超声在肺部疾病中的临床应用
2017-06-05俞万钧
张 磊,俞万钧,马 坚
(1.宁波大学医学院,浙江 宁波 315211;2.宁波大学医学院附属鄞州医院呼吸科,浙江 宁波 315040)
综述
超声在肺部疾病中的临床应用
张 磊1,2,俞万钧2*,马 坚2
(1.宁波大学医学院,浙江 宁波 315211;2.宁波大学医学院附属鄞州医院呼吸科,浙江 宁波 315040)
肺部床旁超声是急诊科及重症监护室用于快速诊断和监测治疗的工具。经胸壁肺部超声(简称肺部超声)在诊断肺炎、气胸、肺栓塞、胸腔积液、肺间质综合征等疾病中有很高的敏感度、特异度和诊断准确率,此外,还可评估从肺间质综合征到肺实变的演变过程,并可对治疗效果提供实时反馈。本文对肺部超声的临床应用进行综述。
肺部疾病;超声检查;诊断显像
20世纪40~50年代超声开始应用于医学领域,于90年代末应用于如急性呼吸衰竭等危重症和急诊患者。随着技术进步,超声可对多种肺部疾病进行快速诊断并进行鉴别诊断[1],具有良好的诊断敏感度和特异度,相比于传统X线胸片及CT,超声检查实时、安全,大大减少了患者的转运风险。本文对经胸壁肺部超声(简称肺部超声)进行综述。
1 超声检查肺部的基本原理和正常肺部超声表现
肺部超声检查现多采用便携式超声设备,此设备参数设置简便、探头较为单一[2],常搭载智能微型凸面探头(2~5 MHz),也可在同一种设备上切换不同频率探头,临床可根据不同受检部位选择合适的探头。超声可对整个胸廓进行探查,临床常采取半侧胸廓6区分法[3],患者常取仰卧位,对前、侧胸壁及后区探查,结合纵向扫查和肋间隙倾斜扫查进行肺部疾病诊断。
肺脏是含有大量气体的脏器,声波穿透胸壁软组织,在探头和肺泡之间发生强烈反射,形成混响伪像,而非肺组织解剖图像[3]。行纵向扫查时可见上下肋骨声影和胸膜线组成的蝙蝠征,是正常的肺部标志性征象;行肋间斜切扫查可方便观察胸膜线,避免肋骨声影干扰。检查中可将两种探查方法相结合,对不同疾病采用不同的扫查区[4-6]。
正常的肺部超声影像包括胸膜线、肺滑征(1ung sliding, LS)、A线和少量B线。胸膜线在超声图像上表现为两条高回声线,分别代表壁层胸膜和脏层胸膜及中间低弱回声的胸膜液。LS是脏层胸膜相对于壁层胸膜随呼吸运动而产生,超声表现为条状高回声胸膜线随呼吸同步滑动[3],M型超声表现为特征性的“沙滩征”。A线(图1A)源于胸膜线,超声表现为自胸膜线下方重复的数条高回声线,随深度的增加而衰减,是正常肺部标志线,相邻A线间距与探头至胸膜的距离大致相等。B线(图1B)为自胸膜线发出垂直于胸膜线,呈条状激光束样高回声直达屏幕底部且无声衰减,可随胸膜线的移动而运动[3],少数正常人下侧胸壁靠近膈肌处重力区可见动态B线。B线被认为是肺间质病变征象,提示肺组织正常气水含量改变,肺组织内气体含量减少、肺组织密度增加时,B线数量增多[7-8],当肺内气体继续减少时,可出现肝样或脾样回声改变。利用这一征象可排除检查区域内气胸,并提示肺间质性病变。肺部超声无法显示肺内部结构,但可用来观察胸膜表面肺组织病理生理改变,反映肺深部组织变化,当疾病累及胸膜表面出现异常现象时[9],采用超声可排除气胸、胸膜炎、肺炎及肺外周梗死[10]。肺部超声还可以反映肺实质的比重[7],近年来越来越受到关注。
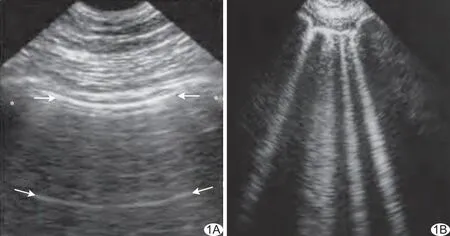
图1 肺部超声表现
2 肺部超声的应用
2.1 气胸 1986年Rantanen[11]首次应用肺部超声诊断气胸,研究[12-13]证实超声诊断气胸具有很高的敏感度和特异度,尤其对隐匿性气胸的诊断明显优于X线胸片。超声可通过LS消失、胸膜线消失、B线消失、肺脉征消失及出现平流征、肺点等征象,诊断或排除气胸[3,14]。当发生气胸时,脏壁层胸膜相对滑动消失,M型超声表现为“平流征”。B线和LS诊断气胸具有很高敏感度,但特异度不高。肺点是诊断气胸的特异性征象,特异度达100%,尤其是隐匿性气胸;呼气末时,肺组织容积缩小,隐匿性气胸可探及肺滑征缺失和平流征;吸气末时,肺组织容积增大,肺表面触及胸壁,可以探及胸膜线、肺滑征及沙滩征,在M型超声上肺点表现为平流征和沙滩征交替出现。需将探头位于同一位置,才能探测到肺点。另外需注意生理性肺点,但在M型超声不会出现平流征[15]。Volpicelli等[16]对比胸片发现在肺点出现的位置可对气胸进行可靠的定量分类;Oveland等[17]应用动物气胸模型阐述了超声可准确评估在抽气过程中气胸量变化的情况。
2.2 肺实变 肺实变由各种原因引起,包括肺炎、肺栓塞、肺癌及转移性肿瘤、肺压缩或阻塞性不张以及肺挫伤等,肺部超声有助于确定实变类型[3]。肺部超声探及肺组织“肝样变结构”时,表明实变发生在整个肺叶,深部边界规整,而局部肺叶实变,表现为碎片征,深部边界不规则;在实变肺组织内动态支气管征、静态支气管征、支气管充液征以及实变区血流信号征象都可被探及[3,18]。肺部超声诊断肺炎累及胸膜明显优于胸片[19],诊断敏感度和特异度与CT相当[20]。肺炎肝组织样结构征中包含动态、静态支气管充气征,也可见支气管充液征,提示支气管内有液体充填[21],实变区内可探及树枝样血管,应用彩色多普勒可分辨血管或充液支气管。肺不张实变的特征性超声征象包括肝组织样结构征、肺滑征缺失、静态支气管充气征[22],如在实变区内探及动态支气管充气征,可除外肺不张,此征象具有很高的特异度。肺不张早期肺泡及支气管内气体减少前可探及到A线或B线,LS被肺搏征取代。
2.3 肺间质综合征 B线起于胸膜线,当近胸膜的小叶间隔发生水肿、纤维化增厚、扩张时,正常的肺组织气水含量改变,肺间质含水量增多,肺气含量减少,声阻抗增加,声波在肺泡气和肺水之间发生强烈混响,此时可探及到弥漫B线或彗尾征、火箭征,提示肺间质性病变,因肺间质性病变多为弥漫性改变,该征象也可反映肺深部间质病理变化,因此肺部超声可用来协助诊断肺间质性疾病,如心源性肺水肿、急性呼吸窘迫综合征等。研究[7,23]表明彗尾征与肺间质综合征相关度良好,前者诊断后者的敏感度和特异度达93%、97%,Al Deeb等[24]研究表明床旁超声确定心源性肺水肿的敏感度和特异度分别为94%、92%。两个或以上肺区内肋间纵切每个切面3条以上且间距<7 mm的B线呈火箭征或每个肺区均呈“白肺样”改变时,称为肺泡-间质综合征[25-26],与CT上克氏B线一致,且与“白肺”相关,该表现在前胸壁及侧胸壁最为明显,B线的数量和肺气的减少相关,但不特异。单侧或局部肺区出现B线多与肺炎、肺不张、肺挫伤相关;彗尾征和弥漫性B线通常与肺水肿和急性呼吸窘迫综合征相关。明确病因需动态观察超声征象,如心源性肺水肿心功能改善肺水肿好转时,B线变化等,必要时要结合多系统超声改变如心脏大血管、肝脏门静脉及腔静脉变化,综合临床体征及症状进行鉴别诊断。
2.4 肺功能 与其他肺部影像学检查相比,肺部超声可实时探测疾病进展状况。心源性肺水肿患者在积极治疗30 min后,超声即可发现B线数量明显减少[27-28]。同理,液体负荷过度患者经透析治疗后,可通过实时B线评估体液容量状态[29],实时反馈指导临床治疗决策。
2.5 呼吸衰竭 急性呼吸衰竭是重症监护室常见病,早期鉴别病因、及时有效干预对预后尤为重要。床旁急性肺部超声方案(BLUE-protocol)可用于急性呼吸衰竭患者病因的鉴别诊断[30]。BLUE-protocol可用于鉴别诊断6类最为常见的急性疾病(心源性肺水肿、慢性阻塞性肺疾病、哮喘、肺栓塞、气胸、肺炎),准确率高达91%[30]。
2.6 胸腔积液 胸腔积液发生在壁层胸膜和脏层胸膜间的重力依赖区,超声表现为无回声结构,吸气和呼气相均存在,M型超声可见特征性的“正弦征”[3,25],由脏层胸膜随呼吸动作向壁层胸膜移动产生;在B超模式下可见特征性“四边征”,由两侧肋骨声影和上下胸膜线形成的大致四边形结构,两种征象特异度达93%。超声检查不能明确积液类型,但一些回声特征可提示积液性质,如漏出液为无回声,渗出液可是弱回声、低回声或无回声内含漂浮物、分隔回声带,血胸呈低回声伴或不伴点状漂浮物。利用超声引导定位胸腔积液可有效避开肺叶、心脏血管等,提高穿刺准确率及安全性。另外超声还可估算积液量,Remerand等[31]提出了一种准确率较高的测量方法:患者取仰卧位,沿一侧胸廓肋间横切扫查,分别探测胸腔积液的上限和下限,呼气末上下限的距离(Lus)及该线中点处胸腔积液横截面呼气末面积(Aus)的乘积即为胸腔积液量,即V(ml)=Lus(cm)×Aus(cm2)。
2.7 肺栓塞 肺栓塞患者的死亡率很高。肺CTA是诊断金标准,但对于血流动力学不稳定、对比剂过敏、肾功能不全、孕妇及一些紧急情况下均不能使用。肺部超声可作为替代检查手段,但需结合其他检查方法如血浆D-dimer、下肢深静脉超声、Wells评分等。当发生栓塞时,超声可探及到肺外围组织实变,由坏死肺组织和肺不张引起,Mathis等[32]研究发现352例可疑肺栓塞患者中,75%以上可通过超声发现胸膜下肺实变。Squizzato等[33]的Meta分析表明对临床怀疑肺栓塞患者采用超声诊断肺栓塞,敏感度和特异度分别为87%、82%。Lichtenstein等[30]研究表明双侧前胸壁A线为主,同时伴下肢深静脉血栓形成,诊断肺栓塞的敏感度和特异度分别为81%、99%。Nazerian等[34]对357例Wells评分>4或血浆D-dimer(+)患者通过床旁超声分别对肺、心脏、下肢深静脉进行检查,发现床旁超声诊断肺栓塞敏感度和特异度分别为90%、86%。
3 局限性与展望
肺部超声主要应用于累及胸膜的病变,对肺组织深部病变未累及胸膜的疾病应用受限,如肿瘤引起肺实变,实变内常混入气体,会阻碍声波传播,图像不清晰甚至探不出实变影像;部分病因导致的间质综合征,在未累及胸膜的情况下,超声的使用也受限。肺外周结节及肺门肿块、深部肺组织病变如结核、结节病等,可应用腔内超声。另外外科敷料、皮下气肿、肥胖等均会阻碍声波传导,不利于超声应用;超声探头选择不当和仪器参数设置不合理、检查条件不理想等也会影响超声功能的发挥[25]。最后,对临床医师进行培训,才可保证充分发挥超声优势[35]。
肺部超声检查方便,无辐射,可于床旁检查,在肺部常见疾病中有较高的诊断敏感度、特异度、准确率,有望成为某些肺部急性和慢性疾病诊断标准,部分取代胸部X线检查甚至CT检查。
[1] Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care, 2014,20(3):315-322.
[2] Lichtenstein DA. How can the use of lung ultrasound in cardiac arrest make ultrasound a holistic discipline. The example of the SESAME-protocol. Med Ultrasond, 2014,16(3):252-255.
[3] Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med, 2012,38(4):577-591.
[4] Miglioranza MH, Gargani L, Sant'Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: A comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging, 2013,6(11):1141-1151.
[5] Trezzi M, Torzillo D, Ceriani E, et al. Lung ultrasonography for the assessment of rapid extravascular water variation: Evidence from hemodialysis patients. Intern Emerg Med, 2013,8(5):409-415.
[6] Barskova T, Gargani L, Guiducci S, et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann Rheum Dis, 2013,72(3):390-395.
[7] Baldi G, Gargani L, Abramo A, et al. Lung water assessment by lung ultrasonography in intensive care: A pilot study. Intensive Care Med, 2013,39(1):74-84.
[8] Soldati G, Copetti R, Sher S. Sonographic interstitial syndrome: The sound of lung water. J Ultrasound Med, 2009,28(2):163-174.
[9] Cardinale L, Ardissone F, Garetto I, et al. Imaging of benign solitary fibrous tumor of the pleura: A pictorial essay. Rare Tumors, 2010,2(1):e1.
[10] Volpicelli G, Cardinale L, Berchialla P, et al. A comparison of different diagnostic tests in the bedside evaluation of pleuritic pain in the ED. Am J Emerg Med, 2012,30(2):317-324.
[11] Rantanen NW. Diseases of the thorax. Vet Clin North Am Equine Pract, 1986,2(1):49-66.
[12] Ianniello S, Di Giacomo V, Sessa B, et al. First-line sonographic diagnosis of pneumothorax in major trauma: Accuracy of e-FAST and comparison with multidetector computed tomography. Radiol Med, 2014,119(9):674-680.
[13] Ojaghi Haghighi SH, Adimi I, Shams Vahdati S, et al. Ultrasonographic diagnosis of suspected hemopneumothorax in trauma patients. Trauma Mon, 2014,19(4):e17498.
[14] Volpicelli G. Sonographic diagnosis of pneumothorax. Intensive Care Med, 2011,37(2):224-232.
[15] Zhang Z, Chen L. A physiological sign that mimics lung point in critical care ultrasonography. Crit Care, 2015,19(1):155.
[16] Volpicelli G, Boero E, Sverzellati N, et al. Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Med, 2014,40(3):1460-1467.
[17] Oveland NP, Lossius HM, Wemmelund K, et al. Using thoracic ultrasonography to accurately assess pneumothorax progression during positive pressure ventilation: A comparison with CT scanning. Chest, 2013,143(2):415-422.
[18] Lichtenstein DA, Lascols N, Meziere G, et al. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med, 2004,30(1):276-281.
[19] Cortellaro F, Colombo S, Coen D, et al. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg Med J, 2012,29(2):19-23.
[20] Parlamento S, Copetti R, Di Bartolomeo S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med, 2009,27(1):379-384.
[21] Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest X-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med, 2014,32(3):115-118.
[22] Acosta CM, Maidana GA, Jacovitti D, et al. Accuracy of transthoracic lung ultrasound for diagnosing anesthesia-induced atelectasis in children. Anesthesiology, 2014,120(1):1370-1379.
[23] Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med, 2006,24(2):689-696.
[24] Al Deeb M, Barbic S, Featherstone R, et al. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: A systematic review and meta-analysis. Acad Emerg Med, 2014,21(3):843-852.
[25] Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care, 2014,4:1.
[26] Bouhemad B, Zhang M, Lu Q, et al. Clinical review: Bedside lung ultrasound in critical care practice. Crit Care, 2007,11(1):205.
[27] Liteplo AS, Murray AF, Kimberly HH, et al. Real-time resolution of sonographic B-lines in a patient with pulmonary edema on continuous positive airway pressure. Am J Emerg Med, 2010,28(1):541.e5-8.
[28] Bouhemad B, Brisson H, Le-Guen M, et al. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med, 2011,183(2):341-347.
[29] Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest, 2009,135(3):1433-1439.
[30] Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest, 2008,134(1):117-125.
[31] Remerand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med, 2010,36(3):656-664.
[32] Mathis G, Blank W, Reissig A, et al. Thoracic ultrasound for diagnosing pulmonary embolism: A prospective multicenter study of 352 patients. Chest, 2005,128(1):1531-1538.
[33] Squizzato A, Rancan E, Dentali F, et al. Diagnostic accuracy of lung ultrasound for pulmonary embolism: A systematic review and meta-analysis. J Thromb Haemost, 2013, 11(3):1269-1278.
[34] Nazerian P, Vanni S, Volpicelli G, et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism. Crit Ultrasound J, 2014,6(1):A25.
[35] Leech M, Bissett B, Kot M, et al. Lung ultrasound for critical care physiotherapists: A narrative review. Physiother Res Int, 2015,20(1):69-76.
Clinical application of ultrasound in pulmonary disease
ZHANGLei1,2,YUWanjun2*,MAJian2
(1.MedicineSchoolofNingboUniversity,Ningbo315211,China; 2.DepartmentofRespiratory,YinzhouHospitalAffiliatedtoMedicineSchoolofNingboUniversity,Ningbo315040,China)
Point-of-care pulmonary ultrasound is a widely used tool for rapid diagnosis and monitoring treatment in emergency departments and intensive care units. Pulmonary ultrasound has high sensitivity, specificity and diagnostic accuracy in identification of pneumonia, pneumothorax, pulmonary-embolism, pleural effusion, alveolar interstitial syndrome, etc. Besides, it can assess the lung aeration from interstitial syndrome to lung consolidation. Additionally, it provides real-time information of treatment response. Lung ulrasound applications in pulmonary disease were reviewed in this article.
Pulmonary diseases; Ultrasonography; Diagnostic imaging
张磊(1983—),男,安徽阜阳人,在读硕士,主治医师。研究方向:呼吸与危重症医学、超声影像学。E-mail: miraclelei@126.com
俞万钧,宁波大学医学院附属鄞州医院呼吸科,315040。
E-mail: nbywj2008@aliyun.com
2016-08-17
2017-02-13
10.13929/j.1003-3289.201608078
R563; R445.1
A
1003-3289(2017)04-0608-04
