograms were recorded.
The retention indices of compounds in the essential oil were determined according to the standard method of Kováts retention indices to support the identification of the compounds.
Iri=100n+(m-n)(tri-trn)/(trm-trn)
(2)
whereIriis the retention index (RI) of consti-tuentiof the essential oil that is being analyzed;nis the number of carbons in the alkane that elutes beforei;mis the number of carbons in the alkane that elutes afteri; andtri,trn, andtrmare retention times ofi, the alkane that elutes beforei, and the alkane that elutes afteri, respectively.
1.4 COSMO-RS approach
The COSMO-RS approach was first established by Klamt and co-workers [18]. These techniques use a quantum chemical approach for solvent screening based on a molecular level. The COSMO-RS can be used to define and calculate thermodynamic properties without experimental data [19-22]. COSMO-RS is a two-step process, (1) structure development of chemical species, and (2) thermodynamic property prediction. In the first step, the COSMO is applied to simulate the conductor environment for the molecule of interest, and the molecule is entrenched into an effective conductor. In this situation, the molecule has a polarized charge density on the line between the compound and the conductor, that is, on the molecule surface. During quantum calculations, the self-constancy algorithm of the solute molecule is correlated with its dynamically optimum state in the conductor with respect to electron density and geometry. In the second step, a statistical thermodynamic calculation is performed. The polarized charge density is used for quantification of the collaborative energy of pair-wise interacting surfaces with regard to the most important molecular interaction methods, that is, hydrogen bonding and electrostatic interactions. The three-dimensional scattering of the polarized charges on the surface of each compound is used to construct a sigma profile (σ-profile). Theσ-profile provides detailed information about compound polarity. The chemical potential of the surface (σ-profile) is calculated from thermodynamics of the molecular interactions. Theσ-profile and sigma potential (σ-potential) show the sites of interaction of the solvent with the surface polarity of the compound. Theσ-profile andσ-potential can be used to understand the interactions between compound(s) and solvent in a mixture.
2 Results and Discussion
2.1 Effect of the treatment method
The efficiencies of hydrodistillation, stirring-assisted hexane extraction, and ultrasonication-assisted hexane extraction for extraction of the essential oil were evaluated. Hydrodistillation was less efficient (essential oil yield 3.37%) than stirring-assisted hexane extraction (essential oil yield 4.0%) or ultrasonication-assisted hexane extraction (essential oil yield 4.33%). The fact that ultrasonication-assisted hexane extraction was more effective than stirring-assisted hexane extraction suggests that stirring is not as effective as ultrasonication for disintegration of the plant matrix. This could be because ultrasonication provides more energy to the system than stirring, and this allows for extraction of more of the essential oil. The effect of ultrasonication treatment can also be attributed to the complete mixing achieved with ultrasonic waves. It is well known that ultrasonication provides energy at the molecular level, and this enhances mixing, dissolution, and interaction of the molecules. Similar effects have been reported in biomass dissolution, mixing of nanomaterial, and synthetic chemistry [23].
2.2 Effect of the solvent and IL
To study the effect of an IL as a solvent for extraction of the essential oil, the IL [C2MIM][Ac] was used in ultrasonication-assisted IL pretreatment followed by hexane extraction (essential oil yield 9.55%) and ultrasonication-assisted aqueous IL extraction (essential oil yield 6.66%). The yields show that IL treatment combined with hexane as a co-solvent instead of water has a positive effect on the extraction. This could be attributed to the polarity of hexane, which is closer to that of the essential oil than water. Three different ILs, [C4MIM][Br], [C4MIM][HSO4], and [C2MIM][Ac], were used in this study. In the ultrasonication-assisted IL pretreatment followed by hexane extraction, the essential oil yields obtained with [C4MIM][Br], [C4MIM][HSO4], and [C2MIM][Ac] were 6.67%, 7.87%, and 9.5, respectively. In the ultrasonication-assisted aqueous IL extraction, the essential oil yields obtained with [C4MIM][Br], [C4MIM][HSO4], and [C2MIM] were 5.54%, 6.0%, and 6.66%, respectively. The higher yields obtained with [C2MIM][Ac] compared with [C4MIM][Br] and [C4MIM][HSO4] could be attributed to its low viscosity, high hydrogen bond basicity, and ability to disintegrate the structural matrix of the plant material [24].
2.3 Effect of temperature
The temperature greatly affects the extraction efficiency of an essential oil. At higher temperatures, the solvent viscosity decreases and diffusion of solvent in plant material increases, which increases the extraction yield. In this study, samples (4.0 g) were treated for 25 min at extraction temperatures of 50, 60, 70, and 80 ℃ using 7 mL of [C2MIM][Ac] and 10 mL of hexane. The extraction efficiency of the essential oil was enhanced when the temperature was increased from 50 to 70 ℃. There was a slight decrease in the extraction efficiency with a further increase in the temperature (from 70 to 80 ℃), which might be caused by degradation of heat-labile compounds. The essential oil yields obtained at the different temperatures were 6.5% (50 ℃), 6.85% (60 ℃), 9.55% (70 ℃), 8.0% (80 ℃), 7.87% (90 ℃), and 6.66% (100 ℃), respectively. Thus, 70 ℃ was identified as the optimum temperature for the extraction process. This is consistent with the optimum temperature identified in microwave treatment of pigeon pea for extraction of essential oil [25].
2.4 Effect of extraction time
Various pretreatment times (10, 15, 20, 25, and 30 min) were evaluated for the extraction of essential oil using ultrasonication-assisted IL pretreatment followed by hexane extraction with [C2MIM][Ac] and an ultrasonication power of 80.0 W. The extraction efficiency of the essential oil increased as the pretreatment time increased. The extraction yields with pretreatment times of 10, 15, 20, 25, and 30 min were 4.4%, 5.9%, 7.7%, 9.55%, and 9.55%, respectively. The maximum yield (9.55%) was obtained with a pretreatment time of 25 min.
2.5 Effect of particle size
The effect of particle size (10-20, 20-40, 40-60, 60-80, and 80-100 mesh) on the extraction efficiency was evaluated in ultrasonication-assisted IL pretreatment followed by hexane extraction. The maximum extraction efficiency was obtained with a particle size of 60-80 mesh. With the smaller particle sizes, excessive grinding of the plant material could cause the loss of bioactive compounds. Moreover, if the particles are too small, the diffusion step in the extraction will be difficult and small particles are not feasible for the extraction of essential oil from plants [26]. However, if the particle size was increased above 80 mesh, the extraction efficiency decreased because of incomplete mixing with the IL solution. The essential oil yields obtained with particles sizes of 20-40, 40-60, 60-80, and 80-100 mesh were 6.6%, 7.9%, 9.55%, and 6.5%, respectively.
2.6 Effect of the IL volume
Different ratios of [C2MIM][Ac] and hexane (IL/hexane=3∶10, 5∶10, 7∶10, 9∶10, v/v) were used for the extraction of essential oil fromP.minorleaves using ultrasonication-assisted IL pretreatment followed by hexane extraction. The essential oil yields obtained with IL∶hexane volume ratios (in mL) of 3∶10, 5∶10, 7∶10, and 9∶10 were 7.95%, 8.25%, 9.55%, and 9.55%, respectively. The yield of essential oil increased when the volume of IL was increased from 3 to 7 mL. Further increases the volume of IL did not greatly increase the yield of essential oil. The highest yield (9.55%) was obtained with an IL∶hexane volume ratio of 7∶10.
2.7 Kinetics study of the extraction of essential oil
Kinetic studies were carried out to evaluate the extraction of essential oil in the presence of an IL. To study the kinetics of essential oil extraction fromP.minor, the rate law for the reaction was expressed as follows:
(3)
whererrepresents the rate, [C] represents the mass concentration (g/L) of essential oil extracted, [ILs] is the mass concentration (g/L) andk*is the equilibrium rate constant.
kis the modified rate constant given by
k=k*[C][ILs]
(4)
Because the IL remains constant,
(5)
which can be rearranged as
(6)
Integration of Eq. 6 gives the following equation:
ln [C]=kt+c
(7)
wherecis the integration constant. If the initial mass concentration of the essential oil is [C0], then Eq. (7) becomes
ln [C0]=k(0)+c
(8)
which could be rearranged to give
ln [C0]=c
(8a)
Substitution of Eq. 8a into Eq. 7 gave
ln [C]=kt+ln [C0]
(9)
which could be rearranged as
ln [C]-ln [C0]=kt
(9a)
or
ln ([C]/[C0])=kt
(9b)
The mass concentration (X) was determined by GC-MS analysis using the percent yield of essential oil. The essential oil was calculated by GC-MS analysis:
(10)
which could be rewritten as
(10a)
Combining Eqs. 9a and 10a gave
ln (X-1)=-kt
(11)
which could be rearranged as
-ln (1-X)=kt
(11a)
A plot of ln (1-X) against time at different temperatures was used to determine the order and rate constant of essential oil extraction (Fig. 2a).
These plots were all linear, which showed it was a first-order reaction.
The activation energy was calculated using the Arrhenius equation [27], which shows the relationship between the activation energy, reaction rate, rate constant, and temperature:
(12)
whereRis universal gas constant,Tis the Kelvin temperature,Eais the activation energy andAeis the pre-exponential factor.
Integration of Eq. 12 gives the following equation:
(13)
In this study, theEaandAewere calculated from the slope and intercept of the graph between lnkversus 1/T×103(Fig. 2b).

Fig. 2 (a) Plot of -ln (1-X) against the reaction time at different temperatures and (b) Arrhenius plots of ln k against 1/T×103 for the extraction of essential oil
The activation energy was 28.76 kJ/mol, which shows the extraction of essential oil is thermodynamically feasible. Krishnan et al. [28] calculated an activation energy for microwave-assisted extraction of flavonoids fromTerminaliabellericaof 12.07 kJ/mol at temperatures between 40-100 ℃ with a solvent-to-solid ratio of 40 mL/g .
2.8 GC-MS analysis with ann-alkane series standard solution
The RI for all the components was determined by co-injection of the samples with standard mixtures containing a homologous series of C9to C24n-alkanes. Identification of the constituents was based on comparison of their RIs on the DB-5MS column with literature data [3,29,30], and comparison of their fragmentation patterns with data from the NIST05 library. The relative quantities of the individual components were calculated based on the essential oil composition calculated from the total ion current chromatograms by a computerized integrator.
The sample from ultrasonication-assisted IL pretreatment followed by hexane extraction was diluted withn-hexane (1∶100, v/v) before GC-MS analysis. The GC-MS chromatogram of theP.minoressential oil obtained by this method (Fig. 3) showed 31 compounds with retention times between 0 and 45 min [31]. In the first 33 min, no compounds were detected. High yields of dodecanal and decanal were obtained (Table 1). Compounds in the spectra could be identified using their retention times and RIs.
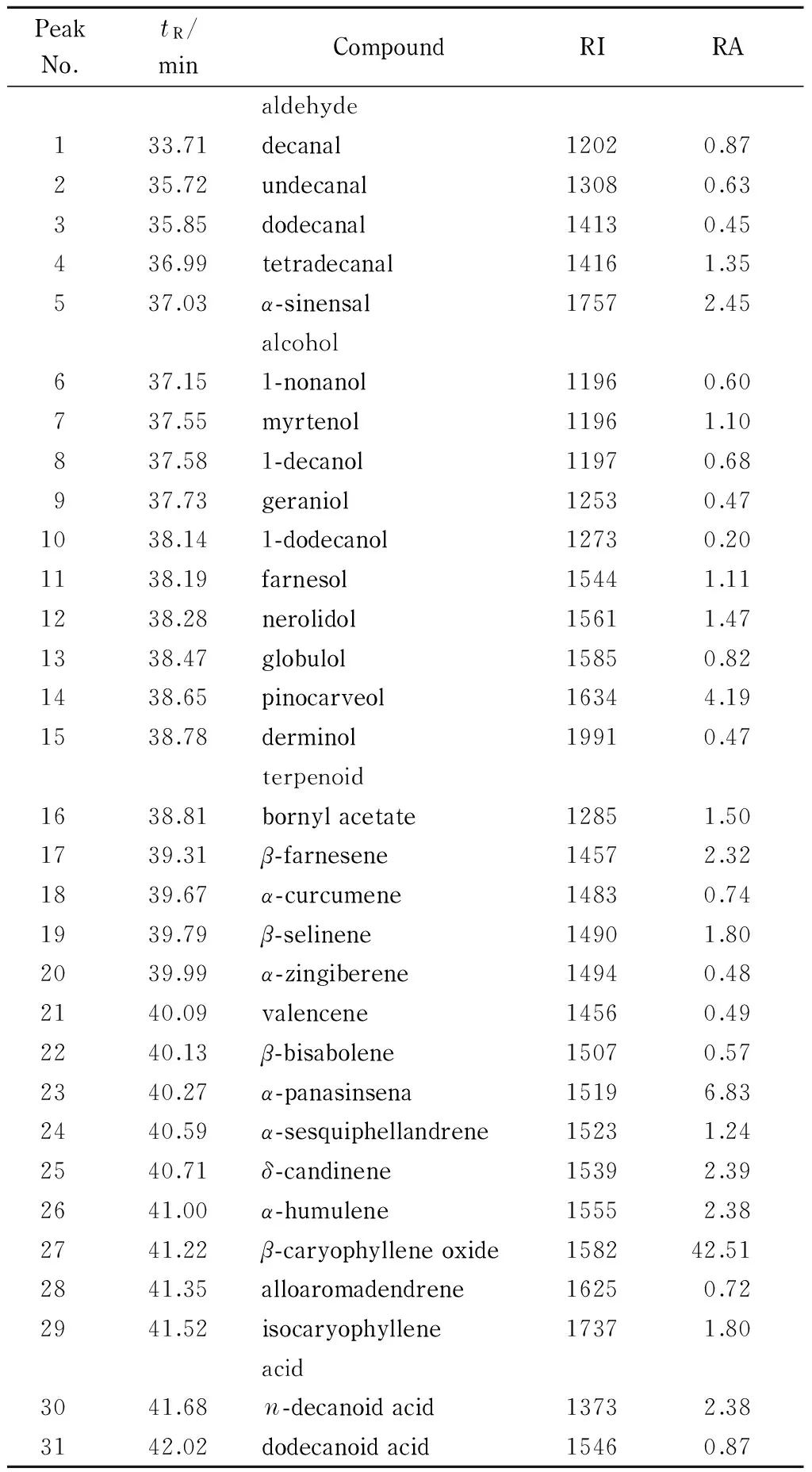
Table 1 Compounds identified in the P. minor essential oil using GC-MS
tR: retention indices relative to C8 to C24n-alkanes on the non-polar DB-5 capillary column. RI: identified by comparison with literature data for retention indices on a non-polar DB-5 capillary column. RA: relative amount (peak area of individual component relative to the total peak areas).

Fig. 3 Chromatogram of essential oil extracted from P. minor leaves
The essential oils extracted by ultrasonication-assisted aqueous IL extraction, stirring-assisted hexane extraction, ultrasonication-assisted hexane extraction, and hydrodistillation were also analyzed by GC-MS. The essential oil obtained by ultrasonication-assisted aqueous IL extraction contained 27 compounds, whereas that from ultrasonication-assisted hexane extraction contained 16 compounds, and the oil from hydrodistillation contained 11 compounds.
2.9 FT-IR analysis
The functional groups in the essential oil were identified by comparing the peaks in the FT-IR spectrum (Fig. 4) with literature data. Characteristic peaks were observed at 2 919, 2 850, and 1 467 cm-1. The peaks at 2 919 cm-1(C-H stre-tching vibration) and 1 467 cm-1(C-H bending) were more intense in the spectrum of the essential oil obtained by ultrasonication-assisted IL pretreatment followed by hexane extraction than in the spectra of any of the essential oils obtained by the other methods. These results suggest that more long chain alkanes are present in this essential oil sample than the other essential oils.
2.10 COSMO-RS analysis
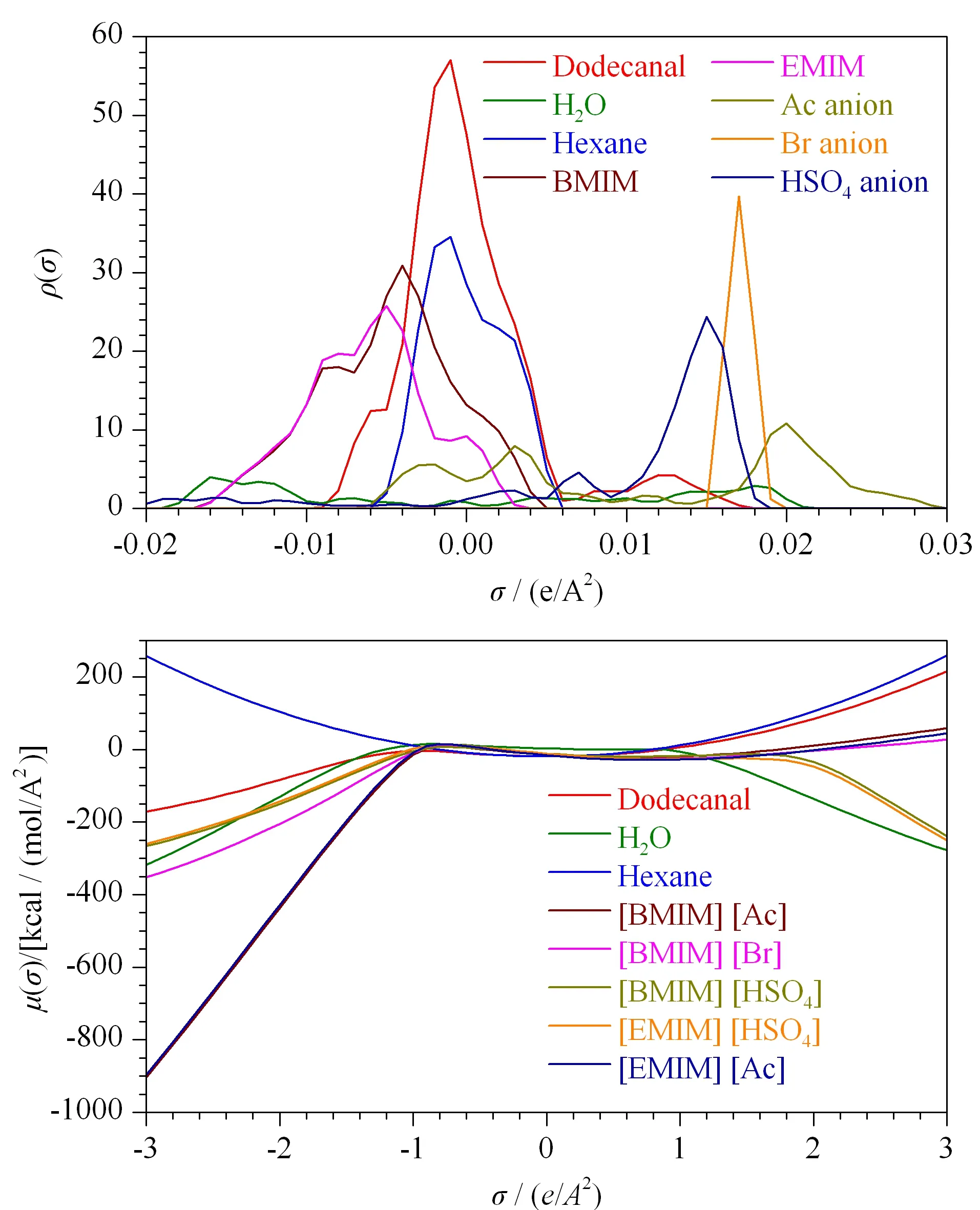
Fig. 5 (a) COSMO-RS σ-profiles and (b) σ-potential of dodecanal, the ionic liquids cations and anions, water and hexane EMIM: 1-ethyl-3-methylimidazolium; BMIM: 1-butyl-3-methylimidazolium.
COSMO-RSσ-profiles of [C4MIM][Br], 1-butyl-3-methylimidazolium ([BMIM])[HSO4], [C2MIM][Ac], hexane, and the model compound dodecanal (the main constituent of the essential oil) were obtained (Fig. 5a). Theσ-profiles andσ-potentials of the constituents of the mixture (compound and solvents), provide important information about the possible interactions in the mixture. Negatively charged parts are located on the right-hand side of the hydrogen bond acceptor and theσvalue will be positive, whereas positively charged parts are located on the left-hand side of the hydrogen bond donor and theσvalue will be negative. Generally, the region around ±1×10-2e/A2(eis energy, andAis area) is considered non-polar. Theσ-profile of hexane contains two peaks, with hydrogen on the negative side and carbons on the positive side. Theσ-potential of hexane is a parabola with its center atσ=0 (Fig. 5b), which is similar to the center of the curve for dodecanal. Even though the polarity of hexane is similar to that of dodecanal, its extraction capacity for the essential oil is not good. This could be caused by trapping of the essential oil in the cellular matrix of the plant, with hexane not able to disintegrate the cellular structure of the plant. In the case of ILs, [C2MIM][Ac] has strong hydrogen bond acceptor and donor sites compared with the other ILs. The hydrogen acceptor site of an IL plays an important role in determining the extraction efficiency. The cellular matrix (cellulose, hemicellulose, and lignin) of the plant are interconnected through hydrogen bonding, and these bonds are disrupted by the interaction of acetate anion of [C2MIM][Ac] with -OH groups in the plant matrix. This results in dissolution of the plant matrix, and facilitates extraction of essential oil. Compared with water as a co-solvent, hexane is a better co-solvent for IL treatment because its polarity is similar to that of dodecanol, which is shown by the overlapping peaks in theσ-profile.
3 Conclusions
Ultrasonication-assisted IL pretreatment followed by hexane extraction is the most efficient technique for extraction of essential oil fromP.minor. This method gives a high yield (9.55%) in a short time (25 min). COSMO-RS is a valuable tool for optimizing experimental parameters, such as the solvent effect. This method could be applied to effective extraction of essential oils from other medicinal plants.
Acknowledgment
The authors acknowledge support provided by Center of Research in Ionic Liquids, Universiti Teknologi PETRONAS, Malaysia.
[1] Vikram P, Chiruvella K K, Ripain H A, et al. Asian Pac J Trop Biomed, 2014, 4: 430
[2] Giarratana F, Muscolino D, Ragonese C, et al. J Essent Oil Res, 2016, 28: 457
[3] Jiao J, Gai Q Y, Zu G Y, et al. Sep Purif Technol, 2013, 107: 228
[4] Shaaban H A, El-Ghorab A H, Shibamoto T, et al. J Essent Oil Res, 2012, 24: 203
[5] Golmakani M T, Moayyedi M. J Essent Oil Res, 2016, 28: 272
[6] Bou Abdallah I, Baatour O, Mechrgui K, et al. J Essent Oil Res, 2016, 28: 545
[7] Sandrine P I, Christian G, Giancarlo C, et al. J Chromatogr A, 2013, 1305: 47
[8] Yang L, Wang H, Zu Y G, et al. Chem Eng J, 2011, 172: 705
[9] Cao Y, Wu J, Zhang T, et al. Chem Eng J, 2009, 147: 13
[10] Zhu S, Wu Y, Wang C, et al. Green Chem, 2006, 8: 325
[11] Xie H, Zhang S, Li S. Green Chem, 2006, 8: 630
[12] Biswas A, Shogren R, Willett J, et al. Carbohyd Polym, 2006, 66: 546
[13] Xie H, Li S, Zhang S. Green Chem, 2005, 7: 606
[14] Yan F, Texter J. Chem Commun, 2006, 25, 2696
[15] Fan J P, Cao J, Zhang X H, et al. Food Chem, 2012, 135: 2299
[16] Benvenuti F, Gironi F, Lamberti L. J Super Fluids, 2001, 20: 44
[17] Chang S, Abbaspour H, Nafchi A M, et al. J Essent Oil Bear Pl, 2016, 19: 1931
[18] Klamt A. Fluid Phase Equilibr, 2003, 206: 223
[19] Benazzouz A, Moity L, Pierlot C, et al. Colloid Surf A-Physicochem Eng Asp, 2014, 458: 101
[20] Durand M, Molinier V, Kunz W, et al. Chem-A Europ J, 2011, 17: 5155
[21] Garcia-Chavez L Y, Hermans A J, Hermans J, et al. Sep Purif Technol, 2012, 97: 2
[22] Zhou T, Qi Z. Chem Eng Sci, 2014, 115: 177
[23] Farhat A, Fabiano-Tixier A S, Visinoni F, et al. J Chromatogr A, 2010, 1217: 7345
[24] Muhammad N, Man Z, Bustam M A, et al. Chem Bio Eng R, 2015, 2: 257
[25] Gardeli C, Vassiliki P, Athanasios C, et al. Food Chem, 2008, 107: 1120
[26] Havlik J, Kokoska L, Valterova I, et al. Flavour Frag J, 2006, 21: 713
[27] Farooq M, Ramli A, Naeem A. RSC Advanc, 2016, 6: 872
[28] Yedhu Krishnan R, Rajan K S. Sep Purif Technol, 2016, 157: 169
[29] Avato P, Raffo F, Vartanian S, et al. Flavour Frag J, 2004, 19: 559
[30] Dallüge J, Beens J, Udo A. J Chromatogr A, 2003, 1000: 69
[31] Rusdi N A, Goh H H, Baharum S N. Plant Omics, 2016, 9: 289
10.3724/SP.J.1123.2017.02002
Received date: 2017-02-01
O658 Document code: A Article IC:1000-8713(2017)06-0656-09
* Corresponding author. Tel: +605-3687635, Fax: +605-3688000, E-mail: habibullah_kust@yahoo.com (Habib ULLAH); E-mail: cecili@utp.edu.my (Cecilia D WILFRED).
