光敏色素的生物学功能及其调控
2017-06-05张诗悦兰海燕
张诗悦,王 娟,2,兰海燕
(1.新疆大学 生命科学与技术学院,新疆生物资源基因工程重点实验室,乌鲁木齐 830046;2.新疆农业科学院经济作物研究所,乌鲁木齐 830091)
光敏色素的生物学功能及其调控
张诗悦1,王 娟1,2,兰海燕1
(1.新疆大学 生命科学与技术学院,新疆生物资源基因工程重点实验室,乌鲁木齐 830046;2.新疆农业科学院经济作物研究所,乌鲁木齐 830091)
光是影响植物生长发育的重要因素,植物为感受光而进化出光受体。光受体分为4类,其中光敏色素研究较为深入,它是红光和远红光受体,在光形态建成过程中发挥着重要作用。近年来的研究阐明了光敏色素的作用模式,以及由其介导的光信号转导途径和植物发育调节过程,如下胚轴延伸、茎分支、生物钟及开花时间控制等。基于目前的研究总结光敏色素的生物学功能及其介导的光信号转导途径,并展望其研究前景,以期为相关领域研究提供参考。
光敏色素; 光信号途径; 生物学功能; 调控
植物在整个生命周期中一直处于变化的光环境下,光质、光强及辐照度的改变均对植物生长发育产生影响[1]。在长期进化过程中,植物在适应光环境变化的同时,还能相互影响改变微生境,即植物能感受光信号也能产生光信号。光信号参与调控种子萌发、幼苗脱黄化、生物钟节律和开花时间等生理过程[2]。植物通过不同种类光受体(photoreceptors)感知光的方向、波长、强度以及光周期等信号,从而调控体内相关基因的表达,光敏色素(phytochrome,phy)是目前研究最为深入的光受体,其生理作用非常广泛,植物从种子萌发到开花、结果及衰老均受其影响[3]。
1 光敏色素的结构和分类
1.1 光敏色素的发现
光敏色素是植物体内普遍存在的红光/远红光受体。Flint等[4]首次发现红光能促进而远红光则抑制种子萌发。随后Butler等[5]发现在红光/远红光照射下蛋白提取液的吸收光谱有显著差异。1960年植物学家Borthwick和物理化学家Hendricks将其正式命名为光敏色素[6]。
1.2 光敏色素的结构特征
光敏色素是一种易溶于水的色素蛋白质,相对分子质量为2.5×105。一个光敏色素单体包含1个线性四吡咯生色团和1个脱辅基蛋白分子,蛋白分子由2个分子质量在120~127 ku 的多肽聚合而成。如图1所示,光敏色素由2个结构域构成:一个是位于N末端的分子质量为70 ku 的光感受域,另一个是 C 末端的分子质量为 55 ku 的光调节域[7]。光敏色素中光感受区包括多个亚结构域。在天然状态下,光敏色素通过 C 末端的氨基酸残基聚合成二聚体[8]。生色团与高度保守的 GAF(cGMP-specific phosphodiesterase/adenylate cyclases/formate hydrogen lyase transcription)区域相结合,当红光或远红光照射时,生色团的线性四吡咯环发生光质异构化,从而形成红光吸收型或远红光吸收型光敏色素[9]。PHY(Phytochrome domain)区域紧邻 GAF 区域的 C 端,是保持吸收光谱完整所必需的组分[10]。光调节区含有2个 PAS(period circadian protein homolog 1 / aryl hydrocarbon nuclear translocator /single-minded gene)同源重复序列和1个组氨酸激酶相关结构域HKRD(Histidine-kinase-related domain),此结构在光信号转导中发挥重要作用[11]。
1.3 光敏色素的分类
光敏色素在植物中的存在和作用并不单一。1989年Sharrock和Quail首次获得直接证据证明存在多种光敏色素基因[12]。随后,Clack等[13]获得光敏色素D(phyD)、光敏色素E(phyE)基因的序列。在玉米、水稻以及小麦中存在3类光敏色素,在松树和银杏中分别存在4种和3种光敏色素[9,14-16]。研究表明,光敏色素家族最早形成2个分支,其中一支很快分成光敏色素 A(phyA)和光敏色素C(phyC),另一分支分为光敏色素 B(phyB)和phyE[11]。phyE在某些类群(如单子叶植物)中丢失,而phyD 则起源于phyB最近的一次基因复制事件[11]。不同种类光敏色素基因的进化速率也不同,phyA、phyB 较保守,而phyC、phyE 的进化速率是phyA、phyB 的1 133倍[17]。
光敏色素按其在光下的状态可分为2类:光不稳定型(如phyA)和光稳定型(如phyB-phyE等)。其在植物中有2种存在形式,分别是红光吸收型(Pr)和远红光吸收型(Pfr)。一般把吸收红光的光敏色素称为Pr型光敏色素,其吸收红光后即转变成吸收远红光的Pfr型光敏色素。在黑暗条件下,Pfr会逆转为Pr,Pfr 浓度降低并被蛋白酶降解[18]。光敏色素主要有3种反应方式,极低辐照度反应(VLFR)、低辐照度反应(LFR)和强辐射反应(HIR)。不同种类光敏色素的反应方式各不相同,其中phyA主要调节VLFR和HIR,phyB 则调节 LFR[19]。
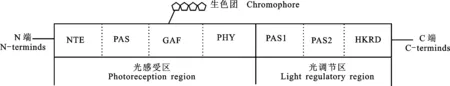
N端: N-terminus; 光感受区: Photoreception region, 包括NTE. N-terminal extension, PAS. period circadian protein homolog 1 / aryl hydrocarbon nuclear translocator /single-minded gene; GAF: cGMP-specific phosphodiesterase/adenylate cyclases/formate hydrogen lyase transcription, PHY: Phytochrome domain; 光调节区: Light regulatory region, 包括PAS1和PAS2: PAS (period circadian protein homolog 1 / aryl hydrocarbon nuclear translocator /single-minded gene) -related domains, HKRD: Histidine-kinase-related domain; 生色团: Chromophore; C端: C-terminus.
2 光敏色素的生物学功能
光敏色素感受光信号并参与调控的生理反应包括种子萌发、幼苗光形态建成、避荫作用、开花时间和昼夜节律响应等[7](表1)。此外,近年研究还发现,光敏色素参与调控植物对非生物胁迫的抗性应答[8]。
2.1 在种子萌发中的功能
Borthwick等[20]的研究首次证明红光/远红光受体能调节种子萌发。对拟南芥突变体的研究表明,至少有 3 种光敏色素(phyA、phyB、phyE)参与拟南芥种子萌发的调控。phyA在不同波长的光照(紫外线、可见光和远红光)下调控不可逆转的VLFR反应,而phyB则调控红光/远红光受体的LFRs反应,2种反应均能促进种子萌发[21]。此外,在连续远红光及强辐射条件下,phyA能促进种子萌发[22]。phyE可能直接参与远红光的光受体响应,或协助phyA调控种子萌发[22]。有趣的是,环境温度参与拟南芥种子萌发的光调控,不同光敏色素在温度变化条件下其功能会相应改变[23]。
2.2 幼苗去黄化
黑暗下生长的幼苗发生黄化,且下胚轴伸长、子叶不展开、原质体发育成白色体。而光下幼苗则发生去黄化反应,植株形态正常且原质体发育为成熟的叶绿体。不同光敏色素(除phyE外)在幼苗去黄化过程中均起到一定作用[24]。
在白光和红光下,phyA 缺失突变体表现为野生型光形态建成表型;而在连续远红光照射时,该突变体表现为暗形态建成表型,表明 phyA 是感知和调节远红光反应的主要光受体[25]。此外,试验证明 phyA 在红光处理下的快速调控基因表达响应中起重要作用[26]。phyB 是主要调控去黄化的光敏色素。但红光处理后,phyB 缺失突变体和野生型在转录水平表达差异不显著[27];phyA/phyB 双突变体与 phyB 单突变体相比,下胚轴明显增长且子叶扩张减少,进一步揭示 phyA 在红光下的作用。
在红光照射下,phyC 也能调控幼苗去黄化反应[28]。但 phyC/phyD 双突变体表型与单突变体表型间无明显差异;在远红光下,phyC 则不能调控幼苗去黄化反应[29]。
此外,phyD 在红光下也能独自调控幼苗去黄化作用[25]。而 phyE 在此过程中的作用则极其微弱。
2.3 避荫反应
植物发育的调控不仅跟光暗有关,还受光质量的影响,特别是由其他植物的阴影带来的光质量变化[30]。因此,植物启动另一种策略即避荫反应,包括茎和叶柄的伸长,开花时间提前,顶端优势增加等[30]。
phyB 是大多数避荫反应的光受体,而 phyA 是避荫环境中光照辐照度变化的敏感感受器。根据对突变体的研究显示,phyD 和 phyE 帮助 phyB 参与避荫反应。

表1 不同种光敏色素在植物生长发育及应对逆境胁迫中的作用
3 光敏色素的调控
目前,已知光信号转导途径的相关基因根据其作用可分为3类:
第1类,参与光敏色素核定位。光敏色素入核是其发挥作用、调控光信号的关键步骤。但不同光敏色素的核内定位机制各不相同[45-46]。早期研究[47-48]显示,持续照射红光和蓝光能有效诱导phyB-phyE 转移到细胞核内,并可以通过远红光照射逆转。近年来研究发现在由黑暗过渡到远红光照射时,phyB 也能快速移位至细胞核内[49],与早期结果矛盾,其潜在原因仍需进一步研究。与 phyB-phyE 不同,phyA 入核不仅需要光信号,还需要2个同源伴侣蛋白协助,即FHY1(Far-red elongated hypocotyl 1)和FHL(fhy1 like)[48, 50-51]。陈芳等[52]研究发现,FHY1是帮助phyA入核、转录因子互作、结合基因启动子等的辅助蛋白。Lin等[53]发现拟南芥中2种光反应关键蛋白:FHY3( far-red elongated hypocotyl3)和FAR1(Far-red impaired response1),两者通过直接激活 FHY1/FHL基因的表达来共同调控 phyA 在核内的积累。Genoud等[54]研究发现细胞核内组成型的 phyA 弥补fhy3突变体的表型。拟南芥 phyA 这种特殊的入核调控方式可能与其适应性进化有关[55]。其他物种不同光敏色素的入核机理是否与拟南芥一致目前还不清楚。
第2类,参与光敏色素信号输出。邓兴旺课题组[56-57]的遗传试验筛选鉴定出一个光形态建成抑制因子,命名为COP/DET/FUS(CONSTITUTIVE PHOTOMORPHOGENESIS/DE- ETIOLATED/FUSCA)基因,这些因子的突变体在暗环境中呈现光形态建成表型。研究表明,HY5(ELONGATED HYPOCOTYL5)[58]、HYH(HY5 Homolog)[59]、LAF1(LONG AFTER FARRED LIGHT1)[60]以及HFR1(LONG HYPOCOTYL IN FAR-RED1)[61-63]等均促进光形态建成蛋白的表达水平增高,进而促进光敏色素的活化,从而抑制 COP/DET/FUS 的活性。此外,这些因子可在植物体内形成不同的复合体,参与泛素化途径。其中 COP1-SPAs 复合体由 COP1及 SPAs(SUPPRESSOR OF PHYA1)家族成员构成,其中 COP1是拟南芥光形态建成的关键抑制因子,在黑暗条件下,能作为E3泛素连接酶降解光形态建成的转录因子,但在光下 COP1活性被抑制且在核内的丰度降低。SPAs 家族是 phyA 信号转导中的另一个负调控因子,通过结合 COP1对目标蛋白进行水解,且作为一个共同作用因子调节 COP1的功能[64]。CSN(COP9 signalosome),由8个高度保守的不同亚基组成,是一个新的 E3泛素连接酶的调节因子,是细胞对外界刺激或胁迫产生响应的调节成分[65-66]。CDD 复合体[COP10, DET1, DDB1 (DNA DAMAGE BINDING PROTEIN1)]的具体功能还不清楚。此外,光信号导致 COP/DET/FUS 失活的具体分子机制也有待进一步研究。
第3类,直接调节光反应。与光受体相互作用因子中,有一类重要的碱性螺旋环螺旋(basic helix-loop-helix, bHLH)类转录因子-PIFs(phytochrome interacting factor)家族蛋白,其主要功能是调节光敏色素介导的暗形态建成到光形态建成转换过程中的信号转导途径[67]。目前,已知的PIFs家族成员有且仅有 PIF1和 PIF3可以与活化的 phyA 相互作用,且 PIF1与 phyA的结合能力比 PIF3强[68-69]。PIF3能与phyC和phyE分别形成异源二聚体[70]。研究发现 PIF3和 phyB可以在体外结合至光响应基因启动子的 G-box 区域,表明光敏色素可以将光信号直接靶向目的基因的启动子调控该基因表达[71-72]。研究证明,在转录因子 PIF3或HY5的协同作用下,phyA-FHY1复合物可以结合至编码查尔酮合酶的CHS(CHALCONE SYNTHASE)基因启动子上,共同调控CHS的转录[73]。Chen等[74]证明在与 phyA 直接发生相互作用的靶基因启动子上存在大量供 PIFs 识别的顺式作用元件。这一发现说明包括 PIFs和 HY5在内的大量转录因子均能以直接或间接的方式在靶基因启动子上与phyA发生相互作用,对众多下游基因进行调控,从而对各种内外信号作出快速反应[75]。拟南芥其他类型的转录因子(如 ARR4、IAAs、ATHB23等)也能以上述方式与光敏色素发生相互作用[75-77]。此外,也有研究认为phyB会阻碍PIFs结合至靶基因的启动子区域[78],并通过一系列体外试验证明,PIF1和 PIF4与靶基因作用后不能与 phyB 或 phyA 同时结合[79-80],其深层次的机理还有待进一步试验证实。
基于以上分析,笔者绘制光敏色素与各相关因子互作的模式图(图2)。

Pr.红光吸收型 Phytochrome red light-obsorbing form; Pfr.远红光吸收型 Phytochrome far-red light-absorbing form; PHYA.Phytochrome A; PHYB.Phytochrome B; FHY1.Far-red elongated hypocotyl 1; PHY.Phytochrome; COP1/DET/FUS.Constitutive photomorphogenesis/de-etiolated/fusca; HY5:Long hypocotyl 5; LAF1:Long after farred light1;HFR1:Long hypocotyl in far-red1; HYH:HY5 Homolog; PIFs:Phytochrome interacting factors; CHS:Chalcone synthase; CCA1:Circadian clock-associated protein 1; ABA:Abscisic acid; CBF.CRT/DRE2 binding factor; STO.Salt tolerance protein;箭头表示正调控作用, T型线表示负调控作用 Arrow represent positive regulation and T-type line represent negative regulation.
4 展 望
不同光敏色素的分子结构和生理功能已得到系统的阐述,其信号传导机制成为近年来研究的重点。目前,基于对光信号、温度及多种植物内源激素共同组成的信号网络有系统认识,一大批与光敏色素相互作用的因子被发现。但因该网络的复杂性,其整体功能以及各信号分子在整个调控网络中的作用还需深入研究。如何系统诠释以光敏色素为中心的信号通路作用机制以及各通路间的相互关系,仍是亟待解决的关键问题。此外,光敏色素还是介导植物应对各种非生物与生物胁迫的重要激素[32],了解其体内的作用方式将为培育适应不同光环境尤其是弱光下的农作物新品种提供有价值的理论参考依据。
Reference:
[1] WHITELAM G C,DEVLIN P F.Light signalling inArabidopsisplant [J].PhysiolBiochem,1998,36(1/2):125-133.
[2] CHORY J,CHATTERJEE M,COOK R K,etal.From seed germination to flowering,light controls plant development via the pigment phytochrome[J].ProceedingsoftheNationalAcademyofSciences,1996,93(22):12066-12071.
[3] JIAO Y,LAU O S,DENG X W.Light-regulated transcriptional networks in higher plants[J].NatureReviewsGenetics,2007,8(30):217-230.
[4] FLINT L H,MCALISTER E D.Wavelengths of radiation in the visible spectrum promoting the germination of light-sensitive lettuce seed[J].IrradiatedLettuceSeed,1937,96(2):1-9.
[5] BUTLER W L,NORROS K H,SIEGELMAN H W,etal.Detection,assay,and preliminary purification of the pigment controlling photoresponsive development of plants[J].ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica,1960,45(12):1703-1708.
[6] BORTHWICK H A,HENDRICKS S B.Photoperiodism in plants [J].Science,1960,132(3435):1223-1228.
[7] QUAIL P H.Phytochrome photosensory signalling networks[J].NatureReviewsMolecularCellBiology,2002,3(3):85-93.
[8] 杨有新,王 峰,蔡加星,等.光质和光敏色素在植物逆境响应中的作用研究进展[J].园艺学报,2014,41(9):1861-1872.
YANG Y X,WANG F,CAI J X,etal.Recent advances in the role of light quality and phytochrome in plant defense resistance against environmental stresses[J].ActaHorticulturaeSinica,2014,41(9):1861-1872(in Chinese with English abstract).
[9] BAE G,CHOI G.Decoding of light signals by plant phytochromes and their interacting proteins[J].AnnualReviewofPlantBiology,2008,59:281-311.
[10] PRATT L H,PARTT M M,KELMENSON P M,etal.The phytochrome gene family in tomato (SolariumlycopersicumL.)[J].PlantCell&Environment,1997,20(6):672-677.
[11] WU S H,LAGARLAS J C.Defining the bilin lyase domain:lessons from the extended phytochrome superfamily[J].Biochemistry,2000,39(44):13487-13495.
[12] SHARROCK R A,QUAIL P H.Novel phytochrome sequences inArabidopsisthaliana:structure,evolution,and differential expression of a plant regulatory family[J].Genes&Development,1989,3(11):1745-1757.
[13] CLACK T,MATHEWS S,SHARROCK R A.The phytochrome apoprotein family inArabidopsisis encoded by five genes:the sequences and expression of PHYD and PHYE[J].PlantMolecularBiology,1994,25(3):413-421.
[14] 周 波,李玉花.植物的光敏色素与光信号转导[J].植物生理学通讯,2006,42(1):134-140.
ZHOU B,LI Y H.Phytochrome and light signal transduction in pants[J].PlantPhysiologyCommunications,2006,42(1):134-140(in Chinese with English abstract).
[15] 张 芳,张晓枫,王进征,等.植物光敏色素PHYA、PHYB研究进展[J].生物学通报,2011,46(1):11-14.
ZHANG F,ZHANG X F,WANG J ZH,etal.Plant phytochrome PHYA,PHYB research progress[J].BiologyReport,2011,46(1):11-14(in Chinese).
[16] MALOOF J N,BOREVITZ J O,WEIGEL D,etal.Natural variation in phytochrome signaling[J].SeminarsinCellandDevelopmentalBiology,2000,11(6),523-530.
[17] MONTGOMERY B L,LAGERLAE J C.Phytochrome ancestry:sensors of bilins and light[J].RendsinBiochemicalSciences,2002,7(8):357-366.
[18] SOMERS D E,SCHULTZ T F,MILNAMOW M,etal.ZEITLUPE encodes a novel clock-associated PAS protein fromArabidopsis[J].Cell,2000,101(3):319-329.
[19] LI J G,LI G,WANG H Y,etal.Phytochrome signaling mechanisms[J].TheArabidopsisBook,2001,3(3):e0148.
[20] BORTHWICK H A,HENDRICKS S B,PARKER M W,etal.A reversible photoreaction controlling seed germination[J].ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica,1952,38(8):662-666.
[21] 陈春峰.水稻phyA基因转化拟南芥的功能研究[D].广州:华南理工大学,2011.
CHENG CH F.Functional analysis of rice phytochrome A inArabidopsis[D].Guangzhou:South China University of Technology,2011(in Chinese with English abstract).
[22] HENNIG L,STODDART W M,DIETERLE M,etal.Phytochrome E controls lightinduced germination ofArabidopsis[J].PlantPhysiol,2002,128(1):194-200.
[23] HESCHEL M S,SELBY J,BUTLER C,etal.A new role for phytochromes in temperature-dependent germination[J].NewPhytologist,2007,174 (4):735-741.
[24] 顾建伟,刘 婧,薛彦久,等.光敏色素在水稻生长发育中的作用[J].中国水稻科学,2011,25(2):130-135.
GU J W,LIU J,XUE Y J,etal.Phytochrome functions in rice development[J].ChineseJournalofRice,2011,25(2):130-135(in Chinese with English abstract).
[25] 李建平.光敏色素D在拟南芥生长和发育中的功能与机理研究[D].北京:中国农业科学院,2013.
LI J P.Mechanisms of phytochrome D on plant growth and development inArabidopsisthaliana[D].Beijing:Chinese Academy of Agricultural Sciences Dissertation,2013(in Chinese with English abstract).
[26] TEPPERMAN J M,HWANG Y S,QUAIL P H.PhyA dominates in transduction of red-light signals to rapidly responding genes at the initiation ofArabidopsisseedling de-etiolation[J].PlantJournal,2006,48(5):728-742.
[27] TEPPERMAN J M,HUDSON M E,KHANNA R,etal.Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red light- regulated gene expression during seedling de-etiolation[J].PlantJournal,2004,38(5):725-739.
[28] FRANKLIN K A,DAVIS S J,STODDART W M,etal.Mutant analyses define multiple roles for phytochrome C inArabidopsisphotomorphogenesis[J].PlantCell,2003,15(9):1981-1989.
[29] MONTE E,ALONSO J M,ECKER J R,etal.Isolation and characterization of phyC mutants inArabidopsisreveals complex crosstalk between phytochrome signaling pathways[J].PlantCell,2003,15(9):1962-1980.
[30] SCHFER E,BOWLER H.Phytochrome-mediated photoperception and signal transduction in higher plants[J].EMBOReports,2002,3(11):1042-1048.
[31] FRANKLIN K A,QUAIL P H.Phytochrome functions inArabidopsisdevelopment[J].JournalofExperimentalBotany,2009,61(1):11-24.
[32] DONOHUE K,HESCHEL M S,BUTLER C M,etal.Diversification of phytochrome contributions to germination as a function of seed-maturation environment[J].NewPhytologist,2008,177 (2):367-379.
[33] HESCHE M S,BUTLER C M,BARUA D,etal.New roles of phytochromes during seed germination[J].InternationalJournalofPlantSciences,2008,169(4):531-540.
[34] TAKANO M,INAGAKI N,XIE X.Distinct and cooperative functions of phytochromes A、B and C in the control of deetiolation and flowering in rice[J].PlantCell,2005,17(12):3311-3325.
[35] SCHFER E,BOWLER H.Phytochrome-mediated photoperception and signal transduction in higher plants[J].EMBOReports,2002,3(11):1042-1048.
[36] TAKANO M,INAGAKI N,XIE X,etal.Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice[J].ProceedingsoftheNationalAcademyofSciences,2009,106(34):14705-14710.
[37] HALLIDAY K J,WHITELAM G C.Changes in photoperiod or temperature alter the functional relationships between phytochromes and reveal roles for phyD and phyE[J].PlantPhysiology,2003,131(4):1913-1920.
[38] BHARDWAJ V,MEIER S,PETERSEN L N,etal.Defence responses ofArabidopsisthalianato infection byPseudomonassyringaeare regulated by the circadian clock[J].PloSOne,2011,6 (10):e26968.
[39] ZHANG C,XIE Q,ANDERSON R G,etal.Crosstalk between the circadian clock and innate immunity inArabidopsis[J].PLosPathogens,2013,9(6):344-351.
[40] BOGGS J Z,LOEWY K,BIBEE K,etal.Phytochromes influence stomatal conductance plasticity inArabidopsisthaliana[J].PlantGrowthRegulation,2010,60 (2):77-81.
[41] INDORF M,CORDERO J,NEUHAUS G,etal.Salt tolerance(STO),a stress-related protein,has a major role in light signalling[J].PlantJournal,2007,51(4):563-574.
[42] FRANKLIN K A,WHITELAM G C.Light-quality regulation of freezing tolerance inArabidopsisthaliana[J].NatureGenetics,2007,39(11):1410-1413.
[43] SYSOEVA M,PATIL G G,SHERUDILO E,etal.Effect of temperature drop and photoperiod on cold resistance in young cucumber plants-involvement of phytochrome B[J].PlantStress,2008,2(1):84-88.
[44] LIU J,ZHANG F,ZHOU J,etal.Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice[J].PlantMolecularBiology,2012,78(3):289-300.
[45] NAGATANI A.Light-regulated nuclear localization of phytochromes[J].CurrentOpinioninPlantBiology,2004,7(6):708-711.
[46] KEVEI E,SCHAFER E,NAGY F.Light-regulated nucleo-cytoplasmic partitioning of phytochromes[J].JournalofExperimentalBotany,2007,58(12):3113-3124.
[47] GIL P,KIRCHER S,ADAM E,etal.Photocontrol of subcellular partitioning of phytochrome-B:GFP fusion protein in tobacco seedlings[J].PlantJournalforCell&MolecularBiology,2000,22(2):135-145.
[48] KIRCHER S,GIL P,KOZMA-BOGNAR L,etal.Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A,B,C,D,and E is regulated differentially by light and exhibits a diurnal rhythm[J].PlantCell,2002,14(7):1541-1555.
[49] ZHENG X,WU S,ZHAI H,etal.Arabidopsisphytochrome B promotes SPA1 nuclear accumulation to repress photomorphogenesis under far-red light[J].PlantCell,2013,25(1):115-133.
[50] HISADA A,HANZAWA H,WELLER J L,etal.Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures[J].PlantCell,2000,12(7):1063-1078.
[51] KIM L,KIRCHER S,TOTH R,etal.Light-induced nuclear import of phytochrome-A:GFP fusion proteins is differentially regulated in transgenic tobacco andArabidopsis[J].PlantJournal,2000,22(2):125-133.
[52] 陈 芳,邓兴旺.远红光受体伴侣蛋白FHY1在介导基因表达和植物生长发育过程中的独特作用[J].遗传,2014,36(9):958.
CHEN F,DENG X W.Far red receptor chaperone FHY1 in mediated gene expression and the unique role in the process of plant growth and development[J].Hereditas,2014,36(9):958(in Chinese).
[53] LIN R,DING L,CASOLA C,etal.Transposase-derived transcription factors regulate light signaling inArabidopsis[J].Science,2007,318(5854):1302-1305.
[54] GENOUD T,SCHWEIZER F,TSCHEUSCHLER A,etal.FHY1 mediates nuclear import of the light-activated phytochrome A photoreceptor[J].PLosGenetics,2008,4(8):338-348.
[55] MATHEWS S,BURLEIGH J G,DONOGHUE M J.Adaptive evolution in the photosensory domain of phytochrome A in early angiosperms[J].MolecularBiologyandEvolution,2003,20(7):1087-1097.
[56] SULLIVAN J A,SHIRASU K,DENG X W.Hediverse roles of ubiquitin and the 26S proteasome in the life of plants[J].NatureReviewsGenetics,2003,4(12):948-958.
[57] DONG J,TANG D F,GAO Z X,etal.ArabidopsisDE-ETIOLATED1 represses photomorphogenesis by positively regulating phytochrome-interacting factors in the dark[J].PlantCell,2014,26(9):3630-3645.
[58] OSTERLUND M T,HARDTKE C S,WEI N,etal.Targeted destabilization of HY5 during light-regulated development ofArabidopsis[J].Nature,2000,405(6785):462-466.
[59] HOLM M,MA L G,QU L J,etal.Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression inArabidopsis[J].GenesDevelopment,2002,16(10):1247-1259.
[60] SEO H S,YANG J Y,ISHIKAWA M,etal.LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1[J].Nature,2003,423(6943):995-999.
[61] DUEK P D,ELMER M V,FANKHAUSER C,etal.The degradation of HFR1,a putative bHLH class transcription factor involved in light signaling,is regulated by phosphorylation and requires COP1[J].CurrentBiology,2004,14(24):2296-2301.
[62] JANG I C,YANG J Y,SEO H S,etal.HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling[J].Genes&Development,2005,19(5):593-602.
[63] YANG J,LIN R,SULLIVAN J,etal.Light regulates COP1-mediated degradation of HFR1,a transcription factor essential for light signaling inArabidopsis[J].PlantCell,2005,17(3):804-821.
[64] HOECKER U,QUAIL P H.The phytochrome A-specific signaling intermediate SPA1 interacts directly with COP1,a constitutive repressor of light signaling inArabidopsis[J].JournalofBiologicalChemistry,2001,276(41):38173-38178.
[65] SCHWECHHEIMER C,SERINO G,CALLIS J,etal.Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIRI in mediating auxin response[J].Science,2001,292(5520):1379-1382.
[66] SERINO G,DENG X W.The COP9 signalosome:regulating plant development through the control of proteolysis[J].AnnualReviewofPlantBiology,2003,54(1):165-182.
[67] WANG H,WANG H Y.Phytochrome signaling:time to tighten up the loose ends[J].MolecularPlant,2015,8(4):540-551.
[68] AL-SADY B,NI W M,KIRCHER S,etal.Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation[J].MolecularCell,2006,23(3):439-446.
[69] SHEN H,ZHU L,CASTILLON A,etal.Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 fromArabidopsisdepend upon its direct physical interactions with photoactivated phytochromes [J].PlantCell,2008,20(6):1586-1602.
[70] CLACK T,SHOKRY A,MOFFET M,etal.Obligate heterodimerization ofArabidopsisphytochromes C and E and interaction with the PIF3 basic helix-loop-helix transcription factor[J].PlantCell,2009,21(3):786-799.
[71] MARTINEZ-GARCIA J F,HUQ E,QUAIL P H.Direct targeting of light signals to a promoter element-bound transcription factor[J].Science,2000,288(5467):859-863.
[72] SHIN J,PARK E,CHOI G.PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters inArabidopsis[J].PlantJournal,2007,49(6):981-994.
[73] CHEN F,SHI X,CHEN L,etal.Phosphorylation of FAR-RED ELONGATED HYPOCOTYL1 is a key mechanism defining signaling dynamics of phytochrome A under red and far-red light inArabidopsis[J].PlantCell,2012,24(5):1907-1920.
[74] CHEN F,DENG X W.Arabidopsisphytochrome A directly targets numerous promoters for individualized modulation of genes in a wide range of pathways[J].PlantCell,2014,26(5):781-785.
[75] SWEERE U,EICHENBERG K,LOHRMANN J,etal.Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling[J].Science,2001,294(5544):1108-1111.
[76] COLON-CARMONA A,CHEN D L,YEH K C,etal.Aux/IAA proteins are phosphorylated by phytochrome in vitro[J].PlantPhysiology,2000,124(4):1728-1738.
[77] CHOI H,JEONG S,KIM D S,etal.The homeodomain-leucine zipper ATHB23,a phytochrome B-interacting protein,is important for phytochrome B-mediated red light signaling[J].PhysiologiaPlantarum,2014,150(2):308-320.
[78] PARK E,PARK J,KIM J,etal.Phytochrome B inhibits binding of phytochrome-interacting factors to their target promoters[J].PlantJournal,2012,72(4):537-546.
[79] HUQ E,AL-SADY B,HUDSON M,etal.Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis[J].Science,2004,305(5692):1937-1941.
[80] HUQ E,QUAIL P H.PIF4,a phytochrome-interacting bHLH factor,functions as a negative regulator of phytochrome B signaling inArabidopsis[J].EMBOJournal,2002,21(21):2441-2450.
(责任编辑:顾玉兰 Responsible editor:GU Yulan)
Biological Functions and Signaling Regulation Network of Phytochromes
ZHANG Shiyue1,WANG Juan1,2and LAN Haiyan1
(1.Xinjiang Key Laboratory of Biological Resources and Genetic Engineering,College of Life Science and Technology,Xinjiang University,Urumqi 830046,China; 2.Institute of Economic Crops,Xinjiang Academy of Agricultural Sciences,Urumqi 830091,China)
Light is essential for plant growth and development.Plants have evolved light receptors (LRs) to accept light signal.So far,four different kinds of LRs have been reported,among these,phytochromes have been well-studied,which act as red and far-red LR and play vital roles in photomorphogenesis.Currently,the action mode of phytochrome and light signal transduction pathway as well as modulation of plant development have been elucidated,e.g.the hypocotyl extension,stem branching,circadian rhythm and flowering time control,etc.In this review,we summarized the biological functions,regulation of phytochrome,and light signal transduction pathways in plant development,which may provide insight for further study in this field.
Phytochrome;Light signal pathways; Biological functions; Regulation
ZHANG Shiyue, female, master student. Research area:plant adversity molecular biology. E-mail: 496689512@qq.com
LAN Haiyan, female,Ph.D,professor.Research area:plant adversity molecular biology. E-mail:lanhaiyan@xju.edu.cn
日期:2017-05-22
2016-03-24
2016-08-08
国家自然科学基金 (31260037; 31460043); 新疆自治区优秀青年科技人才培养项目(2013721013);国家科技部基础研究计划973前期项目 (2012CB722204)。
张诗悦,女,硕士研究生,研究方向为植物抗逆分子生物学。E-mail:496689512@qq.com
兰海燕,女,博士,教授,研究方向为植物抗逆分子生物学。E-mail: lanhaiyan@xju.edu.cn
Q945.43
A
1004-1389(2017)05-0657-08
网络出版地址:http://kns.cnki.net/kcms/detail/61.1220.S.20170522.0856.004.html
Received 2016-03-24 Returned 2016-08-08
Foundation item National Natural Science Foundation of China(No.31260037,No.31460043);Project for Cultivating Young Talents of Xinjiang Uygur Autonomous Region (No.2013721013);Initial Project of 973 Program from the Ministry of Science and Technology of China(No.2012CB722204).
