Chronically denervated distal nerve stump inhibits peripheral nerve regeneration
2017-06-05GiuliaRonchi,StefaniaRaimondo
Chronically denervated distal nerve stump inhibits peripheral nerve regeneration
Schwann cells (SCs) and peripheral nerve regeneration: SCs are the principal glial cells of the peripheral nervous system (PNS). In a healthy nerve, myelinating SCs wrap around larger caliber motor and sensory axons to form the myelin sheath, whereas non-myelinating SCs envelop and support multiple small diameter sensory axons to form Remak bundles. Moreover, they form a basal lamina which surround each SC-axon unit (Hall, 2005).
When a peripheral nerve injury occurs, extensive changes in the differentiation state of both the damaged neurons and the SC distal to the injury site take place. As a response to nerve injury, SC change their function by changing their gene expression: they down-regulate myelin-associated genes (such as myelin protein zero, myelin basic protein, myelin-associated glycoprotein, peripheral myelin protein 22, the Krox-20 transcription factor and periaxin) and up-regulate genes important to support repair and regeneration (such as L1 cell adhesion molecule, neural cell adhesion molecule, p75 neurotrophin receptor p75NTR, and glial fibrillary acidic protein) (Jessen and Mirsky, 2016). They dedifferentiate into immature SC and proliferate, forming columnar structures known as Bands of Büngner that guide axons back to their target organs. These processes, together with the up-regulation of neurotrophic factors and receptors (including glial cell line-derived neurotrophic factor, artemin, brain-derived neurotrophic factor, neurotrophin-3, nerve growth factor, vascular endothelial growth factor, erythropoietin, pleiotrophin, p75NTRand N-cadherin (Jessen and Mirsky, 2016), the phagocytic role of SC to remove axonal and myelin debris and the recruitment of macrophages to help in clearing, create a supportive environment for nerve regeneration (Arthur-Farraj et al., 2012). Moreover, in a recent study, Ronchi et al. (2016) investigated the expression of different neuregulin 1 (NRG1) isoforms (that is one of the most important factors regulating SC activity) in distal rat median nerve samples under regenerating condition and they demonstrated that their expression is specific for distinct and consecutive phases following nerve injury and regeneration. In particular, they showed that soluble isoforms of NRG1 are strongly up-regulated early (few days) after nerve injury, suggesting an active role of this molecules in the response to nerve damage (Ronchi et al., 2016).
Finally, when a successful axonal regeneration has occurred, SC regain their contact with axons and redifferentiate again into myelinating (or non-myelinating) cells.
Due to this surprising degree of plasticity displayed by SC after nerve injury, these cells are considered key factors in promoting nerve regeneration.
The problem of delayed nerve repair: changes occurring in chronically denervated distal nerve stump. Despite the PNS has some ability to regenerate injured axons, the repair is often slow and insufficient, especially when the nerve injury is very proximal (close to the spinal cord) or when the nerve repair is delayed in time.
In clinic, indeed, primary nerve reconstruction is not always possible, especially when other major injuries are concomitant to nerve damage: in these cases a secondary operation is needed to repair the nerve, increasing the delay between nerve injury and repair. Also, if the nerve injury is far from the target organs, axons must regenerate over long distances, increasing the time for nerve regeneration and target reinnervation.
In both cases, the distal nerve stump undergo long-term degeneration and SCs, together with target organs (skeletal muscle and sense organs), remain denervated for a long period (Sulaiman and Gordon, 2013), leading to unsatisfactory functional recovery. This poor outcome can be ascribed to many factors, but recent studies provide evidence that the progressive loss of the SC capability to support regeneration in a long-term degenerated nerve plays the predominant role in the reduced recovery after delayed nerve repair (Sulaiman and Gordon, 2013; Ronchi et al., 2017).
Chronically denervated distal nerve stump undergoes many changes, both morphological and biomolecular.
From a morphological point of view, after long-term denervation (starting from 3 months after axotomy), neither myelinated nor unmyelinated fibers are detectable in the nerve distal stump, and axonal and myelin debris have already been cleared. The chronically denervated nerve is colonized only by atrophic SC and fibroblasts cell-like, together with empty endoneurial tubes (the basal laminae of Schwann cells), which progressively shrink and decrease in diameter (Ronchi et al., 2017).
To give a neurobiological explanation for the poor functional outcome occurring after delayed nerve repair, different studies have focused on the molecular changes in the chronically denervated distal nerve stump. The regeneration-associated genes (α1-tubulin, actin and GAP-43, the increased expression of which immediately after injury is associated with axonal regeneration), show a progressive down-regulation in axotomized motorneurons which lasts also after 6-months (Gordon and Tetzlaff, 2015). Also ciliary neurotrophic factor (whose expression usually decreases after nerve injury and remains low unless the axons begin to regrow) and the transcription factor c‐Jun (that is up-regulated after injury and controls many aspects of Wallerian degeneration) level expression are drastically reduced following long-term degeneration (Sendtner et al., 1992; Jessen and Mirsky, 2016). On the contrary, the neurotrophic factors brain-derived neurotrophic factor and glial cell-derived neurotrophic factor mRNA levels (whose expressions are already up-regulated early after injury) are still elevated at least 6 months after injury, whereas nerve growth factor (that increases after nerve injury) and neurotrophin-3 (whose expression decreases early after injury, returning to normal levels by 1–2 weeks) are present in control levels (Michalski et al., 2008).
Moreover, recent results show a down-regulation of the SCs markers S100 and GFAP (whose expression is up-regulated early after injury) mRNA expression and a strong up-regulation of p75 mRNA (which is already up-regulated early after injury) after longterm degeneration (Ronchi et al., 2017). In the same experimental study, Ronchi et al. (2017) investigated the NRG1/ErbB gliotrophic system, because it is known to be strongly involved in nerve regeneration. They demonstrated that soluble NRG1 mRNA expression (whose expression is strongly up-regulated by SCs in response to acute injury), strongly decreases during chronic degeneration, starting 3 months after nerve axotomy (Ronchi et al., 2017), and its down-regulation increases with time.
Inhibitory effect of chronically denervated SCs on nerve regeneration: Thanks to their plasticity, healthy SCs are essential for a successful nerve regeneration. Chronically denervated SCs are therefore a limiting factor for a positive outcome after nerve injury because they progressively undergo atrophy, down-regulate the expression of factors that permit nerve regeneration, and up-regulate the expression of molecules that inhibit axon regeneration (Hoke, 2011). Recent studies have tried to understand how these changes occurring in chronically denervated SCs affect axonal regeneration, in order to find a therapeutic strategy to improve peripheral nerve regeneration after delayed nerve repair.
In an experimental model in which the chronically denervated common peroneal nerve was cross sutured to the freshly axotomized tibial nerve, it has been demonstrated that denervated SCs lost their ability to support regeneration and create an hostile environment that does not support regeneration (Fu and Gordon, 1995). Moreover, following delayed nerve grafting, the number of regenerating motoneurons and myelinated axons decreases, together with SCs marker expression, with an increase in fibrosis and proteoglycan scar markers in the distal nerve. It has also been demonstrated a correlation between axonal outgrowth and the number of activating transcription factor 3 (ATF3) - stained neurons andSC after delayed nerve repair. Indeed, ATF3 expression, which increases early after injury, decreases in the regenerating distal nerve stump when the repair is delayed (Saito and Dahlin, 2008).
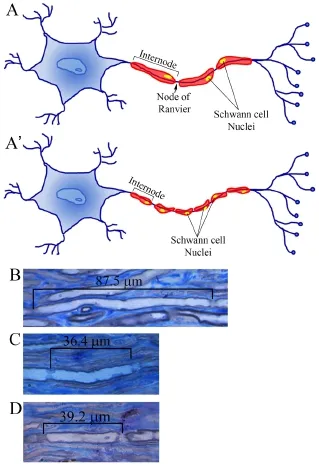
Figure 1 Internodal length in regenerating peripheral nerves.
In a recent study, in which Ronchi and co-workers used the surgical paradigm of the cross suture between the chronically denervated median nerve distal stump and the freshly axotomized ulnar nerve proximal stump, they also demonstrated that chronic degeneration of the distal nerve stump compromised nerve regeneration in terms of functional recovery and number and size of regenerated fibers. Indeed, they did not observe any functional recovery after delayed nerve repair, but they found regenerated myelinated fibers with a lower number and smaller size compared to the nerve immediately repaired. Intriguingly, they found that the number of SCs (in terms of ratio between myelinated fibers and SCs) is higher after delayed nerve repair compared to immediate repair and control nerve. This greater number of SCs could reflect a shorter internodal length, as showed by qualitative images represented in Figure 1. Indeed, it has been already demonstrated that after regeneration internodes are shorter and there is an overproduction of Schwann cells followed by a decrease once nerve regeneration has occurred. Since shorter internodes reflect slower conduction velocity, this might explain the negative functional recovery in the delayed repair groups (Ronchi et al., 2017).
Then, in the same study Ronchi and co-workers also analyzed the delayed nerve regeneration from a biomolecular point of view and they found that p75 was still up-regulated, whereas S100 mRNA expression was still strongly down-regulated compared with healthy control nerves, probably due to an impairment in SCs activities and characteristics. Finally, they showed that the soluble NRG1 is still strongly down-regulated after delayed regeneration (Ronchi et al., 2017), in contrast with an immediate end-to-end repair in which soluble NRG1 expression level was similar to that observed in the healthy control nerve, suggesting that NRG1 could be one of the factors that limits nerve regeneration after delayed repair.
Conclusions:In the last years many researchers have tried to understand what are the factors that limit the regeneration when the nerve repair is delayed in time. Several factors have been identified, and many of them seem to have an important role.
Among these factors, it has been identified the NRG1, because its expression (and therefore its effect on SCs activity) decreases during degeneration and remains low after delayed nerve regeneration. Recent results support the view that soluble NRG1 could be a promising candidate for future therapeutic strategies to improve peripheral nerve regeneration. Indeed, it can be hypothesized that by the manipulation of its expression (in particular by overexpressing NRG1 in chronically denervated distal nerve stump), the regeneration might improve. Future studies are necessary to demonstrate this hypothesis.
Giulia Ronchi*, Stefania Raimondo
Department of Clinical and Biological Sciences, University of Torino, Orbassano (To), Italy; Neuroscience Institute Cavalieri Ottolenghi (NICO), Orbassano (To), Italy
*Correspondence to:Giulia Ronchi, Ph.D., giulia.ronchi@unito.it.
Accepted:2017-04-17
orcid:0000-0002-4795-7024 (Giulia Ronchi) 0000-0002-8907-473X (Stefania Raimondo)
Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633-647.
Fu SY, Gordon T (1995) Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci 15:3886-3895.
Gordon T, Tetzlaff W (2015) Regeneration-associated genes decline in chronically injured rat sciatic motoneurons. Eur J Neurosci 42:2783-2791.
Hall S (2005) The response to injury in the peripheral nervous system. J Bone Joint Surg Br 87:1309-1319.
Hoke A (2011) A (heat) shock to the system promotes peripheral nerve regeneration. J Clin Invest 121:4231-4234.
Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regenerating nerves. J Physiol 594:3521-3531.
Michalski B, Bain JR, Fahnestock M (2008) Long-term changes in neurotrophic factor expression in distal nerve stump following denervation and reinnervation with motor or sensory nerve. J Neurochem 105:1244-1252.
Ronchi G, Haastert-Talini K, Fornasari BE, Perroteau I, Geuna S, Gambarotta G (2016) The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur J Neurosci 43:351-364.
Ronchi G, Cillino M, Gambarotta G, Fornasari BE, Raimondo S, Pugliese P, Tos P, Cordova A, Moschella F, Geuna S (2017) Irreversible changes occurring in long-term denervated Schwann cells affect delayed nerve repair. J Neurosurg doi:10.3171/2016.9.JNS16140.
Saito H, Dahlin LB (2008) Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci 9:88.
Sendtner M, Stockli KA, Thoenen H (1992) Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J Cell Biol 118:139-148.
Sulaiman W, Gordon T (2013) Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner J 13:100-108.
10.4103/1673-5374.206638
How to cite this article:Ronchi G, Raimondo S (2017) Chronically denervated distal nerve stump inhibits peripheral nerve regeneration. Neural Regen Res 12(5):739-740.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial
- Therapeutic opportunities and challenges of induced pluripotent stem cells-derived motor neurons for treatment of amyotrophic lateral sclerosis and motor neuron disease
- Inhibition and enhancement of neural regeneration by chondroitin sulfate proteoglycans
- Collapsin response mediator protein-2 plays a major protective role in acute axonal degeneration
- Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury
- Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury
