Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions
2017-05-29AkbarSoliemanzadehMajidFekri
Akbar Soliemanzadeh*,Majid Fekri
Department of Soil Science,College of Agriculture,Shahid Bahonar University of Kerman,Kerman,Iran
1.Introduction
Although phosphorus is an essential element to all forms of life on Earth,its excessive amounts lead to eutrophication in natural environments such as reservoirs,lakes,and coastal areas[1].Eutrophication is the over enrichment of natural waters with mineral nutrients,particularly phosphorus and nitrogen[2].Phosphorus is introduced to natural waters by severalexogenous sources such as fertilizers,industry,household detergents,and weathering rock[2].Xuet al.[3]reported that eutrophication threshold of total phosphorus(TP)for freshwaters was from 0.02 to 0.10 mg·L-1.Therefore,it is necessary to isolate phosphorus from natural waters to avoid possible hazardous exposure.
In recent years,because of the growing importance of nanotechnologies,nanoscale zero-valent iron(nZVI)has been investigated and used in the removal of phosphorus from aqueous systems due to its large active surface area and high phosphorus sorption capacities[4-6].Chemicaland physicalmethods have been used to synthesize ofnZVI,including top-down and bottom-up methods[7].However,the limitations of these methods are that they are usually expensive,require specific and costly equipment,consume high amount of energy,produce flammable hydrogen gas,and use toxic chemical materials such as sodium borohydride(NaBH4),organic solvents,and stabilizing and dispersing agents[7,8].Therefore,the development of nonhazardous,bio-based,low-cost,simple,and eco-friendly synthesized methods for nZVI is needed.In this approach,the green synthesis of nZVI using extracts of plant products such as green tea leaves[9-11],eucalyptus leaves[12,13],and mintleaves[14],has been developed.The plantextracts are used as reducing and capping agents due to their antioxidantcontents such as polyphenols,reducing sugars,nitrogenous bases,and amino acids[15,16].
In this work,a non-toxic biodegradable,and watersoluble polyphenol extracted from commercial green tea was selected as the reducing and stabilizing agent for nZVI production.Previous studies have reported that green synthesized nZVI was non-toxic and used for the removal of arsenic[17,18],chromium[19],and nitrate[13]from aqueous solutions.
Clay minerals such as zeolite,sepiolite,and bentonite are basically hydrous aluminum silicates having smallparticle sizes(<2μm).Bentonite is a member of smectite family and has unique characteristics such as large specific surface area,high cation exchange capacity(CEC),low-cost,and wide-spread availability,which makes it suitable for hosting nZVI.In addition,previous studies reported that the synthesis of nZVI in the presence of clay minerals(montmorillonite and bentonite)decreases their aggregation by partial dispersion/adsorption onto the clay surface[18,20].
The main objectives of the present work were to investigate(1)the synthesis of green nZVI using commercial green tea leaves in the presence of natural bentonite,(2)characterization of natural bentonite and B-nZVI by infrared spectroscopy(FTIR),scanning electron microscope(SEM),and X-ray diffraction(XRD),and(3)evaluation of the sorption characteristics of phosphorus on natural bentonite and B-nZVI.
2.Materials and Methods
2.1.Materials
All the chemicals used in the present study,including ferrous sulfate heptahydrate(FeSO4·7H2O),sodium hydroxide(NaOH),potassium dihydrogen phosphate(KH2PO4),sulfuric acid(H2SO4),ammonium molybdate(NH4)6Mo7O24),antimony potassium tartrate(K(SbO)·C4H4O6·0.5H2O),and ascorbic acid were of analytical grade(chemical purity>99%,Merck).The commercial leaves of green tea were used as sources of polyphenols.Natural bentonite used in the present study was obtained from Yazd Province,Iran.Based on an XRF analysis,the chemical composition of the natural bentonite sample(%)was:SiO2,66.37;Al2O3,13.24;Fe2O3,2.04;MgO,2.37;CaO,1.79;and Na2O,1.69.
2.2.Preparation of B-nZVI
For the synthesis of B-nZVI,4 g of natural bentonite was added to 50 ml of 0.1 mol·L-1FeSO4·7H2O and the mixture was mixed for 30 min using a magnetic stirrer.Meanwhile,green tea extract was prepared by adding 20 g of leaves of green tea to 1 L of distilled water,and heating at 80°C for 1 h.Then,the extract was filtered and added drop-wise to 0.1 mol·L-1ofFeSO4·7H2Oand naturalbentonite mixture at the volume ratio of 1:1 at room temperature with constant stirring.The immediate color change of the mixture to black indicated the formation of iron nanoparticles.The suspension was centrifuged and washed with ethylene to remove the residual ferrous sulfate heptahydrate.The wet paste was then left to dry overnight.
2.3.Characterization of natural bentonite and B-nZVI
Surface functional groups of natural bentonite,P sorption on natural bentonite,B-nZVI,and P sorption on B-nZVI were examined using KBr pressed disk technique by FT-IR spectroscopy(Model:Bruker,TENSOR 27,Germany).The spectra obtained in the range of 500-4000 cm-1were analyzed.
A KYKY EM-3200 scanning electron microscope(SEM)was used for surface morphological and structural studies of natural bentonite and B-nZVI.
The XRD patterns of natural bentonite and B-nZVI were recorded on B-nZVI were recorded on a Philips X'pert Pro MPD model X-ray diffractometer(Netherlands)using CuKαradiation as the X-ray source.
2.4.Batch experiment
The stock solution containing 1000 mg·L-1phosphorus was prepared by dissolving 4.39 g ofKH2PO4in distilled water,and the desired solutions were prepared by dilution of the stock solution.
The phosphorus sorption isotherms were conducted based on batch equilibrium technique.0.05 g of absorbent samples(natural bentonite or B-nZVI)was added into conical centrifuge tubes with 10 ml of aqueous solution containing various amounts of phosphorus concentrations(10,50,100,200,300,400 and 500 mg)in 0.01 mol·L-1NaCl in triplicate.Then,the suspensions were shaken at room temperature and at the constant agitation rate of 300 r·min-1using a shaker for 24 h.Atthe end ofequilibrium,the suspensions were centrifuged and the equilibrium phosphorus concentrations were measured using the ascorbic acid method[21]and UV-vis spectrophotometer at the wavelength of 880 nm.
In pH studies(adsorbent dose=5 g·L-1;phosphorus initial concentration=100 and 500 mg·L-1),the pH value of reaction mixture was adjusted to the range of 2-10 with 0.1 mol·L-1NaOH or 0.1 mol·L-1HCl,and a pH-meter(Jenway,United Kingdom)was used to determine the pH value.The amount of phosphorus adsorbed per gram adsorbentq(mg·g-1)and removal efficiency(%)of phosphorus can be determined according to the following equations:

whereCiandCe(mg·L-1)are phosphorus initialand equilibrium concentrations,respectively;V(L)is the volume of the solution;W(g)is the dose of natural bentonite or B-nZVI.
The effectofcontacttimesin the range of0-1440 min was investigated with adsorbentdoses of5 g·L-1and phosphorus initialconcentration of500 mg·L-1.The sorption amountattime oft(min),qt(mg·g-1),was determined according to the following equation:

whereCiandCt(mg·L-1)are phosphorus concentrations at first and the time oft,respectively.
2.5.Phosphorus desorption experiment
Desorption experiments were carried out using the method described by[22].Desorption of phosphorus from natural bentonite and B-nZVI was done immediately after sorption at the 500 mg·L-1initial concentration using the successive dilution method.After shaking the phosphorus-sorbent suspensions at room temperature for 24 h,the supernatants were separated by centrifugation.Then,10 ml of the supernatant was removed to measure phosphorus concentration,and replaced with 10 ml of 0.01 mol·L-1NaCl.This desorption cycle was repeated 6 and 9 times for natural bentonite and B-nZVI,respectively,and desorption isotherms were prepared by plotting the phosphorus remained on the adsorbents after each desorption cycleversusthe corresponding equilibrium phosphorus concentrations in the solution.
3.Results and Discussion
3.1.Characterization
The characterization of natural bentonite(a),P sorption on bentonite(b),B-nZVI(c)and P sorption on B-nZVI(d)by FTIR is shown in Fig.1.The IR spectrum of natural bentonite demonstrates that hydrogen-bonded of water H-O-H and H-O-H deformation was at 3427.75 and 1640.96 cm-1,respectively.However,the spectral band of 3630.03 cm-1has been identified to stretching of octahedral O-H groups that attached to Al+3or Mg+2.The Si-O and Si-O-Si groups of the tetrahedral sheet stretching were at 795.04 and 1040.02 cm-1,respectively.The band at 635.33 cm-1is assigned to the out-of-plane vibrations of coupled Al-O and Si-O.Similar functional groups were also reported by other studies[23].When comparing Fig.1a and c,the shift of 1640.96 cm-1band to 1683.31 and the presence of a new band at 1384 cm-1show the loading of nZVI particles to natural bentonite.These resultsare con firmed by otherresearchers[18].The results offig.1d show that the absorption band at1384 cm-1became insignificant,and the intensity of the peak at 1683.31 cm-1was decreased.Probably,the sorption of phosphorus on the B-nZVI resulted in the weakening of the band at 1384 cm-1and decreased the intensity of the peak at 1683.31 cm-1.
The SEM images of natural bentonite and B-nZVI are presented in Fig.2,indicating that the nZVI loaded to the natural bentonite is generally spherical in shape and has an average diameter of 40-60 nm.
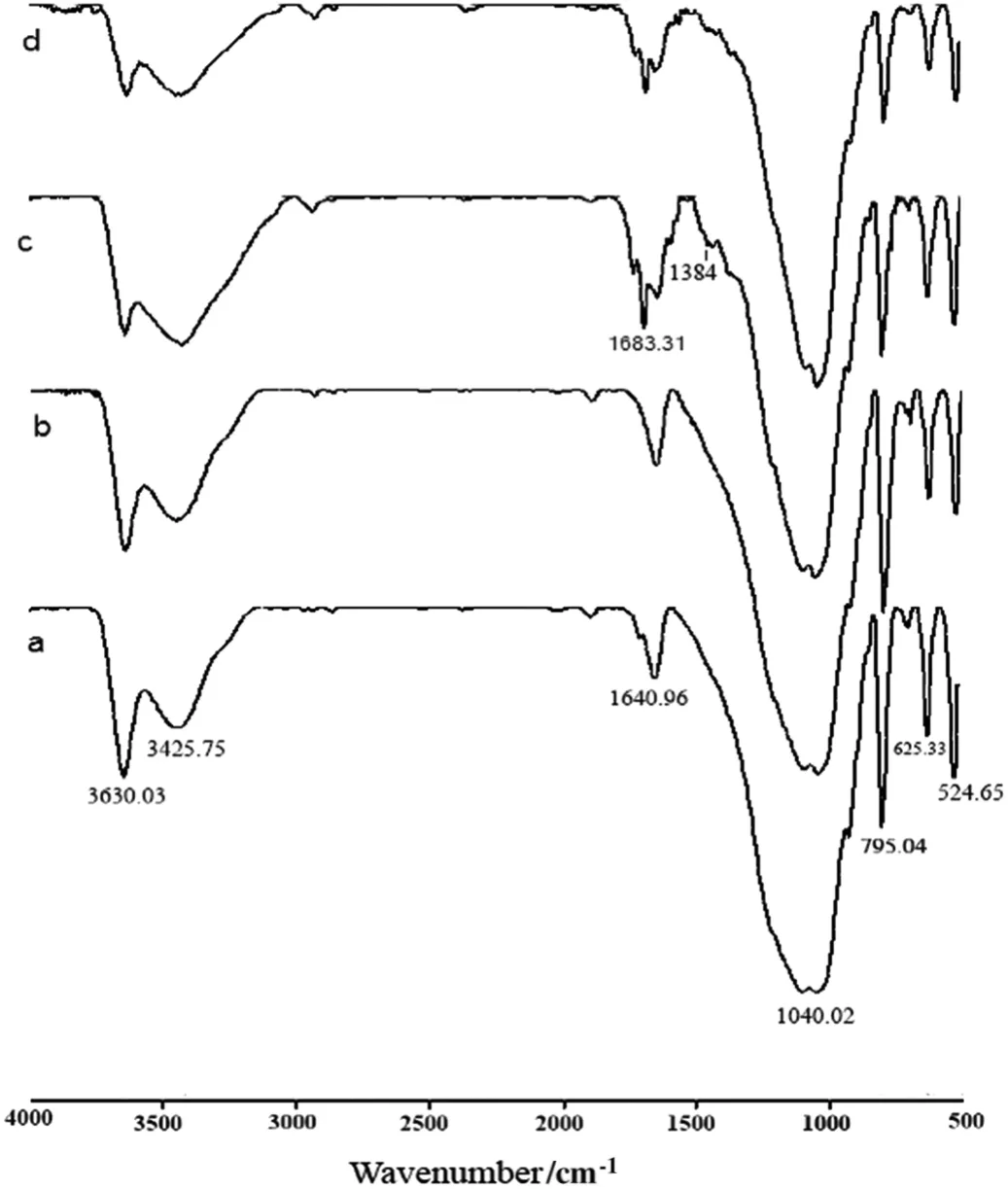
Fig.1.FTIR spectra ofnaturalbentonite(a),P sorption on naturalbentonite(b),B-nZVI(c),and P sorption on B-nZVI(d).
The XRD patterns of natural bentonite(a)and B-nZVI(b)are shown in Fig.3.The XRD pattern of B-nZVI shows the main diffraction peaks at the 2θ value of 44.9°which are related to the dispersion of nZVI to the surface of natural bentonite.As can be seen from Fig.3b,diffraction peaks corresponding to the structure of natural bentonite existed in the XRDpattern ofthe B-nZVI,which shows thatthe bentonite structure is not destroyed after reaction with nZVI[24,25].
3.2.Effect of initial concentration on the removal of phosphorus
B-nZVI(97.78%)and natural bentonite(7.29%)were observed at 50 and 10 mg·L-1of phosphorus initial concentration,respectively.As initial concentrations of phosphorus increased from 50 to 500 mg·L-1,removal efficiency by B-nZVI decreased from 97.78%to 29.25%,while removal by natural bentonite decreased from 7.18%to 2.91%.
It is a well-known fact that the removal efficiency of a sorption phenomenon depends upon the ratio of the number of adsorbate moieties to the available active sites of adsorbent.However,this ratio is related to the adsorbent surface coverage(number of active sites occupied/number of active sites available)that increases with the increase in the number of adsorbate moieties per unit volume of solution at a fixed dose of adsorbent[26].By increasing the initial concentration,the available active sites of adsorbent become saturated by phosphorus
The results of present study showed that the increase in phosphorus initial concentration led to a decrease in the removal efficiency of phosphorus(Fig.4).The removal efficiency of B-nZVI was higher than that of natural bentonite.The maximum percentages ofphosphorus removal by ion which finally results an increase in this ratio and decrease in removal efficiency.

Fig.3.XRD patterns of natural bentonite(a),B-nZVI(b).
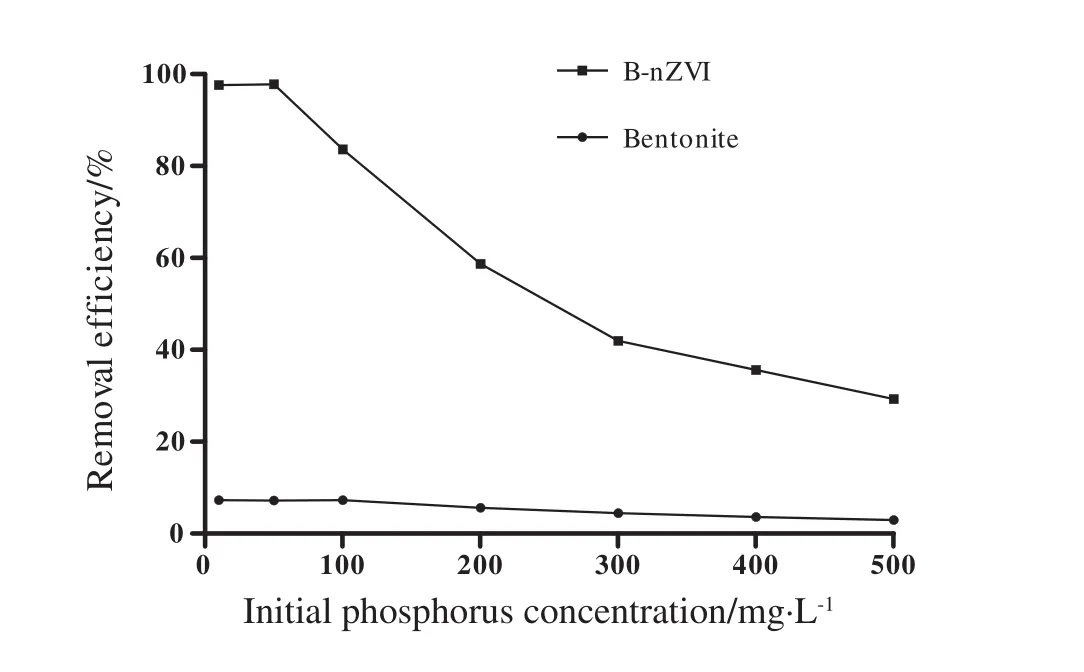
Fig.4.Effect of initial phosphorus concentration on the removal of phosphorus by natural bentonite and B-nZVI.

Fig.2.SEM images of natural bentonite(a)and B-nZVI(b and c).
3.3.Effect of pH on the removal of phosphorus
Experiments were conducted atdifferentranges ofpHincluding 2,4,5,6,8,and 10(Fig.5).The optimum pH for the removal of phosphorus by B-nZVI ranged from 2 to 5 for both 100 and 500 mg·L-1of phosphorus.With an increase of pH from 5 to 8,the sorption capacity decreased from 16.72 to 8.25 and 29.25 to 20.45 mg·g-1for 100 and 500 mg·L-1of phosphorus initial concentration,respectively.The results of the present study showed that the B-nZVI sorption capacity was relatively constant at an acidic solution pH(2-5),whereas B-nZVI sorption capacity decreased sharply as the solution pH approached a highly alkaline condition.

Fig.5.Effect of pH on phosphorus removal by B-nZVI.
The maximum phosphorus removal by B-nZVI was observed at the pH range of 2 to 5,at which interval the dominant phosphorus species is the monovalent H2PO4-1ion.This was due to the change in dominant aqueous phosphorus species as a function of pH.At the lowest range of pH,more H+ions become available on the surface of B-nZVI and the surface becomes more positively charged,leading to the higher adsorption of phosphorus.At the heightrange of pH,the activity of OH-in the solution,which competed with the phosphorus species,becomes higher[27].However,Yan etal.[28]reported thatthe optimalpH for phosphorus adsorption on Al-bentonite and Fe-Al-bentonite ranges from 3 to 5.This agreed with the report of other researchers on the effect of pH on phosphorus sorption which indicates a lower pH being favorable to phosphorus sorption[6,29-31].
3.4.Sorption isotherms
Sorption isotherms are useful tools for understanding the sorption phenomenon by different adsorbents.In the present study,Freundlich(Eq.(4)),Langmuir(Eq.(5)),and Redlich-Peterson(Eq.(6))isotherm models were used to describe the sorption capacity of naturalbentonite and B-nZVI for phosphorus.

WhereCeandqare the equilibrium concentration of phosphorus in the aqueous solution and the amount of phosphorus ions sorbed to the adsorbent,respectively;Ksorband 1/nsorbare Freundlich constants related to sorption capacity and sorption intensity,respectively;KLandQmaxare Langmuir constants related to the affinity of binding sites and maximum sorption capacity,respectively;andA,B,andgare Redlich-Peterson constants related to sorption capacity,affinity of binding sites and sorption intensity,respectively.
The equilibriumisotherm modelparameters are shown in Table 1.For Langmuirisotherm parameters,the values ofQmaxwere determined to be 4.61 and 27.63 mg·g-1for natural bentonite and B-nZVI,respectively.These values indicate that the loading of nZVI to natural bentonite increased the phosphorus sorption capacity by 6 times.In addition,the bonding energy coefficient value(Kl)of B-nZVI is greater than that of natural bentonite,which is related to the specifically sorbed phosphorus at high energy.

Table 1Sorption isotherm parameters of phosphorus onto natural bentonite and B-nZVI
The Langmuir constantKLcan be treated as an empirical equilibrium constant and used in the evaluation of the standard free energy of adsorption using the following equation[17,32]:

where ΔG0is the Gibb's standard free energy,Ris the gas constant(=8.314 J·mol-1·K-1),Tis the temperature(K),andKLis the Langmuir constant(L·mol-1).The ΔG0values for B-nZVI and natural bentonite were-20.57 and-11.94 kJ·mol-1,respectively.These negative values show that the phosphorus sorption phenomenon was spontaneous.
The phosphorus sorption data of natural bentonite and B-nZVI best fitted to the Freundlich model.The value of 1/nsorbin Freundlich isotherm was lower than 1,suggesting that this model is nonlinear,which is a usualbehavior for adsorbents with fixed and limited sorption capacities.For the Freundlich isotherm,theKsorbvalues for natural bentonite and B-nZVI were 0.09 and 8.31,respectively.The value ofKsorbincreased with nZVI loading to natural bentonite surface similar to the Langmuir(QmaxandKL)and Redlich-Peterson(AandB)isotherm constants indicating the increase in capacity of phosphorus sorption on the sorbents.Comparison ofR2values obtained from models shows that the Redlich-Peterson model gave a better fit result than other models.
3.5.Desorption experiment
Comparison of phosphorus sorption-desorption patterns on/from natural bentonite and B-nZVI is shown in Fig.6.Desorption isotherms did not fit with their corresponding sorption isotherms,and sorptiondesorption hysteresis occurred,meaning that irreversibility happened in phosphorus sorption in the time-scale of this experiments.
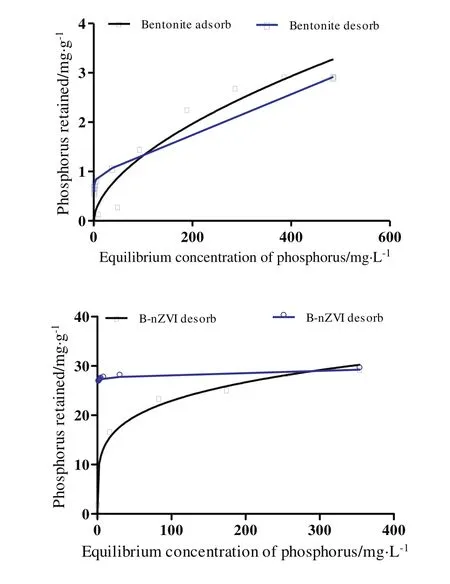
Fig.6.Phosphorus sorption-desorption for the natural bentonite and B-nZVI.
Fig.6 shows that the sorption of phosphorus on natural bentonite was more reversible than that of B-nZVI.The average percentages of the retained phosphorus released from natural bentonite(after six successive desorption steps)and B-nZVI(after nine successive desorption steps),were 80%and 9%,respectively.This can be due to the strongerinteraction between phosphorus anions and the nZVI that were loaded to the naturalbentonite surface.Itmay be concluded thatthe less irreversibility of phosphorus sorption by B-nZVI was due to the inner-sphere binding and covalent bonds of phosphorus to the nZVI surface[33].However,Moharamiand Jalali[29]reported thatmaximumphosphorus desorbed from Al2O3,Fe3O4and TiO2nanoparticles was 6.5%,5.9%and 2.8%,respectively.
As can be seen from Fig.6,after 1 step of desorption,63%of the retained phosphorus was released from the natural bentonite that is equal to 1.85 mg·g-1.This suggests that outer-sphere complexation is the dominant mechanism of sorption reaction between phosphorus and bentonite,and that phosphorus sorption was likely reversible and less specific.These results are in agreement with those obtained by Moharami and Jalali[34]which observed that 41.9%of phosphorus was released from bentonite.
Similar sorption isotherm,the desorption isotherm was calculated using Freundlich equations;
whereKdesorband 1/ndesorbare Freundlich bounding constants for the desorption coefficient.
Various studies showed that Freundlich modelwas the mostpopular one to explain metals sorption-desorption phenomenon by different adsorbents[35-37].For each sorbent,theKvalue(Freundlich constant)calculated from sorption isotherms was lower than that determined from desorption isotherms.Also,this parameter was remarkably higher for B-nZVI than the one obtained from natural bentonite,showing the occurrence of a positive hysteresis and a remarkably lower desorption of sorbed phosphorus from the B-nZVI than from natural bentonite.Dhillon and Brar[38]and Jalaliand NaderiPeikam[39]have also reported hysteresis in phosphorus sorption-desorption phenomenon in soils.
3.6.Sorption kinetics
The pseudo- first-order(Eq.(9)),pseudo-second-order(Eq.(10)),and intra-particle diffusion(Eq.(11))models were applied to describe the sorption kinetics of phosphorus to natural bentonite and B-nZVI(Fig.7).

Whereqtandqmax(sorption capacity)are the amounts of phosphorus sorbed at time the oftand equilibrium,respectively;k1andk2are pseudo- first-order rate constant and pseudo-second-order rate constant,respectively;andkpandCare the intra-particle diffusion rate constant and intercept at the ordinate,respectively.

Fig.7.Kinetic model analyses for natural bentonite(a)and B-nZVI(b).


Table 2Kinetic model parameters for natural bentonite and B-nZVI
The correlation coefficient(r2)of pseudo-second-order equation is higher than those of pseudo- first-order and intra-particle diffusion(Table 2),suggesting that the chemisorption process could be a ratelimiting step[27-29,40,41].In pseudo-second-ordermodelparameters,theqmaxvalue for B-nZVI was higher than that obtained from natural bentonite,indicating that loading nZVI to natural bentonite surface remarkably increased phosphorus sorption capacity.However,the values ofk2decreased with loading nZVI to natural bentonite surface,demonstrating that the time required to reach equilibrium has increased.
The initial sorption constant(h)att→0 was calculated using the following Eq.(12)[40,42]:

The value ofhobtained from B-nZVIwas higherthan the one obtained from natural bentonite,indicating that loading of nZVI to natural bentonite increased sorption at an initial phase of the sorption process.
According to the intra-particle diffusion model(Fig.8),a plot ofqt versus t1/2presented multi-linearity,with an initial linear phase followed by an intermediate linear phase and a plateau,showing that two or more steps govern the sorption phenomenon.

Fig.8.Intra-particle diffusion plots for phosphorus sorption onto naturalbentonite(a)and B-nZVI(b).
The initial sharper phase is attributed to the external surface or the instantaneous sorption,and the second linear phase is attributed to the gradual sorption stage where pore diffusion is rate-controlling[27,42-44].The third phase is attributed to the final sorption equilibrium stage where intra-particle diffusion started to slow down due to the following reasons:a)small pores for diffusion,b)high electrostatic repulsion of the natural bentonite and B-nZVI surface,and c)low concentration of phosphorus in the solution[27,45].Intra-particle diffusion is the sole rate-limiting step ifthe plotofqt versust1/2passes through the origin.In the presentstudy,the plot ofnatural bentonite and B-nZVI did not pass through the origin,indicating that three processes control the sorption rate,but only one is rate-limiting in any particular time range[42].
The values ofkpandCwere determined from the slope ofthe second linear phase(Fig.8,Table 2).In the presentstudy,the values ofkpandCincreased from 0.07 to 0.80 and from 0.37 to 5.59,respectively,as the nZVI loading to the surface of natural bentonite.A higherCvalue shows a greater effect of boundary layer,suggesting that the internal mass transfer is favored over external mass transfer[43,44].
4.Conclusions
In this study,the natural bentonite and B-nZVI were applied to remove phosphorus from aqueous solutions.For both adsorbents,increasing the phosphorus initial concentration decreased removal efficiency.The sorption of phosphorus on B-nZVI was observed to be pH-dependent,with maximum phosphorus removal occurring at the pH range of 2 to 5.The sorption capacity of B-nZVI was higher than that of natural bentonite.Langmuir,Freundlich,and Redlich-Peterson models properly described the sorption isotherm data.The sorptiondesorption of phosphorus by natural bentonite and B-nZVI showed hysteresis.The results indicated that sorption of phosphorus was more reversible on natural bentonite than on B-nZVI.The pseudosecond-order model fitted well to the kinetic data,suggesting that the chemisorption process could be a rate-limiting step.The present study suggests that B-nZVIcan be used as a suitable adsorbentforthe removal of phosphorus from aqueous solutions.
[1]M.A.Tabatabai,D.L.Sparks,L.Al-Amoodi,W.Dick,Chemical Processes in Soils,Soil Science Society of America Inc.,2005
[2]M.Kagami,Y.Hirose,H.Ogura,Phosphorus and nitrogen limitation of phytoplankton growth in eutrophic Lake Inba,Japan,Limnology14(2013)51-58.
[3]H.Xu,H.W.Paerl,B.Qin,G.Zhu,G.Gao,Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu,China,Limnol.Oceanogr.55(2010)420.
[4]H.Liu,T.Chen,X.Zou,Q.Xie,C.Qing,D.Chen,R.L.Frost,Removal of phosphorus using NZVI derived from reducing natural goethite,Chem.Eng.J.234(2013)80-87.
[5]F.Liu,J.Yang,J.Zuo,D.Ma,L.Gan,B.Xie,P.Wang,B.Yang,Graphene-supported nanoscale zero-valent iron:Removal of phosphorus from aqueous solution and mechanistic study,J.Environ.Sci.26(2014)1751-1762.
[6]T.Almeelbi,A.Bezbaruah,Aqueous phosphate removal using nanoscale zero-valent iron,J.Nanopart.Res.14(2012)1-14.
[7]S.Machado,S.Pinto,J.Grosso,H.Nouws,J.T.Albergaria,C.Delerue-Matos,Green production of zero-valent iron nanoparticles using tree leaf extracts,Sci.Total Environ.445(2013)1-8.
[8]L.Huang,X.Weng,Z.Chen,M.Megharaj,R.Naidu,Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green,Spectrochim.Acta A Mol.Biomol.Spectrosc.117(2014)801-804.
[9]M.N.Nadagouda,A.B.Castle,R.C.Murdock,S.M.Hussain,R.S.Varma,In vitro biocompatibility of nanoscale zerovalent iron particles(NZVI)synthesized using tea polyphenols,Green Chem.12(2010)114-122.
[10]L.Huang,F.Luo,Z.Chen,M.Megharaj,R.Naidu,Green synthesized conditions impacting on the reactivity of Fe NPs for the degradation of malachite green,Spectrochim.Acta A Mol.Biomol.Spectrosc.137(2015)154-159.
[11]C.Mystrioti,N.Papassiopi,A.Xenidis,D.Dermatas,M.Chrysochoou,Column study for the evaluation of the transport properties of polyphenol-coated nanoiron,J.Hazard.Mater.281(2015)64-69.
[12]Z.Wang,Iron complex nanoparticles synthesized by eucalyptus leaves,ACS Sustain.Chem.Eng.1(2013)1551-1554.
[13]T.Wang,X.Jin,Z.Chen,M.Megharaj,R.Naidu,Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater,Sci.Total Environ.466(2014)210-213.
[14]K.S.Prasad,P.Gandhi,K.Selvaraj,Synthesis of green nano-iron particles(GnIP)and their application in adsorptive removal of As(III)and As(V)from aqueous solution,Appl.Surf.Sci.317(2014)1052-1059.
[15]V.V.Makarov,S.S.Makarova,A.J.Love,O.V.Sinitsyna,A.O.Dudnik,I.V.Yaminsky,M.E.Taliansky,N.O.Kalinina,Biosynthesis of stable iron oxide nanoparticles in aqueous extracts ofHordeum vulgareandRumex acetosaplants,Langmuir30(2014)5982-5988.
[16]S.Quideau,D.Deffieux,C.Douat-Casassus,L.Pouységu,Plant polyphenols:chemical properties,biological activities,and synthesis,Angew.Chem.Int.Ed.50(2011)586-621.
[17]N.Horzum,M.M.Demir,M.Nairat,T.Shahwan,Chitosan fiber-supported zerovalent iron nanoparticles as a novel sorbent for sequestration of inorganic arsenic,RSC Adv.3(2013)7828-7837.
[18]P.K.Tandon,R.C.Shukla,S.B.Singh,Removal of arsenic(III)from water with claysupported zerovalent iron nanoparticles synthesized with the help of tea liquor,Ind.Eng.Chem.Res.52(2013)10052-10058.
[19]M.Chrysochoou,C.P.Johnston,G.Dahal,A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysul fide and green-tea nanoscale zero-valent iron,J.Hazard.Mater.201(2012)33-42.
[20]R.Abbassi,A.K.Yadav,N.Kumar,S.Huang,P.R.Jaffe,Modeling and optimization of dye removal using “green”clay supported iron nano-particles,Ecol.Eng.61(2013)366-370.
[21]E.W.Rice,L.Bridgewater,A.P.H.Association,Standard Methods for the Examination of Water and Wastewater,American Public Health Association Washington,DC2012.
[22]S.Bakhtiary,M.Shirvani,H.Shariatmadari,Adsorption-desorption behavior of2,4-D on NCP-modified bentonite and zeolite:Implications for slow-release herbicide formulations,Chemosphere90(2013)699-705.
[23]K.Bukka,J.D.Miller,J.Shabtai,FTIR study of deuterated montmorillonites;Structural features relevant to pillared clay stability,Clay Clay Miner.40(1992)92-102.
[24]L.Chen,Y.Huang,L.Huang,B.Liu,G.Wang,S.Yu,Characterization of Co(II)removal from aqueous solution using bentonite/iron oxide magnetic composites,J.Radioanal.Nucl.Chem.290(2011)675-684.
[25]A.Soliemanzadeh,M.Fekri,The application of green tea extract to prepare bentonite-supported nanoscale zero-valent iron and its performance on removal of Cr(VI):Effect of relative parameters and soil experiments,Microporous Mesoporous Mater239(2017)60-69.
[26]P.Mondal,C.B.Majumder,B.Mohanty,Effects of adsorbent dose,its particle size and initial arsenic concentration on the removal of arsenic,iron and manganese from simulated ground water by Fe3+impregnated activated carbon,J.Hazard.Mater.150(2008)695-702.
[27]Z.Wang,E.Nie,J.Li,M.Yang,Y.Zhao,X.Luo,Z.Zheng,Equilibrium and kinetics of adsorption of phosphate onto iron-doped activated carbon,Environ.Sci.Pollut.Res.19(2012)2908-2917.
[28]L.G.Yan,Y.Y.Xu,H.Q.Yu,X.D.Xin,Q.Wei,B.Du,Adsorption of phosphate from aqueous solution by hydroxy-aluminum,hydroxy-iron and hydroxy-iron-aluminum pillared bentonites,J.Hazard.Mater.179(2010)244-250.
[29]S.Moharami,M.Jalali,Effect of TiO2,Al2O3,and Fe3O4nanoparticles on phosphorus removal from aqueous solution,Environ.Prog.Sustain.Energy33(2014)1209-1219.
[30]D.Wu,Y.Shen,A.Ding,M.Qiu,Q.Yang,S.Zheng,Phosphate removal from aqueous solutions by nanoscale zero-valent iron,Environ.Technol.34(2013)2663-2669.
[31]A.Soliemanzadeh,M.Fekri,S.Bakhtiary,M.H.Mehrizi,Biosynthesis of iron nanoparticles and their application in removing phosphorus from aqueous solutions,Chem.Ecol.32(2016)286-300.
[32]Y.Liu,Some consideration on the Langmuir isotherm equation,Colloids Surf.A Physicochem.Eng.Asp.274(2006)34-36.
[33]Z.Wen,Y.Zhang,C.Dai,Removal of phosphate from aqueous solution using nanoscale zerovalent iron(nZVI),Colloids Surf.A Physicochem.Eng.Asp.457(2014)433-440.
[34]S.Moharami,M.Jalali,Removal of phosphorus from aqueous solution by Iranian natural adsorbents,Chem.Eng.J.223(2013)328-339.
[35]M.Kragović,A.Daković,M.Marković,J.Krstić,G.D.Gatta,N.Rotiroti,Characterization of lead sorption by the natural and Fe(III)-modified zeolite,Appl.Surf.Sci.283(2013)764-774.
[36]M.Hamidpour,M.Kalbasi,M.Afyuni,H.Shariatmadari,P.E.Holm,H.C.B.Hansen,Sorption hysteresis of Cd(II)and Pb(II)on natural zeolite and bentonite,J.Hazard.Mater.181(2010)686-691.
[37]Y.Aşçi,Ü.Açikel,Y.S.Açikel,Equilibrium,hysteresis and kinetics of cadmium desorption from sodium-feldspar using rhamnolipid biosurfactant,Environ.Technol.33(2012)1857-1868.
[38]N.Dhillon,B.Brar,In fluence of long-term use of fertilizers and farmyard manure on the adsorption-desorption behaviour and bioavailability of phosphorus in soils,Nutr.Cycl.Agroecosyst.75(2006)67-78.
[39]M.Jalali,E.N.Peikam,Phosphorus sorption-desorption behaviour of river bed sediments in the Abshineh river,Hamedan,Iran,related to their composition,Environ.Monit.Assess.185(2013)537-552.
[40]S.Y.Yoon,C.G.Lee,J.A.Park,J.H.Kim,S.B.Kim,S.H.Lee,J.W.Choi,Kinetic,equilibrium and thermodynamic studies for phosphate adsorption to magnetic iron oxide nanoparticles,Chem.Eng.J.236(2014)341-347.
[41]S.Benyoucef,M.Amrani,Adsorption of phosphate ions onto low cost Aleppo pine adsorbent,Desalination275(2011)231-236.
[42]H.K.Boparai,M.Joseph,D.M.O'Carroll,Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano-zerovalent iron particles,J.Hazard.Mater.186(2011)458-465.
[43]W.Liu,J.Zhang,C.Zhang,Y.Wang,Y.Li,Adsorptive removal of Cr(VI)by Fe-modified activated carbon prepared fromTrapa natanshusk,Chem.Eng.J.162(2010)677-684.
[44]R.K.Bharali,K.G.Bhattacharyya,Biosorption of fluoride on Neem(Azadirachta indica)leaf powder,J.Environ.Chem.Eng.(2015).
[45]H.Koyuncu,A.R.Kul,An investigation of Cu(II)adsorption by native and activated bentonite:Kinetic,equilibrium and thermodynamic study,J.Environ.Chem.Eng.2(2014)1722-1730.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Elevating the flexibility and operability of dividing-wall distillation columns via feed thermal condition adjustment☆
- Hydrodynamic dispersion ofreactive solute in a Hagen-Poiseuille flow of a layered liquid
- Interpenetrating polymers supported on microporous polypropylene membranes for the transport of chromium ions☆
- Suppression of gold nanoparticle agglomeration and its separation via nylon membranes☆
- Horizontal gas mixing in rectangular fluidized bed:A novel method for gas dispersion coefficients in various conditions and distributor designs
- Experimental detection of bubble-wall interactions in a vertical gas-liquid flow☆
