Enthalpy of phase transition of isonicotinic acid
2017-05-29DongfangZhaoGuanghuiLiuJianSunLishengWang
Dongfang Zhao,Guanghui Liu,Jian Sun,Lisheng Wang*
School of Chemical Engineering and Environment,Beijing Institute of Technology,Beijing 100081,China
1.Introduction
Chemically isonicotinic acid is pyridine-4-carboxylic acid(CAS number 55-22-1).It occurs as a fine-crystalline cream-yellow powder,and it is insoluble in water and sparingly soluble in alcohols and acetone;its molecular mass is 123.11[1].Isonicotinic acid is widely used as an anticorrosion reagent,plating additive,photosensitive resin stabilizer,and food additive[2,3].Moreover,it is an important intermediate in the synthesis of anti-tuberculosis drug isoniazid(isonicotinic acid hydrazide)[1,2,4,5],againstPlasmodium gallinaceumandPlasmodium berghei.This attractive compound also plays an important role in the synthesis of inaben fide(a plant growth regulator),terefenadine(an antihistamine),nialamide(an antidepressant)and other pharmaceutically important drugs[5].
It is surprising that there are only a few thermochemical data on isonicotinic acid,even though it is an essential chemical due to its pharmaceutical and analytical applications.Its thermochemical property of fusion has received little attention in the literature.The calculation of phase change enthalpies is often necessary for the energy balances design in chemical and petrochemical processes.Furthermore,accurate predictions for the phase change enthalpy are basically for those phase change materials which are used to prevent over-heat in aerospace or high supersonic flight[6].
Generally speaking,the fusion enthalpy can be determined with convenience and accuracyviadifferential thermal analysis(DTA)or DSC[7-10].The values obtained from direct calorimetry method reveal a higher reliability as the development of modern flow calorimeters in recent years.But even with today's modern instrumentation,it would be physically impossible to measure the thermochemical properties for more than 6 million distinct known chemical substances,let alone the seemingly in finite number of possible binary,ternary and higherorder multicomponent mixtures[11].Therefore,researchers turn to a predictive method as an approach to get desired thermochemical properties from structural information.Techniques for predicting fusion enthalpy can play several useful roles.Because they provide a numerical value that can be used in cases when there are no experimental data,and they are also useful in selecting the most probable experimental value in cases where two ormore values are in significantdisagreement with each other[12].Furthermore,the predictive method can also be used to test the reliability of experimental values in the literature.
Many researchers are dedicated to the prediction ofthermochemical properties of chemical compound by using different methods[13-16].One of the most important methods is group contribution method due to its accuracy and comprehensiveness[17-29].The group contribution method could be de fined as estimation procedure for the thermochemical property of a chemical compound,where values assigned to small groups of atoms constituting the molecule are summed to estimate their contribution to the inherent physicochemical properties of the molecule[30].Chickoset al.[12,31-33]have done a lot of works in this field,and it has been proved that the group values provided by him are credible.
In this study,fusion enthalpy of isonicotinic acid has been calculatedviathe Chickos's group contribution method.However,the result obtained was significantly lower compared with the value determined by DSC and the data published in the literature.Fusion enthalpies of other compounds,which have similar structures with isonicotinic acid,also have been calculated in the same way to prove the reliability of this method,and all of these results are in good agreement with experimental data.In addition,the thermal decomposition process of isonicotinic acid was studied by Py-GC/MS and these results were verified.
2.Experimental Section
2.1.Material
Isonicotinic acid(99%)was commercially available from Sinopharm Chemical Reagent Co.,Ltd.,and it was used as received without further purification.
2.2.Estimation of fusion enthalpy of isonicotinic acid
Fusion enthalpy(ΔfusHm)and fusion entropy(ΔfusSm)are related to each other through the following equation:


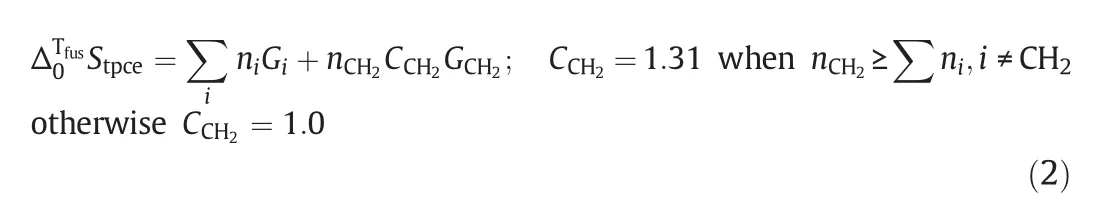
wherenirefers to the number ofitype group de fined in Table 1;Githe group value ofitype group de fined in Table 1;nCH2the number ofmethylene groups in the molecule;andGCH2the group value associated with a CH2group.
The parameters used for estimating Δ0TfusStpceof isonicotinic acid and its analogs are listed in Table 1,and the group coefficients are used to modify group values whenever a functionalgroup is attached to the carbon in question.Once Δ0TfusStpceof isonicotinic acid and its analogs are calculated and the fusion temperature are measured,the fusion enthalpy(ΔfusHm)can be calculatedviaEq.(1).
2.3.TGA and DSC analysis
Thermogravimetric analysis(TGA)was implemented using a SDT Q600(TA Instruments,USA)thermogravimetric analyzer at a heating rate of 10 K·min-1.Isonicotinic acid was analyzed in an atmosphere of dry nitrogen at a flow rate of 100 ml·min-1.
DSC was implemented using a DSC-60(Shimadzu,Japan)differential scanning calorimetry analyzer at a heating rate of 10 K·min-1.Isonicotinic acid was analyzed under dry nitrogen atmosphere with a flow rate of 50 ml·min-1.
2.4.Py-GC/MS analysis
Py-GC/MS was carried out using a GCMS-QP2010SE(Shimadzu,Japan)equipped with a microfurnace pyrolyzer(Model EGA/PY-3030D,Frontier Lab,Japan).About 1 mg of isonicotinic acid sample was loaded into the pyrolysis cup and then introduced into the heatcenter of pyrolyzer.The pyrolysis was performed at 600°C for 60 s,using 1 ml·min-1Helium(He)carrier gas.The GC/MS instrument, fitted with an Ultra alloy-5 column(30 m × 0.25 mm i.d.,0.25 μm in film thickness)and a mass selective detector,was used to separate and detect the compounds from pyrolysis gases and vapors.The GC/MS conditions were the following:inlet temperature at 280°C,split ratio 50:1.The GC oven temperature was programmed from 50°C(hold for 1 min)to 180 °C at 5 °C·min-1and then to 280 °C(hold for 10 min)at 10 °C·min-1.The mass spectrometer conditions:ion source temperature 250°C and electron impact ionization at 70 eV.Electro Ionization(EI)mass spectra were obtained fromm/z33 to 600 every 0.2 s.Finally,the compounds from pyrolysis gases and vapors were identified by comparing their mass spectra with stored data(F-Search System,Frontier Lab,Japan)which include a National Institute of Standards and Technology(NIST)library and previously published mass spectra.
3.Results and Discussion
3.1.Fusion enthalpy of isonicotinic acid calculated by group contribution method
The estimation of Δ0TfusStpcefor isonicotinic acid follows directly from Eq.(2).The molecule contains four kinds of groups:four tertiary benzenoid sp2C(═CH--),one quaternary benzenoid sp2C adjacent to an sp2atom(═C(R)--),one carboxylic acid(R--C(═O)OH),and one aromatic heterocyclic nitrogen(═N--).Group coefficient identified as B36 in Table 1 is used for the carboxylic acid since isonicotinic acid would be considered to contain only two functional groups.Additionally,there are no methylene groups,resulting in the absence ofnCH2CCH2GCH2in Eq.(2).Δ0TfusStpcefor isonicotinic acid was calculated by summing these group values listed in Table 1(4×A10+A12+A41+A36×B36=49.2 J·mol-1K-1).ΔfusSmfor isonicotinic acid is numerically equal to Δ0TfusStpcesince no extra solid phase transitions can be observed from the DSC curve in Fig.1.In consideration of the melting point ofisonicotinic acid(593 K),a numerical value of 29.2 kJ·mol-1was calculated for the fusion enthalpy of isonicotinic acid using Eq.(1).
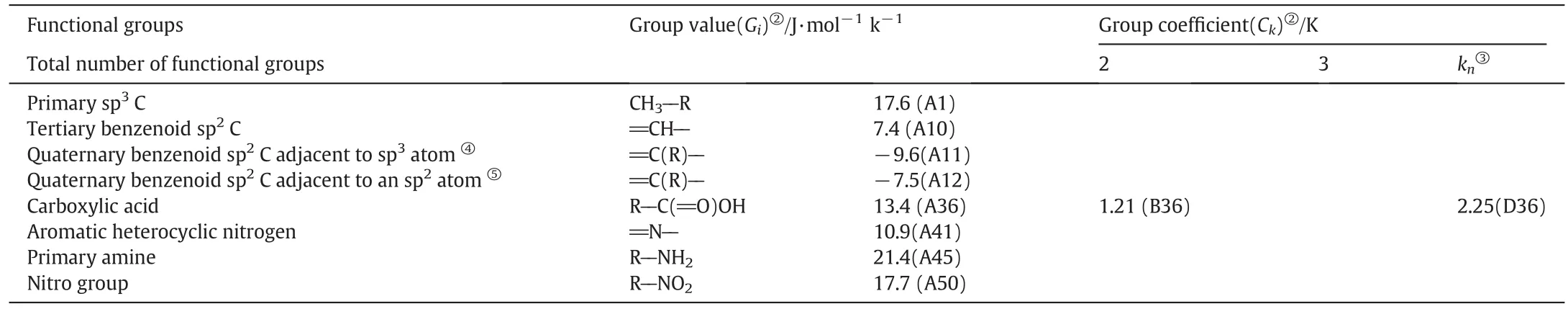
Table 1Contributions of the hydrocarbon and the functional group portions of isonicotinic acid molecule and its analogues①
To demonstrate the validity of this group contribution method,fusion enthalpies of several compounds were calculated by using the method mentioned above.Calculated results are compared with data described in the literature,and the percent of error[(calculatedexperimental)/experimental]×100,are listed in Table 2.As indicated in Table 2,the results of group contribution method are in good agreement with experimental data.These calculated compounds are similar in structure with isonicotinic acid.In view of this,we consider that the fusion enthalpy of isonicotinic acid obtained by group contribution method is credible,even though the obtained result shows a large difference with the value described in the literature.
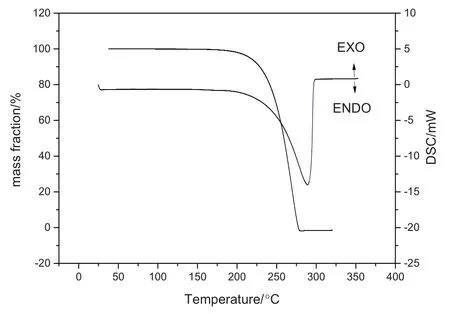
Fig.1.TG-DSC plot of isonicotinic acid.
3.2.Fusion enthalpy of isonicotinic acid by DSC
Fig.1 shows the TG and DSC curves of isonicotinic acid.The TGcurve elucidates that isonicotinic acid undergoes a single mass loss step from 187.27 °C to 277.47 °C with total elimination of the sample.The DSC curve shows an endothermic peak at288.9°C,and the endothermic process is observed in the range 188-298°C.In this case,the enthalpy was determined by using a straightbaseline for integration,and the value of the phase transition enthalpy is 128.03 kJ·mol-1.
The thermal analysis studies of isonicotinic acid were done by Allan[38],and a similarvalue of135 kJ·mol-1wasobtained forthe fusion enthalpy of isonicotinic acidviaDTA.In addition,this value was extracted as the data on enthalpy of phase transition from solid to liquid by Domalski[39],and the data was evaluated as A(high quality)based upon the method used,purity of the sample,the number of measurements,the details of the measurements as reported,calibrations,and corrections applied to the data.
Although the two values mentioned above are similar to each other,neither of the two values determined by DSC and DTA could be considered as the correct data on fusion enthalpy of isonicotinic acid.Both of the two values show a large difference with the value estimated by the group contribution method,and the group contribution method was demonstrated to be validity in a preceding part of this paper.Moreover,the pyrolytic decomposition ought to be accompanied by significant exothermic or endothermic peaks,but no extra exothermic or endothermic peaks can be observed except that caused by phase transition at 288.9°C from the DSC curve in Fig.1.Therefore,we have everyreason to believe that the sample undergoes a sublimation step instead of a decomposition one from 187.27 °C to 277.47 °C,as shown in Fig.1.DSC and DTAare the mostwidely used thermalanalyticaltechnique,but are used almost exclusively under ambient conditions.The isonicotinic acid sample undergoes a sublimation step under the test conditions.Therefore,the value determined by DSC or DTA is notthe fusion enthalpy of isonicotinic acid.That is to say,the fusion enthalpy of isonicotinic acid cannot be determined by DSC or DTA.The fusion enthalpy of isonicotinic acid determined by DSC or DTAis thus not credible.In practice,the fusion enthalpy of a compound is not easily determined by the experiment,especially for the compound that will be sublimed before melting point temperature.We have already searched extensive literature and unfortunately we could not find any other experimental methods to determine the fusion enthalpy of isonicotinic acid.
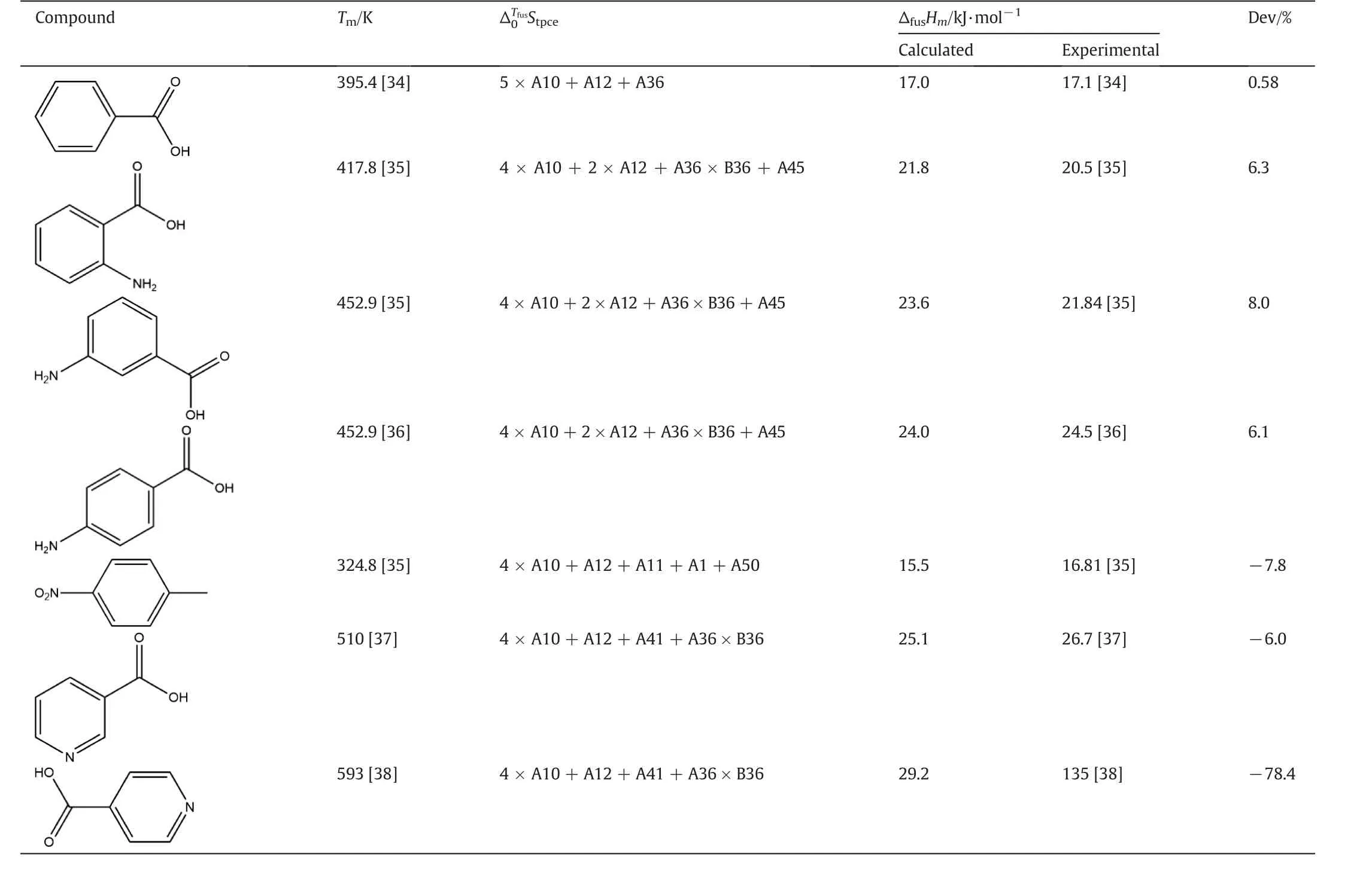
Table 2Experimental and calculated fusion enthalpy of isonicotinic acid and its analogs
Very few general techniques have been developed for directly estimating fusion enthalpy,in part,as a consequence of the complex phase behavior exhibited by some compounds.By contrast,the group contribution method provides a numerical value for fusion enthalpy of isonicotinic acid in cases when there are no experimental data.The reliability of this group contribution method has been proved in a preceding part of this paper.The same conclusion also has been drawn in other literatures.[17-29]In view ofthis,we consider thatthe fusion enthalpy of isonicotinic acid obtained by group contribution method is credible.
3.3.Py-GC/MS Analysis
The Py-GC/MS pyrogram for isonicotinic acid is presented in Figs.2-5.As shown in Fig.2,three main peaks are observed at 1.705 min,3.075 min and 18.770 min respectively.The three compounds were clearly identified by comparing these mass spectra with stored data(F-Search System,Frontier Lab,Japan).
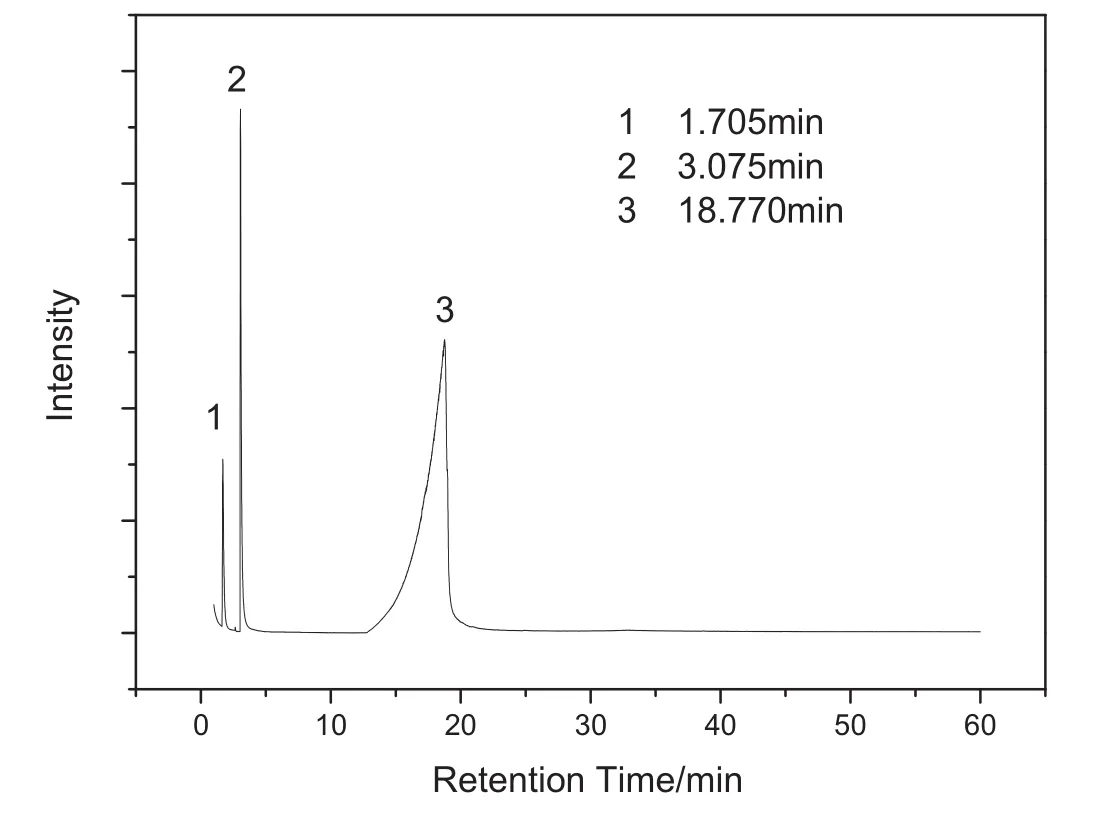
Fig.2.Mass chromatogram resulting from the pyrolysis of isonicotinic acid at 600°C for 60 s.
As shown in Figs.3-5,both of detected mass spectra(a)and mass spectra published by NIST(b)are presented.The mass spectrum at retention time 1.705 min,Fig.3(a),shows a diagnostic peak atm/z44,and it is in good agreement with the value for carbon dioxide published by NIST,Fig.3(b).The mass spectrum at retention time 3.075 min,Fig.4(a),shows a diagnostic peak atm/z79,and it is in good agreement with the value for pyridine published by NIST,Fig.4(b).The mass spectrum at retention time 18.770 min,Fig.5(a),shows a diagnostic peak atm/z123,and it is in good agreement with the value for isonicotinic acid published by NIST,Fig.5(b).As indicated above,the characteristic peaks were assigned as follows:retention time 1.705 min,carbon dioxide;3.075 min,pyridine;18.770 min,isonicotinic acid.
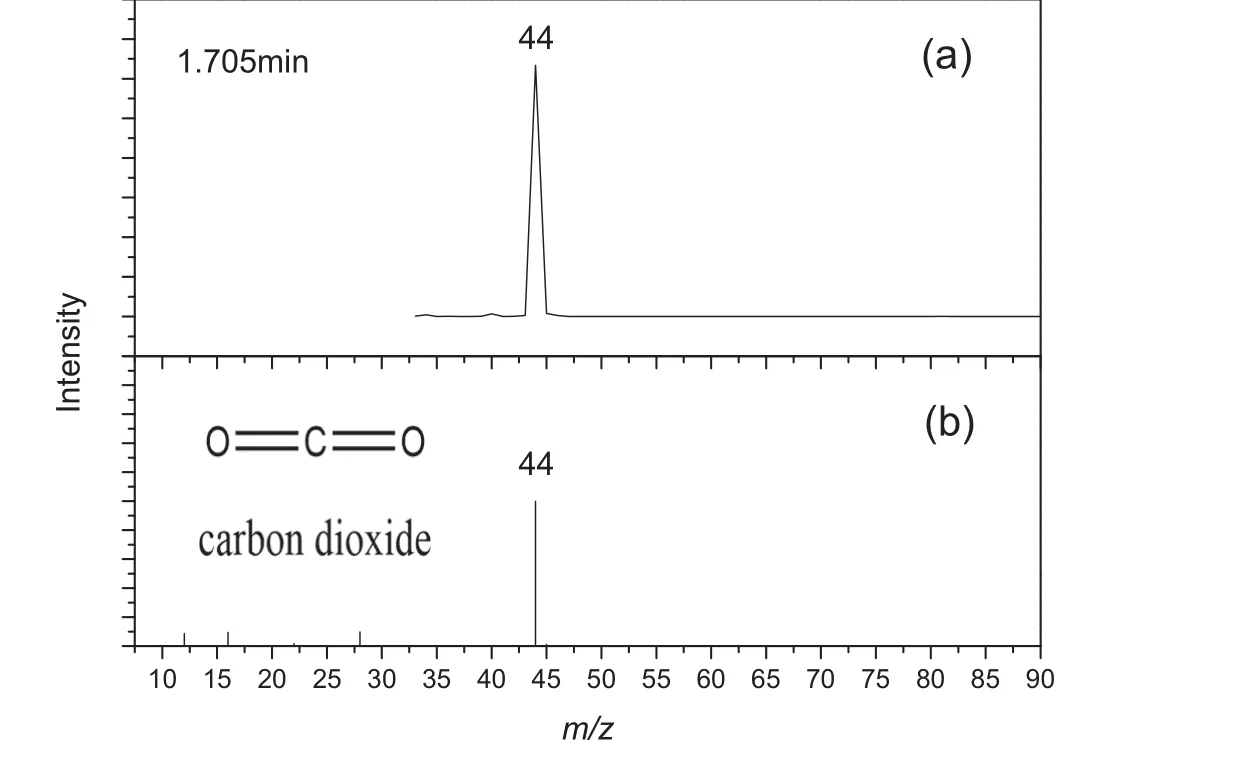
Fig.3.Mass spectrum of peak number 1 from the pyrogram shown in Fig.2,identified as carbon dioxide.
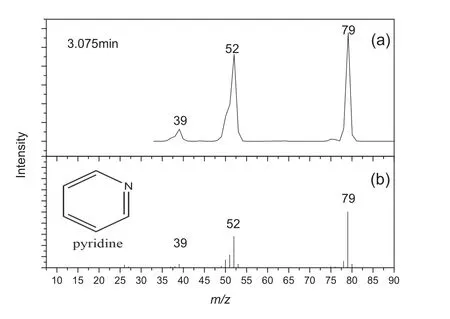
Fig.4.Mass spectrum of peak number 2 from the pyrogram shown in Fig.2,identified as pyridine.
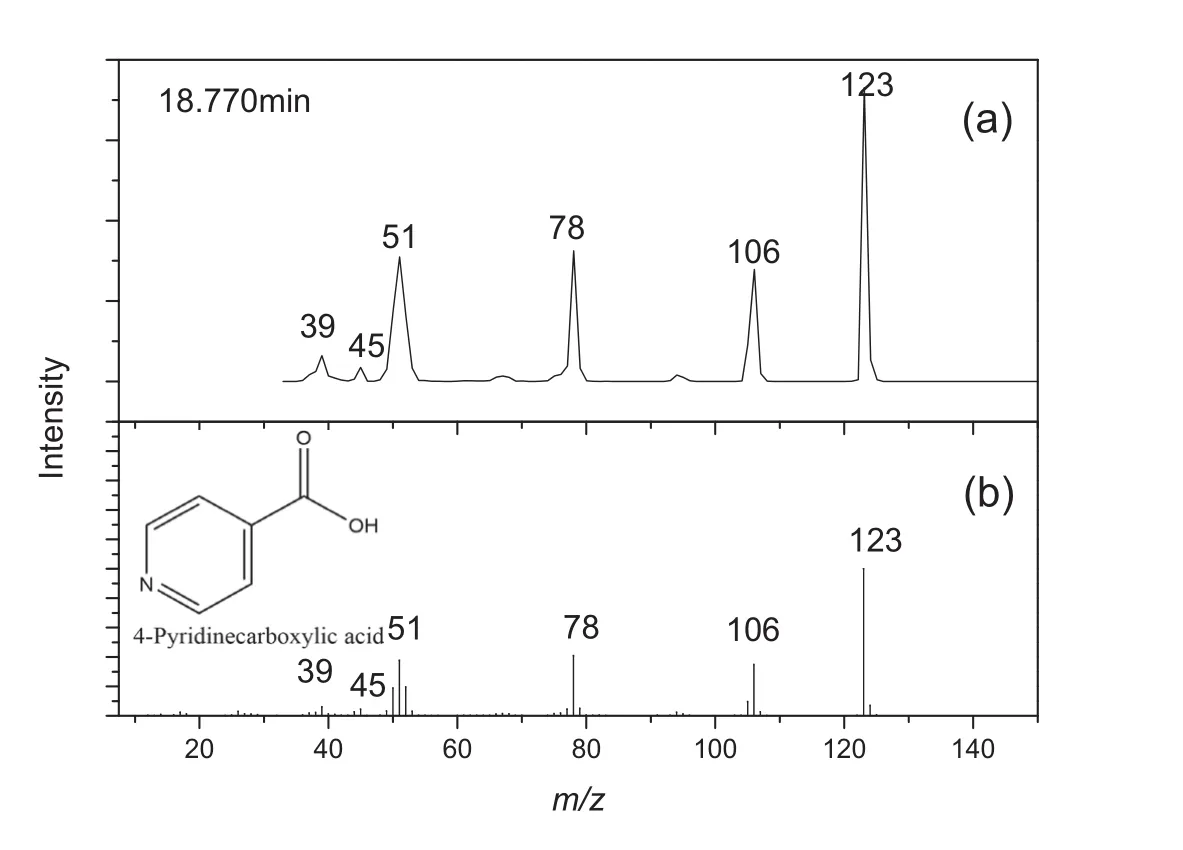
Fig.5.Mass spectrum of peak number 3 from the pyrogram shown in Fig.2,identified as isonicotinic acid.
Peak area is an indication ofthe quantities ofthe pyrolyzed products.As shown in Fig.2,carbon dioxide and pyridine occur in minute amounts in all pyrolysis products,and meanwhile almost all of the pyrolysis products are isonicotinic acid,elucidating that pyrolytic decomposition of isonicotinic acid sample does not occur at 600°C.Moreover,this result reinforces our view that the isonicotinic acid sample undergoes a sublimation step under the test conditions.Therefore,the fusion enthalpy of isonicotinic acid determined by DSC or DTA is simply not credible.By contrast,the group contribution method provides a reliable value for the fusion enthalpy of isonicotinic acid.
4.Conclusions
In this study,the value for the fusion enthalpy of isonicotinic acid determined by using DTA or DSC was proved incorrect.The DSC/TG measurementand Py-GC/MS measurementwere done to show this conclusion.A numericalvalue of29.2 kJ·mol-1was calculated forthe fusion enthalpy of isonicotinic acid by using group contribution method.The value and this group contribution method were proved credible.Phase transition of isonicotinic acid from solid to gas occurred at 187.27°C,and the sample underwent a sublimation process from 187.27°C to 277.47 °C with a sublimation enthalpy of 128.03 kJ·mol-1.
[1]G.V.Tsarichenko,V.I.Bobrov,M.V.Smarkov,Toxicity of isonicotinic acid,Pharm.Chem.J.11(1977)481-483.
[2]L.C.Wang,H.Ding,J.H.Zhao,C.Y.Song,J.S.Wang,Solubility of isonicotinic acid in 4-methylpyridine plus water from(287.65 to 361.15)K,J.Chem.Eng.Data53(2008)2544-2546.
[3]M.H.Abraham,W.E.Acree,On the solubility of nicotinic acid and isonicotinic acid in water and organic solvents,J.Chem.Thermodyn.61(2013)74-78.
[4]M.Arai,Y.I.Alavi,J.Mendoza,O.Billker,R.E.Sinden,Isonicotinic acid hydrazide:An anti-tuberculosis drug inhibits malarial transmission in the mosquito gut,Exp.Parasitol.106(2004)30-36.
[5]P.K.Mehta,S.K.Bhatia,R.K.Bhatia,T.C.Bhalla,Thermostable amidase catalyzed production of isonicotinic acid from isonicotinamide,Process Biochem.(Oxford,U.K.)50(2015)1400-1404.
[6]T.D.Swanson,G.C.Birur,Nasa thermal control technologies for robotic spacecraft,Appl.Therm.Eng.23(2003)1055-1065.
[7]D.G.Archer,S.Rudtsch,Enthalpy of fusion of indium:A certified reference material for differential scanning calorimetry†,J.Chem.Eng.Data48(2003)1157-1163.
[8]J.A.Wilson,J.S.Chickos,Vapor pressures and vaporization,sublimation,and fusion enthalpies of some fatty acids,J.Chem.Eng.Data58(2013)322-333.
[9]J.Troncoso,C.A.Cerdeirina,Y.A.Sanmamed,L.Romani,L.P.N.Rebelo,Thermodynamic properties of imidazolium-based ionic liquids:Densities,heat capacities,and enthalpies of fusion of[bmim][pf6]and[bmim][ntf2],J.Chem.Eng.Data51(2006)1856-1859.
[10]G.Nichols,S.Kweskin,M.Frericks,S.Reiter,G.Wang,J.Orf,B.Carvallo,D.Hillesheim,J.Chickos,Evaluation of the vaporization,fusion,and sublimation enthalpies of the 1-alkanols:The vaporization enthalpy of 1-,6-,7-,and 9-heptadecanol,1-octadecanol,1-eicosanol,1-docosanol,1-hexacosanol,and cholesterol att=298.15 K by correlation gas chromatography,J.Chem.Eng.Data51(2006)475-482.
[11]W.E.Acree,Thermodynamic properties oforganic compounds.Part4.First update of enthalpy of fusion and melting point temperature compilation,Thermochim.Acta219(1993)97-104.
[12]J.S.Chickos,W.E.Acree,Total phase change entropies and enthalpies.An update on fusion enthalpies and their estimation,Thermochim.Acta495(2009)5-13.
[13]J.Lohmann,R.Job,J.Gmehling,Estimation of enthalpies of fusion,melting temperatures,enthalpies of transition,and transition temperatures of pure compounds from experimental binary solid-liquid equilibrium data of eutectic systems,J.Chem.Eng.Data42(1997)1176-1180.
[14]R.M.Dannenfelser,S.H.Yalkowsky,Estimation of entropy of melting from molecular structure:A non-group contribution method,Ind.Eng.Chem.Res.35(1996)1483-1486.
[15]A.S.Hukkerikar,B.Sarup,A.Ten Kate,J.Abildskov,G.Sin,R.Gani,Groupcontribution+(gc+)based estimation of properties of pure components:Improved property estimation and uncertainty analysis,Fluid Phase Equilib.321(2012)25-43.
[16]E.A.Espinosa-Fuentes,J.R.Castro-Suarez,D.Meza-Payares,L.C.Pacheco-Londono,S.P.Hernández-Rivera,Sublimation enthalpy of homemade peroxide explosives using a theoretically supported non-linear equation,J.Therm.Anal.Calorim.119(2014)681-688.
[17]M.H.Keshavarz,Approximate prediction of melting point of nitramines,nitrate esters,nitrate salts and nitroaliphatics energetic compounds,J.Hazard.Mater.138(2006)448-451.
[18]K.M.Klincewicz,R.C.Reid,Estimation of critical properties with group contribution methods,AIChE J.30(1984)137-142.
[19]L.Constantinou,S.E.Prickett,M.L.Mavrovouniotis,Estimation of properties of acyclic organic-compounds using conjugation operators,Ind.Eng.Chem.Res.33(1994)395-402.
[20]J.Marrero-Morejon,E.Pardillo-Fontdevila,Estimation of pure compound properties using group-interaction contributions,AIChE J.45(1999)615-621.
[21]K.G.Joback,R.C.Reid,Estimation of pure-component properties from groupcontributions,Chem.Eng.Commun.57(1987)233-243.
[22]L.Constantinou,S.E.Prickett,M.L.Mavrovouniotis,Estimation of thermodynamic and physical-properties of acyclic hydrocarbons using the abc approach and conjugation operators,Ind.Eng.Chem.Res.32(1993)1734-1746.
[23]J.Marrero,R.Gani,Group-contribution based estimation of pure componentproperties,Fluid Phase Equilib.183-184(2001)183-208.
[24]L.Constantinou,R.Gani,New group contribution method for estimating properties of pure compounds,AIChE J.40(1994)1697-1709.
[25]L.Zong,S.Ramanathan,C.C.Chen,Predicting thermophysical properties of monoand diglycerides with the chemical constituent fragment approach,Ind.Eng.Chem.Res.49(2010)5479-5484.
[26]C.K.Kim,K.A.Lee,K.H.Hyun,H.J.Park,I.Y.Kwack,C.K.Kim,H.W.Lee,B.S.U.Lee,Prediction of physicochemical properties of organic molecules using van der waals surface electrostatic potentials,J.Comput.Chem.25(2004)2073-2079.
[27]J.D.Dyekjaer,S.O.Jonsdottir,Qspr models based on molecular mechanics and quantum chemical calculations.2.Thermodynamic properties of alkanes,alcohols,polyols,and ethers,Ind.Eng.Chem.Res.42(2003)4241-4259.
[28]M.Goodarzi,T.Chen,M.P.Freitas,Qspr predictions of heat of fusion of organic compounds using Bayesian regularized artificial neural networks,Chemom.Intell.Lab.Syst.104(2010)260-264.
[29]S.Puri,J.S.Chickos,W.J.Welsh,Three-dimensional quantitative structure-property relationship(3d-qspr)models for prediction of thermodynamic properties of polychlorinated biphenyls(pcbs):Enthalpies of fusion and their application to estimates of enthalpies of sublimation and aqueous solubilities,J.Chem.Inf.Model.43(2003)55-62.
[30]M.H.Keshavarz,H.R.Pouretedal,New approach for predicting melting point of carbocyclic nitroaromatic compounds,J.Hazard.Mater.148(2007)592-598.
[31]J.S.Chickos,W.E.Acree,J.F.Liebman,Estimating solid-liquid phase change enthalpies and entropies,J.Phys.Chem.Ref.Data28(1999)1535.
[32]J.S.Chickos,C.M.Braton,D.G.Hesse,J.F.Liebman,Estimating entropies and enthalpies of fusion of organic compounds,J.Organomet.Chem.56(1991)927-938.
[33]J.S.Chickos,W.E.Acree,Total phase change entropies and enthalpies:An update on their estimation and applications to the estimations of amphiphillic fluorocarbonhydrocarbon molecules,Thermochim.Acta395(2003)59-113.
[34]S.Roy,A.T.Riga,K.S.Alexander,Experimental design aids the development of a differential scanning calorimetry standard test procedure for pharmaceuticals,Thermochim.Acta392-393(2002)399-404.
[35]W.E.Acree,Thermodynamic properties of organic compounds:Enthalpy of fusion and melting point temperature compilation,Thermochim.Acta189(1991)37-56.
[36]M.K.Rotich,B.D.Glass,M.E.Brown,Thermal studies on some substituted aminobenzoic acids,J.Therm.Anal.Calorim.64(2001)681-688.
[37]A.El Moussaoui,A.Chauvet,J.Masse,Solid-state interaction of nordazepam-iii and nicotinic-acid,J.Therm.Anal.39(1993)619-632.
[38]J.R.Allan,W.C.Geddes,C.S.Hindle,A.E.Orr,Thermal analysis studies on pyridine carboxylic acid complexes of zinc(ii),Thermochim.Acta153(1989)249-256.
[39]E.S.Domalski,W.H.Evans,E.D.Hearing,Heat capacities and entropies of organic compounds in the condensed phase,J.Phys.Chem.Ref.Data19(1984)881-1047.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Suppression of gold nanoparticle agglomeration and its separation via nylon membranes☆
- Effects of solubility parameter differences among PEG,PVP and CA on the preparation of ultra filtration membranes:Impacts of solvents and additives on morphology,permeability and fouling performances
- Experimental detection of bubble-wall interactions in a vertical gas-liquid flow☆
- Horizontal gas mixing in rectangular fluidized bed:A novel method for gas dispersion coefficients in various conditions and distributor designs
- Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions
- Hydrodynamic dispersion ofreactive solute in a Hagen-Poiseuille flow of a layered liquid
