Acupuncture with reinforcing and reducing twirling manipulation inhibits hippocampal neuronal apoptosis in spontaneously hypertensive rats
2017-06-05JuanLuYanGuoChangqingGuoXueminShiNingyuDuRuiliZhaoWenpingDuJingrongLiangShipengZhuHuanChen
Juan Lu, Yan Guo, Chang-qing Guo, Xue-min Shi, Ning-yu Du Rui-li Zhao Wen-ping Du, Jing-rong Liang Shi-peng Zhu, Huan Chen
1 First Hospital Affiliated to Tianjin University of Traditional Chinese Medicine, Tianjin, China
2 Collage of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, China
3 Shijiazhuang Hospital of Traditional Chinese Medicine, Shijiazhuang, Hebei Province, China
4 Second School of Clinical Medicine of Nanjing University of Traditional Chinese Medicine, Nanjing, Jiangsu Province, China
5 Department of Acupuncture and Moxibustion, Jiangsu Province Hospital, Nanjing, Jiangsu Province, China
Acupuncture with reinforcing and reducing twirling manipulation inhibits hippocampal neuronal apoptosis in spontaneously hypertensive rats
Juan Lu1,#, Yan Guo2,#, Chang-qing Guo1,*, Xue-min Shi2,*, Ning-yu Du1, Rui-li Zhao1, Wen-ping Du3, Jing-rong Liang1, Shi-peng Zhu4, Huan Chen5
1 First Hospital Affiliated to Tianjin University of Traditional Chinese Medicine, Tianjin, China
2 Collage of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, China
3 Shijiazhuang Hospital of Traditional Chinese Medicine, Shijiazhuang, Hebei Province, China
4 Second School of Clinical Medicine of Nanjing University of Traditional Chinese Medicine, Nanjing, Jiangsu Province, China
5 Department of Acupuncture and Moxibustion, Jiangsu Province Hospital, Nanjing, Jiangsu Province, China
How to cite this article:Lu J, Guo Y, Guo CQ, Shi XM, Du NY, Zhao RL, Du WP, Liang JR, Zhu SP, Chen H (2017) Acupuncture with reinforcing and reducing twirling manipulation inhibits hippocampal neuronal apoptosis in spontaneously hypertensive rats. Neural Regen Res 12(5):770-778.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This study was supported by the National Natural Science Foundation of China, No. 81072861, 81373727.
Graphical Abstract

Different acupuncture manipulations on hypertension and protection of target organs
To observe the effects of different acupuncture manipulations on blood pressure and target organ damage in spontaneously hypertensive rats (SHRs), this study used the reinforcing twirling method (1.5–2-mm depth; rotating needle clockwise for 360° and then counter clockwise for 360°, with the thumb moving heavily forward and gently backward, 60 times per minute for 1 minute, and retaining needle for 9 minutes), the reducing twirling method (1.5–2-mm depth; rotating needle counter clockwise for 360° and then clockwise for 360°, with the thumb moving heavily backward and gently forward, 60 times per minute for 1 minute, and retaining needle for 9 minutes), and the needle retaining method (1.5–2-mm depth and retaining the needle for 10 minutes). BilateralTaichong(LR3) was treated by acupuncture using different manipulations and manual stimulation. Reinforcing twirling, reducing twirling, and needle retaining resulted in a decreased number of apoptotic cells, reduced Bax mRNA and protein expression, and an increased Bcl-2/Bax ratio in the hippocampus compared with the SHR group. Among these groups, the Bcl-2/Bax protein ratio was highest in the reducing twirling group, and the Bcl-2/Bax mRNA ratio was highest in the needle retaining group. These results suggest that reinforcing twirling, reducing twirling, and needle retaining methods all improve blood pressure and prevent target organ damage by increasing the hippocampal Bcl-2/Bax ratio and inhibiting cell apoptosis in the hippocampus in SHR.
nerve regeneration; spontaneously hypertensive; acupuncture; reinforcing and reducing twirling manipulation; Taichong (LR3); hippocampal CA1 area; blood pressure; apoptosis; neural regeneration
Introduction
Hypertension is one of the most common disorders, affecting 26.4% of the adult population worldwide (Macmahon et al., 2008; Peiris et al., 2015). Long-term hypertension, which causes brain ischemia and hypoxia and damages morphology and functions of brain cells, is a major risk factor for stroke (Tayebati et al., 2012; Gorgui et al., 2014). Previous studies showed that hypertension induces apoptosis in brain neurons and damages target organs before it presents as a cerebrovascular disease (Mignini et al., 2004; Tayebati et al., 2012). Several epidemiological studies indicated a correlation between blood pressure level and cognitive decline (Starr et al., 1993; Posner et al., 2002; Hanon et al., 2003). It is well accepted that a reduced number of small arterioles and capillaries per volume of tissue (rarefaction) plays a major role in elevated vascular resistance and, consequently, blood pressure (Boudier, 2002). Conversely, impaired cerebral perfusion resulting from rarefaction contributes to brain hypoperfusion, which leads to neuronal dysfunction and progressive cognitive failure (Zhang et al., 1994; Ringelstein and Nabavi, 2005). Cognitive decline is dependent on hippocampal functions.
The hippocampus is closely connected to the cardiovascular center and plays an important role in blood pressure regulation (Ueno et al., 2004; Pietranera et al., 2012). One of the hippocampal subfields, the cornu ammonis (CA) 1, is the brain region that is vulnerable to neuronal death (Wong et al., 2005) and is particularly sensitive to hypoxia and ischemia (Raz et al., 2015). Neuronal loss occurs in conjunction with reduced grey matter volume in the CA1 subfield and dentate gyrus of the hippocampus in spontaneously hypertensive rats (SHRs) during the initial stages of hypertension (Sabbatini et al., 2000, 2002). In humans, even relatively brief episodes of transient ischemia result in highly localized CA1 lesions that convey negative cognitive consequences. Thus, vascular risks may affect the CA1 region more than other hippocampal subfields (Bartsch et al., 2011).
Acupuncture is an effective method for treating primary hypertension. Numerous clinical and experimental studies have shown that acupuncture not only lowers blood pressure, but also reduces damage to target organs (Shao et al., 2009; Li et al., 2014). Acupuncture also promotes recovery of damaged brain tissues by improving cerebral blood flow (Kim et al., 2013), inhibiting neuronal apoptosis (Feng et al., 2013), enhancing neuronal plasticity (Tan et al., 2014), and regulating brain metabolism (Tseng et al., 2005). The reinforcing and reducing method affects the curative effect of acupuncture. Twirling is the most commonly used manipulation for the reinforcing or reducing method. Recent studies found that reinforcing and reducing methods through the use of twirling have different curative effects on hypertension (Wang et al., 2011; Liu et al., 2015). Acupuncture is described inNeijing(Internal Classic) as; “wrong application of reinforcing and reducing method will have exactly the reverse effect.” According to traditional Chinese medicine theory,Taichong(LR3) is a commonly used acupoint on the liver meridian of Foot-Jueyin, which can be used to treat hypertension with liverYanghyperactivity syndrome by pacifying the liver to subdueYangand promotingQicirculation to resolve depression. Acupuncture at LR3 activates mainly the liver meridians and can improve mental and physical symptoms of hypertension (Hanon et al., 2003; Ringelstein and Nabavi, 2005).
Previous studies (Mensah et al., 2002; Ashoorkhani et al., 2016; Mallat et al., 2016; Novak et al., 2016) that treated primary hypertension were more focused on anti-hypertensive treatment, improvement of related complications, and protection of target organs, and are applied during the middle and late stages. However, fewer studies have focused on treating hypertension during the early stage and the effects of different acupuncture manipulations (reinforcing method by twirling and reducing method by twirling) on hippocampal neuronal cells. To better understand the effects of different acupuncture manipulations on hypertension and protection of target organs, we measured blood pressure in SHRs, quantified neuronal cells, and analyzed changes in Bax and Bcl-2 expression in the hippocampal CA1 region.
Materials and Methods
Animal preparation
A total of 48 12-week-old, male SHRs and twelve 12-weekold male Wistar-Kyoto (WKY) rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. in China (License No. SCXK (Jing) 200223). Experimental animals were raised in clean facilities with free access to food and water. A controlled environment at 20 ± 1°C, 50% humidity, and a 12-hour light/dark cycle was maintained throughout the study. All procedures for animal experiments were conducted in accordance with World Health Organization’s International Guiding Principles for Biomedical Research Involving Animals, and were approved by the Animal Care and Use Committee at Beijing University of Chinese Medicine (BUCM-4-2015091002-3004).
A total of 48 12-week-old SHRs were randomly divided into four groups (n= 12/group): SHR group, reinforcing twirling, reducing twirling, and needle retaining. Twelve 12-week-old WKY rats served as the blank control group (WKY group).
Acupuncture and moxibustion treatment
The LR3 is located in the dorsum of the foot, in the depression anterior to the junction of the first and second metatarsals. Conscious rats were loosely immobilized in a specially designed restrainer with the hind legs exposed.
In the reinforcing twirling group, the needles (0.16 mm × 7 mm, purchased from Suzhou Acupuncture Goods Co., Ltd., Suzhou, Jiangsu Province, China) were directly inserted bilaterally into LR3 for 1.5–2 mm. The reinforcing twirling (rotate the needle clockwise for 360° and then counter clockwise for 360°, with the thumb moves heavily forward and gently backward, 60 times per minute) was applied for 1 minute, and the needle was retained for 9 minutes aftermanipulation.
In the reducing twirling group, the needles were directly inserted bilaterally into LR3 for 1.5–2 mm. The reducing twirling method (rotate the needle counter clockwise for 360° and then clockwise for 360°, with the thumb moves heavily backward and gently forward, 60 times per minute) was applied for 1 minute and the needle was retained for 9 minutes after manipulation.
In the needle retaining group, the needles were directly inserted bilaterally into LR3 for 1.5–2 mm and retained for 10 minutes with no manipulation.
In the SHR group and WKY group, rats were immobilized in the same restrainer for 10 minutes without acupuncture intervention. The treatment was initiated at 08:00 for 10 minutes, 6 days per treatment, with a 1-day interval between every two courses. The entire treatment lasted for 9 weeks.
Blood pressure measurement
Blood pressure was measured between 20:00 and 00:00. After 1 week of adaptive feeding, systolic pressure and diastolic pressure of the caudal artery were measured using a noninvasive blood pressure instrument (BP-6, Chengdu Thai Union Biological Instrument Co., Ltd., Chengdu, China). Each rat was measured three times, and the average blood pressure recorded served as the baseline value. Rats that did not meet diagnostic criteria for hypertension were excluded. Subsequently, blood pressure was measured in all rats at 12, 15, 18, and 21 weeks.
Sample collection
Rat caudal artery blood pressure was measured at 21 weeks after blood pressure measurements. Subsequently, the rats were sacrificed after anesthesia with an intraperitoneal injection of 100 g/L chloral hydrate (500 mg/kg). A total of 6 rats/group were randomly selected for brain harvesting. The brains were then fixed in 4°C 40 g/L paraformaldehyde for terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay. For rats in the other groups, hippocampi were obtained and placed on ice, washed in 4°C saline, dried, fixed in liquid nitrogen, and stored at −80°C for western blot assay and reverse transcription-polymerase chain reaction (RT-PCR).
TUNEL assay
The TUNEL assay was applied to detect apoptosis in the rat hippocampal CA1 area. Samples were paraffin-embedded, sectioned, and processed using the TUNEL kit (TACS. XL DAB In Situ Apoptosis Detection Kit, Cat. No. 4828-30-DK, TREVIGEN, New York, NY, USA). Apoptotic cells were characterized by a dark-brown staining of the nucleus and nuclear membrane. Quantification was performed by counting the number of positive cells in four randomly chosen fields within each 400×-frame using an Olympus optic microscope (BX43, Olympus, Japan). Image-pro plus 6.0 software (Media Cybernetics, Boston, MA, USA) was used to quantify the TUNEL-positive cells.
Western blot assay
The western blot assay was used to measure Bcl-2 and Bax protein expression in the rat hippocampus. The tissues were homogenized in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate) and centrifuged at 12,000 r/min for 10 minutes at 4°C in a microcentrifuge (LEGEND MICRO, Thermo, Waltham, MA, USA). Protein concentrations were estimated using the Pierce BCA Protein Assay Kit (Thermo Scientific, Cat. No. 23228). Equal amounts of protein were electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gels at 100 V for 1.5 hours in a Mini PROTEAN Tetra Cell (BioRad Laboratories Inc, Hercules, CA, USA). Proteins were transblotted onto polyvinylidene fluoride membranes (Mini Trans-Blot Cell, BioRad Laboratories Inc., Hercules, CA, USA) and incubated in anti-Bcl-2 polyclonal antibody (1:2,000; Abcam, Cambridge, MA, USA), anti-Bax polyclonal antibody (1:1,000; Abcam), and GAPDH polyclonal antibodies (1:1,250; MILLIPORE, Darmstadt, Germany) at 4°C overnight, followed by incubation in the corresponding secondary antibodies (horseradish-peroxidase goat anti-rabbit IgG; 1:100,000; 40 minutes, 37°C). The protein bands were detected using enhanced chemiluminescence-chromogenic detection and luminescent substrate A/B (Engree Biosystem Co., Ltd., Beijing, China). Bcl-2, Bax, and GAPDH protein bands were scanned using Image Lab 4.0.1 software (Sausage Software, Blaine, WA, USA) and integrated density values were calculated using Gel-Pro analyzer4 software (Media Cybernetics, Bethesda, MD, USA) and were normalized to GAPDH expression.
RT-PCR
RT-PCR was used to measure Bcl-2 and Bax mRNA expression in the rat hippocampus. Tissues were harvested and total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was reverse-transcribed to cDNA using a reverse transcription kit (Promege, Madison, WI, USA) according to manufacture instructions. The resulting cDNA PCR fragments were amplified in a DNA Engine thermal cycler (Bio-Rad, T100, Richmond, CA, USA) at 1 cycle of 10 minutes at 95°C, 35 cycles of 45 seconds at 95°C, 45 s at 64°C, and 1 minute at 72°C. After cycling, a melting protocol was performed for 7 minutes at 72°C and then held at 12°C. The PCR products were separated by 1.2% agarose gel electrophoresis. According to GenBank sequences, Oligo7.37 software (Molecular Biology Insights, Colorado Springs, CO, USA) was used to design primers (Table 1). Quantity One V4.62 (Bio-Rad, Hercules, CA, USA) was used to analyze the electrophoresis gray level of Bcl-2 and Bax mRNA and to calculate relative expression. Relative expression amount was equal to the experimental gray level/internal reference grey level. GAPDH was utilized as an internal reference.
Statistical analysis
Data, presented as the mean ± SD, were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Differences among groups were examined using one-way analysis of variance,followed by the Student-Newman-Keuls test. A value ofP< 0.05 was considered statistically significant.

Table 1 Primer sequences employed for reverse transcriptionpolymerase chain reaction and anticipated product size
Results
Effects of reinforcing and reducing twirling manipulation on systolic blood pressure in SHR
Systolic blood pressure in rats was measured to determine the effects of different acupuncture manipulations on blood pressure. Systolic blood pressure in WKY rats was maintained at a normal level at 12, 15, 18, and 21 weeks. Compared with the WKY group, systolic blood pressure in the SHR group was significantly increased at 15, 18, and 21 weeks (P< 0.01). Compared with the SHR group, systolic blood pressure in the reducing twirling group and needle retaining group was significantly decreased at 15, 18, and 21 weeks (P< 0.01). Compared with the SHR group, systolic blood pressure in the reinforcing twirling group was decreased at 15 (P< 0.01) and 18 weeks (P< 0.05), with no significant difference at 20 weeks. Compared with the reinforcing twirling group, systolic blood pressure in the reducing twirling group was significantly decreased at 15 (P= 0.019), 18 (P< 0.01), and 21 (P< 0.01) weeks, and systolic blood pressure in the needle retaining group was significantly decreased at 18 and 21 weeks (P< 0.01). Compared with the reducing twirling group, systolic blood pressure in the needle retaining group was significantly increased at 18 (P= 0.036) and 21 weeks (P< 0.01; Figure 1).
Effects of reinforcing and reducing twirling manipulation on hippocampal neuronal apoptosis in the hippocampal CA1 area in SHR
In the WKY group, neurons in the hippocampal CA1 area were stained blue with few TUNEL-positive cells, whereas, in the SHR group, TUNEL-positive cells were widely distributed. There were fewer TUNEL-positive cells in the reducing twirling group, reinforcing twirling group, and needle retaining group (Figure 2A–E). The quantitative TUNEL results are shown in Figure 2F. The SHR group exhibited significantly increased apoptosis compared with the WKY group (P< 0.05). There was significantly less apoptosis in the reducing twirling group, reinforcing twirling group, and needle retaining group compared with the SHR group (P< 0.05), with no significant difference between the three groups (P> 0.05).
Effects of reinforcing and reducing twirling manipulation on Bax and Bcl-2 protein expression and the Bcl-2/Bax ratio in the hippocampus of SHR
As important biological indexes of the apoptosis response, Bax and Bcl-2 promote or inhibit neuronal apoptosis by transducing the apoptosis signal (Wang et al., 2016). The ratio of Bcl-2/Bax is regarded as an index of the overall trend of cell apoptosis (Mohammadi et al., 2016). Compared with the WKY group, the SHR group showed a significant increase in protein expression of Bax (P< 0.01) and Bcl-2 (P< 0.01) and a significant decrease in the Bcl-2/Bax ratio (P< 0.01). Compared with the SHR group, the reducing twirling, reinforcing twirling, and needle retaining groups exhibited significantly decreased protein expression of Bax (allP< 0.01) and an increased Bcl-2/ Bax ratio (allP< 0.01). Bcl-2 protein expression in the reinforcing twirling group and needle retaining group was significantly decreased compared with the SHR group (P< 0.01), although there was no significant difference between the SHR group and reducing twirling group (P> 0.05). Compared with the reducing twirling group, Bcl-2 protein expression and the Bcl-2/Bax ratio were significantly decreased in the reinforcing twirling and needle retaining groups (P< 0.01; Figure 3).
Effects of reinforcing and reducing twirling manipulation on Bax and Bcl-2 mRNA expression and the Bcl-2/Bax ratio in the hippocampus of SHR
Compared with the WKY group, the SHR group showed a significant increase in Bax and Bcl-2 mRNA expression (P< 0.01) and a significant decrease in Bcl-2/Bax mRNA ratio (P< 0.01). Compared with the SHR group, the reducing twirling group, reinforcing twirling group, and needle retaining group showed a significant decrease in Bax mRNA expression (P< 0.01) and a marked increase in the Bcl-2/ Bax mRNA ratio (P< 0.01). Bcl-2 mRNA expressions in the reinforcing twirling group and needle retaining group were significantly decreased compared with the SHR group (P< 0.01), with no significant difference between the SHR group and reducing twirling group (P> 0.05). Compared with the reinforcing twirling group, Bcl-2 mRNA expression in the reducing twirling group was markedly increased (P= 0.022), and the Bcl-2/Bax mRNA ratio in needle retaining group was significantly increased (P< 0.01). No obvious difference in Bax mRNA expression was found among the reducing twirling group, reinforcing twirling group, and needle retaining group (P> 0.05; Figure 4).
Discussion
Hypertension is commonly known to have unfavorable effects on health status (Cook et al., 2016). Long-term hypertension leads to a continuous shortage of blood, oxygen, and energy supply, which induces neuronal apoptosis in the brain. Studies have shown that the hippocampus is the most sensitive brain region to ischemia and anoxia (Lipton andLobner, 1990; Park et al., 2016; Yoo et al., 2016). Closely connected to the cardiovascular center, the hippocampus plays an important role in blood pressure regulation (Ueno et al., 2004; Pietranera et al., 2012). Furthermore, hypertension induces apoptosis in brain neurons during the early stages of hypertension and prior to the symptoms of cerebrovascular disease (Mignini et al., 2004).
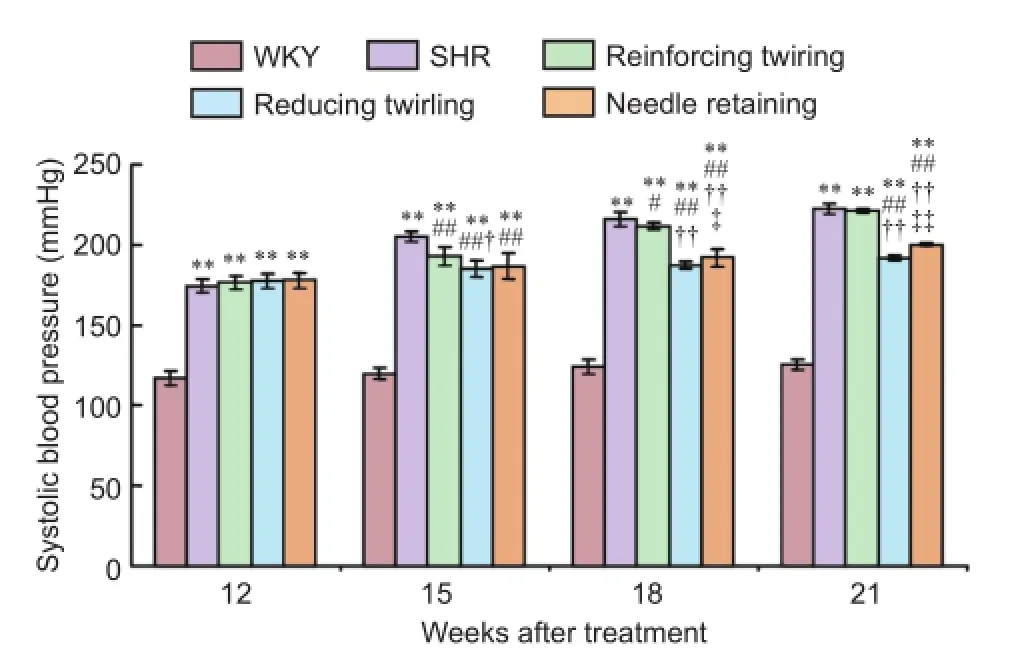
Figure 1 Changes in systolic blood pressure after different acupuncture manipulations in SHR.
SHRs are the result of selective mating from WKY rats and are internationally recognized and widely used in basic hypertension research (Tayebati et al., 2012). Compared with WKY rats of the same age, early-stage SHRs (from 7 to 12 weeks) are more aggressive and more easily irritated, with red and protruding eyes, dry stool, and yellow urine (Hui-Hua et al., 2008). According to the theory of traditional Chinese medicine, SHRs during this stage exhibit liver-yang hyperactivity syndrome (Zhong et al., 2015). Blood pressure is normal at birth, but continuously rises from 3 to 6 months. The features of SHRs hypertension are similar to humans with regard to increased time-dependent arterial blood pressure, brain atrophy, neuronal loss, and glial cell reactions. Therefore, SHRs are often used to study brain damage caused by hypertension (Amenta et al., 2010). Previous studies have shown that hippocampal dysfunction already occurs during the initial stages of hypertension in SHR. With the development of hypertension, volumes of CA1, CA3, dentate gyrus, corpus callosum, and the hippocampal external capsule gradually shrink and the area of neuronal apoptosis gradually expands in SHR (Mignini et al., 2004; Lanari et al., 2007; Amenta et al., 2010; Fotuhi et al., 2012; Tayebati et al., 2012). In the present study, compared with the WKY group, systolic blood pressure in the SHR group significantly increased at 12 weeks, indicating successful model establishment. Results also showed a greater number of widely distributed TUNEL-positive cells in the SHR group compared with the WKY group, indicating that hypertension increased hippocampal neuronal apoptosis. This finding was consistent with the other studies showing that hypertension leads to brain damage (Mignini et al., 2004; Amenta et al., 2010; Fotuhi et al., 2012; Tayebati et al., 2012).


Figure 2 Effects of reinforcing and reducing twirling manipulation on cell apoptosis in the hippocampal CA1 area of SHR.

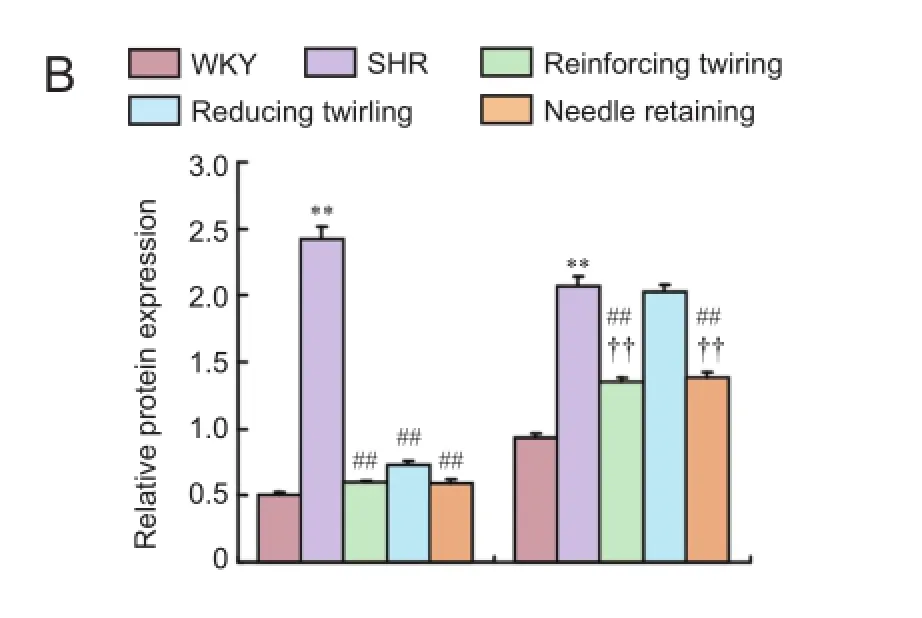

Figure 3 Protein expression of Bax and Bcl-2 in the hippocampus of rats (western blot assay).


Figure 4 Reverse transcription-polymerase chain reaction for mRNA expression of Bax and Bcl-2 in the hippocampus of rats (RT-PCR).
Anti-apoptotic Bcl-2 and pro-apoptotic Bax are expressed in specific areas of the brain and exhibit antagonistic properties (Yang et al., 2016). As important biological indexes of the apoptotic response, they promote or inhibit neuronal apoptosis. Bcl-2 exerts anti-apoptotic effects by inhibiting the effects of oxygen-free radicals on lipid peroxidation, while Bax counteracts Bcl-2 functions, thereby accelerating apoptosis (Rincheval et al., 2012). Under normal circumstances, products of these two genes remain relatively balanced. An imbalance in expression can lead to promotion or inhibition of cell apoptosis. Therefore, the ratio of Bcl-2/Bax can be used as an index of cell apoptosis (Oltvai and Korsmeyer, 1994; Croker et al., 2011). Studies have shown that long-term hypertension increases Bax expression, thereby decreasing the ratio of Bcl-2/Bax and inducing excessive apoptosis in rat brain cells. This consequently results in decreased learning and memory abilities (Wernig and Xu, 2002; Li et al., 2012). In the present study, western blot assay and RT-PCR results revealed the same trend compared with the WKY group; Bax and Bcl-2 mRNA and protein expression significantly increased, and the Bcl-2/Bax ratio significantly decreased in the SHR group. These results suggested that high blood pressure led to increased Bax mRNA expression and brain damage in the SHR group. The endogenous protective system was likely activated to antagonize Bax and inhibit neuronal apoptosis by promoting Bcl-2 expression.
Previous studies have shown that acupuncture is an effective method for treating primary hypertension (Lai et al., 2012; Cevik and Iseri, 2013; Longhurst and Tjen-A-Looi, 2013; Li et al., 2014). Some studies have shown that acupuncture at the LR3 acupoint alleviates high blood pressure induced by essential hypertension (Zhang et al., 1994). Modern studies indicate that the central mechanism of acupuncture for anti-hypertension lies in activation of the hypothalamic arcuate nucleus and periaqueductal gray in the anterior ventral region, as well as inhibition of expression of cardiovascular sympathetic neurons and Apelin (multifunctional peptide that regulates blood pressure and heart function) in the rostral ventrolateral medulla (Michikami et al., 2006; Guo and Longhurst, 2010; Zhang et al., 2013a). Furthermore, by regulating Bcl-2 and Bax expression and reducing the ratio of Bax/Bcl-2 in the brain, acupuncture could inhibit excessive apoptosis of brain cells, thereby protecting against hypertension-induced damage (Wu and Wang, 2011).
Reinforcing and reducing manipulation affects the curative effect of acupuncture. The application of correct reinforcing or reducing method, according to deficiency or excess of disease, is a prerequisite for curative effects. Twirling is the most commonly used method for the reinforcing or reducing method in clinical practice. Previous studies primarily applied acupuncture therapy during the middle or late stages of hypertension. However, the study utilized different reinforcing and reducing methods during a relatively early stage (12 weeks old). Our results indicated that acupuncture had beneficial effects on blood pressure in SHR, with the effects differing according to the manipulations applied (reinforcing twirling, reducing twirling, and needle retaining). Compared with the SHR group, systolic blood pressure significantly decreased in the reducing twirling group, reinforcing twirling group, and needle retaining group at 15 weeks. The antihypertensive effect of acupuncture in the reducing twirling group and needle retaining group was maintained for 21 weeks. However, this effect was no longer observed in the reinforcing twirling group at 21 weeks. Additionally, compared with the reinforcing twirling group and needle retaining group, the reducing twirling group showed superior antihypertensive effects from 15 to 21 weeks. Therefore, we concluded that among the three acupuncture treatment methods, reducing twirling results in the best antihypertensive effects. Furthermore, the effects of reducing twirling and needle retaining lasted longer than reinforcing twirling. All results were consistent with previous findings (Wang et al., 2011; Liu et al., 2015; Zhong et al., 2015).
Compared with the SHR group, the number of TUNEL-positive cells significantly decreased in the hippocampal CA1 area in the reducing twirling group, reinforcing twirling group, and needle retaining group, which suggested that acupuncture had a protective effect on hippocampal neurons. These results were consistent with previous findings showing that acupuncture exerts neuroprotective effects by inhibiting cellular apoptosis in the rat hippocampus (Zheng et al., 2007; Zhang et al., 2015).
Moreover, acupuncture exhibited significant regulatory effects on Bax and Bcl-2 mRNA and protein expression, as well as the Bcl-2/Bax ratio. Compared with the SHR group, Bax and Bcl-2 mRNA and protein expression significantly decreased in the reinforcing twirling group and needle retaining group; Bax mRNA and protein expression drastically decreased, and Bcl-2 mRNA expression was slightly lowered in the reducing twirling group. All acupuncture treatments resulted in a significantly increased Bcl-2/Bax ratio compared with the SHR group. This result was not consistent with previous findings showing that acupuncture counteracts apoptosis by up-regulating Bcl-2 mRNA and protein expression (Wu and Wang, 2011; Zhang et al., 2013b). These results revealed decreased Bax expression in the reinforcing twirling group, reducing twirling group, and needle retaining group, which suggests that accelerated apoptosis was restricted in the brains of hypertensive rats. It is possible that Bcl-2 expression was automatically reduced, thereby maintaining a relative balance between Bax and Bcl-2. Moreover, some studies showed that the regulatory effect of acupuncture on Bax and Bcl-2 expression not only reduced Bax expression, but increased Bcl-2 expression; acupuncture also results in altered expression of one or both genes (Zhao et al., 2015). The increased ratio of Bcl-2/Bax is the determining factor for inhibiting apoptosis and protecting target organs (Siddiqui et al., 2015). We hypothesized that the effect of acupuncture regulation on Bax and Bcl-2 expression is a comprehensive and bidirectional regulatory process. Bax and Bcl-2 both relate mutually and oppose mutually, and maintain the dynamic balance under normal states. Pathological conditions may upset the balance between pro-apoptotic genes and apoptosis suppressor genes, thereby decreasing the Bcl-2/ Bax ratio. That is considered the imbalance ofYinandYang, which accelerates the apoptosis. Acupuncture intervention can harmonizeYinandYang, and inhibit excessive apoptosis of brain cells, thereby protecting against hypertension-induced damage.
Results from our study showed that different acupuncture manipulation can trigger different types of central responses, which was consistent with previous results (MacPherson et al., 2008; Park et al., 2011; Hu et al., 2014). The Bcl-2/Bax ratio of mRNA and protein expression significantly increased with reducing twirling compared with reinforcing twirling. This suggested that the twisting reducing method is better than the twisting reinforcing method in protecting brain neurons by regulating Bax and Bcl-2 expression and the Bcl-2/Bax ratio in the rat hippocampus. Nevertheless, future studies are needed to determine whether other apoptotic mechanisms are involved in hypertension.
In summary, reinforcing twirling, reducing twirling, and needle retaining methods may all improve blood pressure and prevent target organ damage by increasing the Bcl-2/Bax ratio and inhibiting apoptosis of hippocampal neurons in SHR. Reducing twirling significantly increased the mRNA and protein Bcl-2/Bax ratio compared with reinforcing twirling.
Author contributions:JL and YG wrote the paper, were responsible for the design and performance of the main experiments. CQG and XMS were responsible for the design and guidance in all works. NYD took charge of western blot assay and RT-PCR. RLZ took charge of TUNEL assay. WPD helped with data analysis. JRL recorded the blood pressure of rats. SPZ and HC modified language expressions throughout the article. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Amenta F, Tayebati SK, Tomassoni D (2010) Spontaneously hypertensive rat neuroanatomy: applications to pharmacological research. Ital J Anat Embryol 115:13-17.
Ashoorkhani M, Bozorgi A, Majdzadeh R, Hosseini H, Yoonessi A, Ramezankhani A, Eftekhar H (2016) Comparing the effectiveness of the BPMAP (Blood Pressure Management Application) and usual care in self-management of primary hypertension and adherence to treatment in patients aged 30-60 years: study protocol for a randomized controlled trial. Trials 17:511.
Bartsch T, Dohring J, Rohr A, Jansen O, Deuschl G (2011) CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci U S A 108:17562-17567.
Boudier HA (2002) Hypertension and microcirculation. Arch Mal Coeur Vaiss 95 Spec No 6:17-22.
Cevik C, Iseri SO (2013) The effect of acupuncture on high blood pressure of patients using antihypertensive drugs. Acupunct Electrother Res 38:1-15.
Cook NR, Appel LJ, Whelton PK (2016) Sodium intake and all-cause mortality over 20 years in the trials of hypertension prevention. J Am Coll Cardiol 68:1609-1617.
Croker BA, O’Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang JG, White M, Lackovic K, Cengia LH, O’Reilly LA, Bouillet P, Cory S, Strasser A, Roberts AW (2011) Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci U S A 108:13135-13140.
Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L (2013) Electroacupuncture ameliorates cognitive impairment through inhibition of NF-kappaB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep 7:1516-1522.
Fotuhi M, Do D, Jack C (2012) Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 8:189-202.
Gorgui J, Gorshkov M, Khan N, Daskalopoulou SS (2014) Hypertension as a risk factor for ischemic stroke in women. Can J Cardiol 30:774-782.
Guo ZL, Longhurst JC (2010) Activation of reciprocal pathways between arcuate nucleus and ventrolateral periaqueductal gray during electroacupuncture: involvement of VGLUT3. Brain Res 1360:77-88.
Hanon O, Seux ML, Lenoir H, Rigaud AS, Forette F (2003) Hypertension and dementia. Curr Cardiol Rep 5:435-440.
Hu NJ, Lin C, Li J, Zhang P, Yuan HW, Qi DD, Hao J, Xin SY, Liu YQ, Li CH, Wang P, Zhu J (2014) Remarks on the relationship between deqi and effect of acupuncture. Zhongguo Zhen Jiu 34:413-416.
Hui-Hua QU, Zhao Y, Rong-Bo QU, Tang EQ, Zhou G, Xie T, Wang QG (2008) Study on the syndrome difference of two rat models of hypertension. Acta Laboratorium Animalis Scientia Sinica. 16:31-35.
Kim JH, Choi KH, Jang YJ, Bae SS, Shin BC, Choi BT, Shin HK (2013) Electroacupuncture acutely improves cerebral blood flow and attenuates moderate ischemic injury via an endothelial mechanism in mice. PLoS One 8:e56736.
Lai X, Wang J, Nabar NR, Pan S, Tang C, Huang Y, Hao M, Yang Z, Ma C, Zhang J, Chew H, He Z, Yang J, Su B, Zhang J, Liang J, Sneed KB, Zhou SF (2012) Proteomic response to acupuncture treatment in spontaneously hypertensive rats. PLoS One 7:e44216.
Lanari A, Silvestrelli G, De Dominicis P, Tomassoni D, Amenta F, Parnetti L (2007) Arterial hypertension and cognitive dysfunction in physiologic and pathologic aging of the brain. Am J Geriatr Cardiol 16:158-164.
Li DZ, Zhou Y, Yang YN, Ma YT, Li XM, Yu J, Zhao Y, Zhai H, Lao L (2014) Acupuncture for essential hypertension: a meta-analysis of randomized sham-controlled clinical trials. Evid Based Complement Alternat Med 2014:279478.
Li Y, Duan Z, Gao D, Huang S, Yuan H, Niu X (2012) The new role of LOX-1 in hypertension induced neuronal apoptosis. Biochem Biophys Res Commun 425:735-740.
Lipton P, Lobner D (1990) Mechanisms of intracellular calcium accumulation in the CA1 region of rat hippocampus during anoxia in vitro. Stroke 21:Iii60-64.
Liu W, Zhu LQ, Chen SS, Lu SC, Tang J, Liu QG (2015) Effect of twirling-reinforcing-reducing needling manipulations on contents of serum acetylcholine and arterial NOS and cGMP in stress-induced hypertension rats. Zhen Ci Yan Jiu 40:136-140.
Longhurst JC, Tjen-A-Looi S (2013) Acupuncture regulation of blood pressure: two decades of research. Int Rev Neurobiol 111:257-271.
Macmahon S, Alderman MH, Lindholm LH, Liu L, Sanchez RA, Seedat YK (2008) Blood-pressure-related disease is a global health priority. J Hypertens 26:2071-2072.
MacPherson H, Green G, Nevado A, Lythgoe MF, Lewith G, Devlin R, Haselfoot R, Asghar AU (2008) Brain imaging of acupuncture: comparing superficial with deep needling. Neurosci Lett 434:144-149.
Mallat SG, Tanios BY, Itani HS, Lotfi T, Akl EA (2016) Free versus fixed combination antihypertensive therapy for essential arterial hypertension: a systematic review and meta-analysis. PLoS One 11:e0161285.
Mensah GA, Croft JB, Giles WH (2002) The heart, kidney, and brain as target organs in hypertension. Cardiol Clin 20:225-247.
Michikami D, Kamiya A, Kawada T, Inagaki M, Shishido T, Yamamoto K, Ariumi H, Iwase S, Sugenoya J, Sunagawa K, Sugimachi M (2006) Short-term electroacupuncture at Zusanli resets the arterial baroreflex neural arc toward lower sympathetic nerve activity. Am J Physiol Heart Circ Physiol 291:H318-326.
Mignini F, Vitaioli L, Sabbatini M, Tomassoni D, Amenta F (2004) The cerebral cortex of spontaneously hypertensive rats: a quantitative microanatomical study. Clin Exp Hypertens 26:287-303.
Mohammadi A, Yaghoobi MM, GholamhoseynianNajar A, Kalantari-Khandani B, Sharifi H, Saravani M (2016) HSP90 inhibitor enhances anti-proliferative and apoptotic effects of celecoxib on HT-29 colorectal cancer cells via increasing BAX/BCL-2 ratio. Cell Mol Biol (Noisy-Le-Grand) 62:62-67.
Novak K, Vrdoljak D, Jelaska I, Borovac JA (2016) Sex-specific differences in risk factors for in-hospital mortality and complications in patients with acute coronary syndromes: An observational cohort study. Wien Klin Wochenschr doi: 10.1007/s00508-016-1105-7.
Oltvai ZN, Korsmeyer SJ (1994) Checkpoints of dueling dimers foil death wishes. Cell 79:189-192.
Park JJ, Akazawa M, Ahn J, Beckman-Harned S, Lin FC, Lee K, Fine J, Davis RT, Langevin H (2011) Acupuncture sensation during ultrasound guided acupuncture needling. Acupunct Med 29:257-265.
Park SM, Park CW, Lee TK, Cho JH, Park JH, Lee JC, Chen BH, Shin BN, Ahn JH, Tae HJ, Shin MC, Ohk TG, Cho JH, Won MH, Choi SY, Kim IH (2016) Effect of ischemic preconditioning on antioxidant status in the gerbil hippocampal CA1 region after transient forebrain ischemia. Neural Regen Res 11:1081-1089.
Peiris D, Thompson SR, Beratarrechea A, Cardenas MK, Diez-Canseco F, Goudge J, Gyamfi J, Kamano JH, Irazola V, Johnson C, Kengne AP, Keat NK, Miranda JJ, Mohan S, Mukasa B, Ng E, Nieuwlaat R, Ogedegbe O, Ovbiagele B, Plange-Rhule J, et al. (2015) Behaviour change strategies for reducing blood pressure-related disease burden: findings from a global implementation research programme. Implement Sci 10:158.
Pietranera L, Brocca ME, Cymeryng C, Gomez-Sanchez E, Gomez-Sanchez CE, Roig P, Lima A, De Nicola AF (2012) Increased expression of the mineralocorticoid receptor in the brain of spontaneously hypertensive rats. J Neuroendocrinol 24:1249-1258.
Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R (2002) The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology 58:1175-1181.
Raz N, Daugherty AM, Bender AR, Dahle CL, Land S (2015) Volume of the hippocampal subfields in healthy adults: differential associations with age and a pro-inflammatory genetic variant. Brain Struct Funct 220:2663-2674.
Rincheval V, Bergeaud M, Mathieu L, Leroy J, Guillaume A, Mignotte B, Le Floch N, Vayssiere JL (2012) Differential effects of Bcl-2 and caspases on mitochondrial permeabilization during endogenous or exogenous reactive oxygen species-induced cell death: a comparative study of H(2)O(2), paraquat, t-BHP, etoposide and TNF-alpha-induced cell death. Cell Biol Toxicol 28:239-253.
Ringelstein EB, Nabavi DG (2005) Cerebral small vessel diseases: cerebral microangiopathies. Curr Opin Neurol 18:179-188.
Sabbatini M, Strocchi P, Vitaioli L, Amenta F (2000) The hippocampus in spontaneously hypertensive rats: a quantitative microanatomical study. Neuroscience 100:251-258.
Sabbatini M, Catalani A, Consoli C, Marletta N, Tomassoni D, Avola R (2002) The hippocampus in spontaneously hypertensive rats: an animal model of vascular dementia? Mech Ageing Dev 123:547-559.
Shao Y, Lai XS, Guan CW, Xie LL, Wu LN, Tang CZ (2009) Effect of electroacupuncture on learning-memory ability of vascular dementia rats with concomitant hypertension and hyperlipemia. Zhen Ci Yan Jiu 34:368-375.
Siddiqui WA, Ahad A, Ahsan H (2015) The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol 89:289-317.
Starr JM, Whalley LJ, Inch S, Shering PA (1993) Blood pressure and cognitive function in healthy old people. J Am Geriatr Soc 41:753-756.
Tan F, Chen J, Liang Y, Gu M, Li Y, Wang X, Meng D (2014) Electroacupuncture attenuates cervical spinal cord injury following cerebral ischemia/reperfusion in stroke-prone renovascular hypertensive rats. Exp Ther Med 7:1529-1534.
Tayebati SK, Tomassoni D, Amenta F (2012) Spontaneously hypertensive rat as a model of vascular brain disorder: microanatomy, neurochemistry and behavior. J Neurol Sci 322:241-249.
Tseng CS, Shen WC, Cheng FC, Chen GW, Li TC, Hsieh CL (2005) Dynamic change in energy metabolism by electroacupuncture stimulation in rats. Am J Chin Med 33:767-778.
Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang CL, Kanenishi K (2004) Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol 107:532-538.
Wang L, Jing MX, Zhi JM, Lu J, Wang CY, Liu QG (2011) Effects of reinforcing and reducing methods by twirling and rotating the needle on contents of CGRP and NO in rats with stress-induced hypertension. Zhongguo Zhen Jiu 31:337-341.
Wang X, Xie Y, Zhang T, Bo S, Bai X, Liu H, Li T, Liu S, Zhou Y, Cong X, Wang Z, Liu D (2016) Resveratrol reverses chronic restraint stress-induced depression-like behaviour: Involvement of BDNF level, ERK phosphorylation and expression of Bcl-2 and Bax in rats. Brain Res Bull 125:134-143.
Wernig F, Xu Q (2002) Mechanical stress-induced apoptosis in the cardiovascular system. Prog Biophys Mol Biol 78:105-137.
Wong LF, Ralph GS, Walmsley LE, Bienemann AS, Parham S, Kingsman SM, Uney JB, Mazarakis ND (2005) Lentiviral-mediated delivery of Bcl-2 or GDNF protects against excitotoxicity in the rat hippocampus. Mol Ther 11:89-95.
Wu DH, Wang GY (2011) Effect of mild hypothermia in combination of acupuncture on Bcl-2 and Bax protein expressions of local cerebral ischemia/reperfusion rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 31:1506-1509.
Yang RJ, Yin WY, Li H (2016) Expression levels of Bax, Bcl-2 and brain-derived neurotrophic factor in the rat brain motor cortex after over fatigue-induced sudden death. Zhongguo Zuzhi Gongcheng Yanjiu 20:7377-7383.
Yoo DY, Lee KY, Park JH, Jung HY, Kim JW, Yoon YS, Won MH, Choi JH, Hwang IK (2016) Glucose metabolism and neurogenesis in the gerbil hippocampus after transient forebrain ischemia. Neural Regen Res 11:1254-1259.
Zhang CR, Xia CM, Jiang MY, Zhu MX, Zhu JM, Du DS, Liu M, Wang J, Zhu DN (2013a) Repeated electroacupuncture attenuating of apelin expression and function in the rostral ventrolateral medulla in stress-induced hypertensive rats. Brain Res Bull 97:53-62.
Zhang WW, Ma KC, Andersen O, Sourander P, Tollesson PO, Olsson Y (1994) The microvascular changes in cases of hereditary multi-infarct disease of the brain. Acta Neuropathol 87:317-324.
Zhang Y, Lan R, Wang J, Li XY, Zhu DN, Ma YZ, Wu JT, Liu ZH (2015) Acupuncture reduced apoptosis and up-regulated BDNF and GDNF expression in hippocampus following hypoxia-ischemia in neonatal rats. J Ethnopharmacol 172:124-132.
Zhang ZN, Li JY, Zhao Y, Wang JQ, Huang C, Fan GQ (2013b) Effects of Tongnao Huoluo acupuncture therapy on Caspase-3 and Bcl-2 of rats with acute cerebral infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi 33:646-650.
Zhao XW, Dong Q, Shen MH (2015) Study of acupuncture on cerebral ischemia after apoptosis-related genes Bcl-2,Bax regulatory role. Jilin Zhongyiyao 35:730-732.
Zheng YQ, Liu JX, Wang JN, Xu L (2007) Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res 1138:86-94.
Zhong G, Xiang L, Hu J, Yin Y, Chen Q, Fang X (2015) Effect of Pinggan Qianyang recipe on the expression of Tpx II HSP27 and ANXA1 in the hypothalamus of spontaneously hypertensive rats with hyperactivity of liver-YANG syndrome. Zhong Nan Da Xue Xue Bao Yi Xue Ban 40:136-143.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Chang-qing Guo or Xue-min Shi, guochangqing5901@163.com or shixuemin56789@163.com.
Chang-qing Guo or Xue-min Shi, guochangqing5901@163.com or shixuemin56789@163.com.
#These authors contributed equally to this study.
orcid: 0000-0003-0414-3423 (Xue-min Shi)
10.4103/1673-5374.206648
Accepted: 2017-04-20
杂志排行
中国神经再生研究(英文版)的其它文章
- Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial
- Therapeutic opportunities and challenges of induced pluripotent stem cells-derived motor neurons for treatment of amyotrophic lateral sclerosis and motor neuron disease
- Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans
- Collapsin response mediator protein-2 plays a major protective role in acute axonal degeneration
- Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury
- Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury
