Effects of solubility parameter differences among PEG,PVP and CA on the preparation of ultra filtration membranes:Impacts of solvents and additives on morphology,permeability and fouling performances
2017-05-29KibromAlebelGebruChandanDas
Kibrom Alebel Gebru,Chandan Das*
Department of Chemical Engineering,Indian Institute of Technology Guwahati,781039,Assam,India
1.Introduction
Phase-inversion is a suitable method for the preparing of polymeric membranes having all types of morphological structures.In this technique,a casting solution comprised of polymer and solvent is immersed into a non-solvent coagulation bath,and a polymer solution is phase separated into polymer rich and polymer lean phases in a controlled manner[1].The solidification process is very frequently started by the change from one liquid phase into two liquid phases(liquid-liquid demixing).At certain period during de-mixing,the polymer rich phase solidifies so that a solid membrane matrix is formed[1,2].Membrane morphologies,particularly the pore size distributions can be controlled by selecting the different solvent,non-solvent,polymer,pore former,and fabrication parameters depending on the particular application[3-6].Cellulose acetate is broadly applicable for the synthesis of membranes because ofhaving tough,biocompatible,hydrophilic characteristics,good desalting nature,high flux,and relatively less expensive[7-9].Cellulose acetate membranes with differentpolyvinylpyrrolidone(PVP)concentration and coagulation-bath-temperature were prepared by a group of researchers[10].They have found that the addition of 0 to 3 wt%of PVP to the polymer solution increased the macro-void development and consequently the pure water flux was raised.Nevertheless,due to further addition of PVP to 6 wt%the macro-void formation was suppressed and pure water flux(PWF)was reduced.On the other hand,increasing in coagulation bath temperature up to 25°C resulted in increasing of the macro-void formation.On the other hand,Chakrabortyet al.have prepared flat sheet polysulfone membranes from casting solutions by using NMP and DMAc solvents[11].Polyethylene glycol(PEG)of average molecular weights of 400,6000,and 20000 were used.The PWF andPmwere enhanced significantly as the PEG molecular weight was increased.A substantial increase in rejection of bovine serum albumin solution was also reported due to an increasing of the molecular weight of PEG from 400 to 6000.This increasing tendency of the membrane with PEG 6000 may be due to the spongelike and a dense structure which can offermore resistance to the protein molecules.They have also reported that BSA rejection was observed to decrease when the PEG molecular weight was beyond 6000(i.e.20000).The high porosity and presence of finger-like cavities in the sub layer formembranes with PEG 20000 have allowed more BSAtransmission withoutmuch resistance and resulted in less BSA rejection.Kimet al.have also investigated the effect of PEG as a pore-former on polysulfone/N-methyl-2-pyrrolidone(NMP)membrane formation using phase inversion process[12].They have found that the coagulation value was decreased as the molecular weight(Mw)of the PEG was increased.They have also observed that the membrane surface pore size becomes larger,and the top layer appears more porous and the distance from the top surface to the starting pointof macro-void developmentbecomes greater.On the other hand,they have reported that with increasing of the PEG additiveMw,water flux was increased and PEG solute(PEG of 12000 and 35000 g·mol-1used as solutes)rejections were observed to decrease.Solute transmission increases if the electrostatic interaction is insignificant,where in turn solute rejection is expected to decrease with increasing in PEG molecular weight.
Generally,polymeric additives are essential to improve the membrane properties[11-14]mainly pore structure,mechanical stability,thermal stability,hydrophilicity, flux and rejection capabilities[10,15-17].
Based on the above literature,it seems that so far no work has been reported on the effects of solubility parameter differences among PEG,PVP,and CA on the preparation of ultra filtration CA membranes and comparison of the effects of PEG and PVP additives on morphological structures and performances of the membrane.The previous studies have focused on the individual effects rather than comparing their effects atthe same processing condition fora single polymer.Consequently,an effort has been made to explore the effect of PEG and PVP additives,and the effect of solvents in the mixtures of CA/Ac:DMAc and CA/DMF solution discretely.In this study,CA was used as polymer and PVP and PEG were used as additives and pore forming agents to compare their effects on the morphological structure and permeability properties of the membrane.On the other hand,we put our effort to investigate the effect of the two solvents on the morphological structure and permeability properties of the membrane.All the experiments in this study were performed for three times and all the graphs are presented with error bars.Moreover,allthe experimentalresults were consistent with the membrane properties and agreed with each other.
2.Experimental
2.1.Materials
Cellulose acetate and polyvinyl pyrrolidone(PVP,Mwof 40000)polymers were purchased from Loba Chemie,India.The two solvents,namely,N,N-Dimethyl acetamide(DMAc)and acetone(both with an analytical purity of 99%);N,N-Dimethyl formamide(DMF)with 99.5%purity;polyethylene glycol 4000 were all obtained from Merck Specialities Private Limited,Mumbai,India.The non-solvent in the coagulation bath used throughoutthis experimentwas DIwater after purifying with Millipore system.BSA withMwof 66000 was purchased from SRL,India.
2.2.Membrane preparation
Phase inversion technique using immersion precipitation was used for the fabrication of asymmetric ultra filtration membrane from cellulose acetate polymer.Initially 10.5 wt%of CA powder was dissolved in acetone:DMAc(70 wt%/30 wt%)and DMF separately with continuous magnetic stirring at room temperature of(26 ± 2)°C.A particular attention was given to acetone and DMAc where the two solvent properties were then mixed to attain enhanced CA solubility due to synergistic effectand we have selected the mixture ratio according to the literature.On the other hand,DMF was selected due to its good solvent nature for CA where it avoids rapid evaporation of the solution[18,19].After getting a uniform solution of the CA/solvent,a fixed amount of each additive(5 wt%)was added and stirred continuously at least for 1 day until the solution was fully dissolved and finally homogeneous casting solution was obtained.The amount of polymer and additives were chosen based on the literature[13,16].Membranes with differentcomposition were designated as,CA0,CA1,CA2,CA3,CA4,and CA5.Table 1 shows the solution casting compositions ofpolymeric additives with CA.
The membrane preparation process is shown in Fig.1.The casting solution was stirred overnight using a magnetic stirrer at room temperature.After that,a homogeneous solution of solvent,polymer,and the additives were attained and kept for 24 h at room temperature.Consequently,the casting solutions were poured consistently on a glass sheet with the help of a casting knife keeping an allowance of roughly 0.25 mm between the knife and the glass plate.Then,the resultant films were exposed to air for about 30 s earlier to soaking in the coagulation bath containing DIwater atroom temperature.In the coagulation bath,the cast solution turned from transparent to white color and the thin film detached from the glass plate.The membrane sheets were kept for 30 min in the coagulation bath.After that,the fabricated membranes were reserved in DI water beakers until use.Finally,the membrane sheet was cut into a circular disk to put in the filtration cell for pure water and BSA filtration experiments.
2.3.Characterization of membranes
2.3.1.Morphological studies
Morphological studies of phase inverted cellulose acetate membranes were done using a high-resolution field emission scanning electron microscopy(FESEM,ZEISS,USA),which provides visual information of the topographic view besides the cross-sectional structure of the membrane.A gold coating for the samples was done using a high-resolution sputter coater,Quorum,to protect the membranes from charging during the image analysis.Finally,the pieces ofthe membraneswere attached to a plate holderusing double-sided adhesive carbon tape in a horizontal position.
2.3.2.Pure waterflux and hydraulic permeability
In this study,a 400 ml stirred batch cell experimental set-up was used for the permeability experiments,which is presented schematically in Fig.2.This experimental set up comprised of(1)a pressure source to supply the pressure required for the filtration experiment;(2)feed tank where the feed is collected;(3) filtration cell,(4)membrane piece,and(5)permeate collector after membrane filtration.Membranes with the circular shape of 7×10-2m diameter and with an effective filtration area of 3.848×10-3m2were employed for this experiment.Compaction studies of each of the prepared membranes were done using DI water for 2 h using a fixed pressure of 300 kPa and PWFs were recorded with 10 min interval.The membrane compaction factors(CF)were found by calculating the ratio of initial PWF(Jwi)to steady state PWF(Jwf).PWF results were calculated using the following equation:

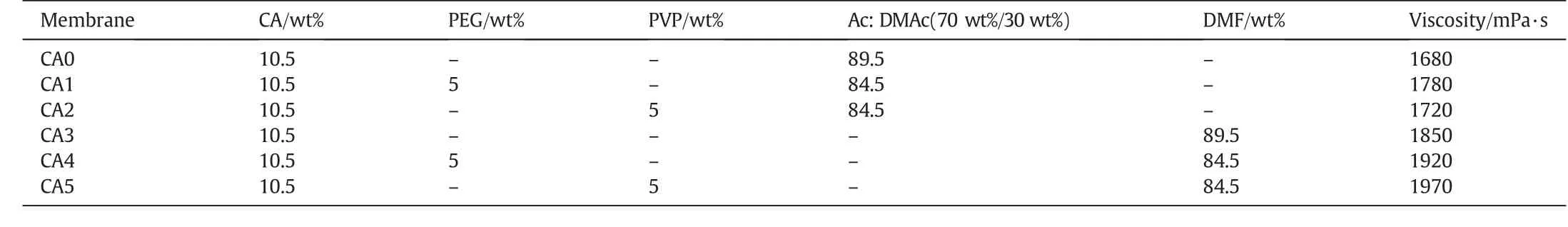
Table 1Compositions and viscosity of the casting solution of polymeric additives with CA
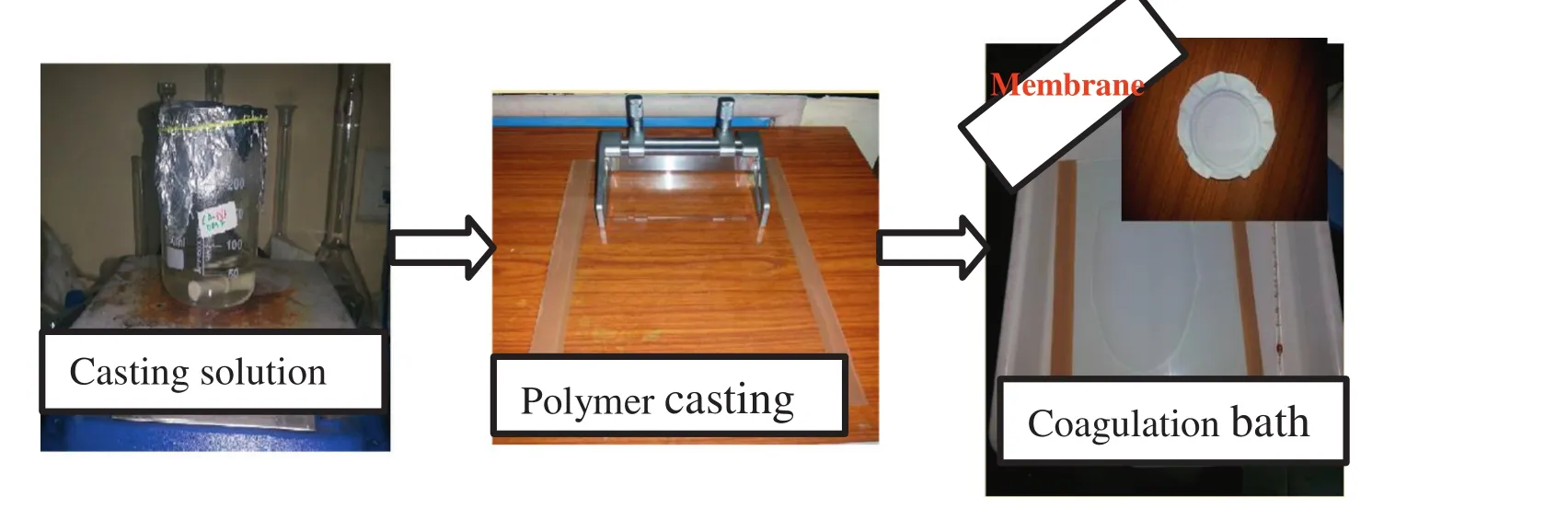
Fig.1.Membrane preparation process.
where,Jwis the PWF(L·m-2·h-1),Qis the volume of water permeated(L),Ais the effective membrane area(m2)andΔtis the permeation time(h).Thus,PWFs were evaluated by passing DI water through the membranes.PWF values at different operating pressures(ΔP)(ranging from 100 to 300 kPa)were recorded.The hydraulic permeability(Pm)(L·m-2·h-1·kPa-1)ofthe membranes was calculated from the inverse slope ofJwversusΔPplot.The calculations forthe hydraulic permeability were done as follows:

The resistances(Rm)ofthe prepared membranes were calculated according to Darcy's law(Eq.(3)):

where μ is the water viscosity(8.9 × 10-4)(Pa·s)
2.3.3.Hydrophilicity and porosity measurements
To study the membranes'hydrophilicity nature and porosity,three parameters were applied namely,water contact angle(°),equilibrium water content(EWC),and membrane porosity(ε).Both the ε and hydrophilicity play a significant role in the permeability and rejection nature of the membrane.The water contact angle(WCA)measurements ofthe prepared membranes were conducted on a contactangle measuring instrument(Kruss,advance drop shape analysis,Germany).On the other hand,the EWC and ε were evaluated using a simple gravimetric method,where each membrane samples(2.5×2.5 cm2)were immersed in DI water beakers for a specified period.Then,the samples were dabbed with dry filter paper and weighed immediately(Ww).Finally,the membranes were kept inside vacuum atmosphere for 24 h at 50°C.The final dry mass of the samples(Wd)have taken again.The membrane porosities were calculated by dividing the volume of the pores by the total volume of the membrane.Therefore,the results are found using the following equation[20,21],

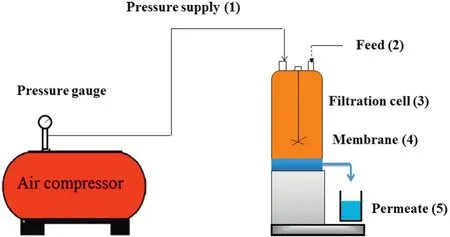
Fig.2.Schematic diagram of the batch cell ultra filtration set-up.
where,Wwis the wetmembrane mass(g),Wdis the dry membrane mass(g),ρwis the pure waterdensity atworking condition(g·cm-3),andρpis the polymer density(g·cm-3).EWC results were calculated using the subsequent equation[5],

The average values ofEWC and membrane porosity were taken after measuring of five different samples to reduce the error of the balancing measurement.
2.3.4.Membrane fouling and rejection experiments
Membrane fouling experimentations were done using a stirred batch cell specified before to investigate the effect of PEG and PVP additives and effect of the two solvents on BSA rejection and permeate fluxes of the fabricated membranes.The BSA solution(1 g·L-1)was prepared using deionized water as solvent.The pH of the BSA solutions was kept at 7.9.The flux values were calculated using Eq.(1)and the percentage rejection of BSA solutions was evaluated by the next equation[22]:

where,CfandCpare the BSA concentrations(mg·L)on the feed side and the permeate side,respectively.The ultra filtration experiments for BSA solution were achieved through all prepared membranes at an operating pressure of 150 kPa.After that,the permeates were collected using a measuring container at a constant interval of time(20 min)to calculate flux values and the length of each filtration test was for 2 h.Finally,the permeate concentration of BSA solution was evaluated by using UV-vis spectrophotometer(Thermofisher Scientific,UV2300,India)ata wavelength of278 nm.After filtration ofBSAsolutions,fouled membranes were soaked in DI water and rinsed for 30 min and the water flux of the membranes were measured.These experimentations were repeated for three times and the graphs including error bars are presented.To investigate the anti-fouling properties of the prepared membranes,the flux losses due to irreversible fouling(Fir),reversible fouling(Fr),and total fouling(Ft)of all the experiments were calculated using the following equations,respectively[23,24].


where the fouling resistant capacity of the prepared membranes was evaluated using normalized flux ratio(NFR)as shown in Eq.(10).

whereJwfis the flux of the membrane after fouling(2 h)andJwiis the flux of the membrane found at the start of the fouling stage.Normally,greater NFR value(next to 1)indicates better anti-fouling nature of the membranes.
2.3.5.Average pore radius determination
Water filtration velocity method was employed to determine the average pore size of the membranes,and the results were calculated at constant operating pressure(300 kPa).Generally,membrane average pore radius(rm)is considered as an approximation of true pore size and it denotes the average pore radius throughout the membrane's thickness(ζ).Average pore radius can be estimated by using the Guerout-Elford-Ferry equation[21,25],
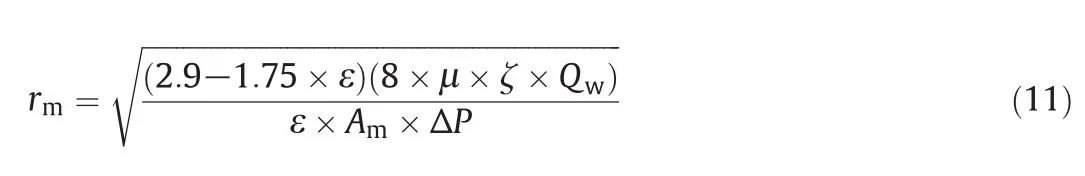
where,μis the water viscosity(8.9 × 10-4)in Pa·s,Qwis the water flow(m3·s-1)and ΔPis the operating pressure(0.3 MPa).
3.Results and Discussion
During the preparation of phase inverted membranes using immersion precipitation process,a ternary and multicomponent system were used.In ternary system,scheme consisting ofa solvent,a polymer,and a non-solvent was applied where the non-solvent and solvent are miscible.On the other hand,the multicomponent system(polymer/solvent/non-solvent/additive)was also investigated.In immersion precipitation,a homogenous mixture of the solvent and polymer are cast to a thin film on the glass plate and then immersed in a non-solvent(waterin thiscase)bath.Asseen in Fig.3,the non-solvent(water)starts to diffuse into the cast film(J1)and the solvent diffuses into the coagulation bath(J2).After a specified time,the exchange of non-solvent and solvent has continued until the solution becomes thermodynamically unstable and liquid-liquid de-mixing occurs.Finally,a polymeric film is obtained with an asymmetric structure.
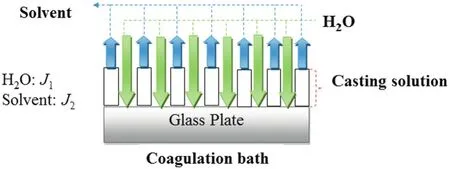
Fig.3.Schematic representation ofcasting film and coagulation bath interface:solvent(J2)and non-solvent(J1).
3.1.Morphological study
The FESEM images of(a)top layer surface view and(b)the crosssectional view of the prepared membranes by dissolving the polymer and additives in two different solvents(DMF and Ac:DMAc)are presented in Fig.4.As clearly shown from the figures,the prepared membranes are asymmetric in structures comprising a porous sublayer and a dense top layer for all types of the membranes(i.e.for CA/DMF and CA/Ac:DMAc).The sublayer portion of the membranes without additives(CA0 and CA3)seems to have finger-like voids as well as macro
void structures.According to the literature,there are so many parameters,such as evaporation step[26]and coagulation bath temperature(CBT)[13]that allow to the development of macro-voids during membrane formation using immersion precipitation process.Generally,the development of macro-voids happen under rapid precipitation condition,and the precipitations are quicker at higher coagulation temperatures.In the current study,the coagulation bath temperature and evaporation step(i.e.before immersion)are fixed to 25°C and 30 s,respectively,during the preparation of all the membranes.Another important factor which affects the formation or suppression of macrovoids is the type of de-mixing which occurred during the phase separation process.Furthermore,the instantaneous de-mixing and the delayed de-mixing depend on the mutual affinity of the non-solvent and solvent in the ternary system[1].The solvents used in this study(DMF and Ac:DMAc)have high mutual affinity with water.Therefore,apparently,the formation of the finger-like voids and macro-voids in the sublayerof the membranes without additives is due to the instantaneous de-mixing.On the other hand,when the polymeric additives are added to the ternary(polymer/solvent/non-solvent)system the formations of macro-voids are suppressed for membrane prepared using PEG(i.e.CA1 and CA4).However,for membranes prepared using PVP the formation of some macro-voids are still maintained(i.e.CA2 and CA5).However,the formation of microporous structures was observed for both the additives.In a quaternary system(polymer/solvent/nonsolvent/additive),nevertheless,the exchange process of water and solventbetween the coagulation bath and the casting solution taking place right upon immersing is considerably faster than the separation of the two polymers.However,after some instants of immersion process,polymer-polymer movement may possibly occur.This is because of the separation of the two polymers(membrane forming and additive)that involves the movement of one polymer with respect to the other[27].Therefore,a binary phase arises from the phase separation,where one involves of the CA(i.e.membrane-forming polymer),Ac:DMAc/DMF(i.e.solvents)and non-solvent(i.e.water)and the second contains the polymer additives(i.e.PEG or PVP),solvents(i.e.Ac:DMAc or DMF)and non-solvent(i.e.water).The membrane forming polymer and additives have a driving force to separate completely into a CA-rich and a PEG-rich or PVP-rich phase.Consequently,the type of de-mixing process in this case can be in fluenced by the diffusion/movement of the two polymers on each other.Therefore,this phenomenon should be slow compared to the diffusion of solvent and non-solvent in the polymer solution.To maintain this,a polymeric additive with a certain minimum molecular weight in the system is required[28].
3.1.1.Effect of additives
Generally,the introduction of hydrophilic additives which are soluble in the non-solvent(i.e.water)with high affinity to the solvents and low affinity to the membrane forming polymer,tends to increase the thermodynamic instability of the casting film which further leads to instantaneous de-mixing to occur in the coagulation bath.This result encourages the development of macro-voids in the membrane structure.On the other hand,the presence of the polymeric additives increases the concentration/viscosity of the casting solution which may diminish the diffusional exchange rate of the solvent and non-solvent during the membrane formation process.Consequently,this may hinder the instantaneous liquid-liquid de-mixing process which suppresses the development of macro-voids[10,13,29].It is evidently clear from the figures that the finger-like voids in the sublayer are considerably reduced with the adding of polymeric additives(PEG and PVP)for both solvents.Particularly,for CA/Ac:DMAc/PEG scheme(CA1)and CA/DMF/PEG(CA4),the membranes are found to have almost macro-void free cross-sectional structures.On the other hand,the CA/Ac:DMAc/PVP system(CA2)and CA/DMF/PVP system(CA5)membranes showed the development of some finger-like and macrovoids.These results lead to the conclusion,although the instantaneous de-mixing is yet continued,the effect of those additives has considerable part on the suppression or development of macro-voids.The FESEM images of the top view of all the prepared membranes are presented in Fig.4(a).Some of the open pore structures developed in the membranes are formed by nucleation and development of the polymer-lean phase in the metastable area between the bi-nodal and the spinodal curve[28,30].Nevertheless,the top layer structure of the ultra filtration membrane frequently doesn't reveal an open pore structures and not a completely uniform gel-layer,which is stated as a nodularstructure.The developmentofnodularstructurescan'tbe described by nucleation ofthe polymer lean part.Itis also notcommon thatnucleation of the polymer-rich part happens because this only occurs at initially low polymer concentrations,below the critical point.A possible explanation for the formation of a nodular structure on the top surface of the membranes could be due to the spinodal de-mixing because the diffusion process throughout the development of the top layer is faster for the homogenous system to develop greatly unstable and crosses the spinodal curvature[4,28].The top surface with improved interconnected pore structures is observed more prominently in the case of PEG(i.e.CA1)which is attained because of the spinodal decomposition.Therefore,the inter-connected pores may be regarded as a continuous CA-lean(PEG-rich)phase tangled by a constant CA-rich(PEG-lean)phase which produces membrane with a uniform matrix.On the other hand,in the case of PVP(CA2),a non-uniform pore structure was observed.However,the remaining membrane surfaces(i.e.CA0,CA3,CA4,and CA5)depicted dense top surface structures.
3.1.2.Effect of solvents
The effects of solvent:non-solvent and solvent:polymer interactions are also very important parameters on the final morphological structures of the membranes.Therefore,the tendency of mixing of the solvent with non-solvent,the degree of swelling of the pure polymerin the non-solventand the solubility of the membrane forming polymer in the solventshould be considered during the investigation ofthe effect of different parameters on the final structure of the membranes.From the FESEM images,Fig.4(b),it is clearly observed that the macro-void formation in the CA/DMF system is greater than Ac:DMAc system.This can be due to the transition from instantaneous to delayed onset of de-mixing attained by changing the type of solvents[5,27],which is also complemented by the diminishing of development of macrovoids.The miscibility of the solvent:non-solvent mixture,employed for the preparation of the membranes,has a significant effect on the if nal structure of the membrane[30].According to Reuverset al.[31],the delay time for the onset of liquid-liquid de-mixing increases with decreasing the tendency of mixing solvent with the non-solvent.The current observations are completely in agreement with these explanations that the absence or less macro-voids formation in the case of Ac:DMAc/CA system is due to the delayed onset of de-mixing.On the other hand,development of macro-voids or finger-like cavities is observed for DMF/CA systems.These results are because DMF has greater tendency of miscibility with water than that of Ac/DMAc and therefore the instantaneous de-mixing occurs which is responsible for the formation of macro-voids.The effect of these solvents can be explained from another point of view that the top layer thickness of the crosssectional views depicted a visible difference.Therefore,the delayed and instantaneous de-mixing processes can affect the top layer surface of the finally prepared membrane.In this case,the membrane formed from DMF/CA system gives membranes with relatively lesser top layer thickness than the membranes prepared using AC:DMAc/CA system.However,the total thickness of the AC:DMAc/CA membranes are lesser than DMF-CA system membranes as seen from Table 2.It is clear that a thicker top layer is due to the delayed onsetde-mixing during the phase separation.One point which should be clarified here is that the delayed de-mixing,in this case,is not extended delayed de-mixing.Normally,if the delayed de-mixing is extended,the polymer film thickness will decrease considerably which may cause a decrease in the porosity of the sublayer membranes[31].This result may further prevent the formation of interconnected pore structures which in turn can form membranes with homogenous and with dense layer structure.However,for the membrane prepared from Ac:DMAc/CA system(CA0,CA1,and CA2)the pores in the sublayer are interconnected due to the development of aggregates associated with the gelation of sublayer.Therefore,the resistance of the sublayer of these membranes is much lower than that of membranes could prepare from extended delayed de-mixing(dense membranes).
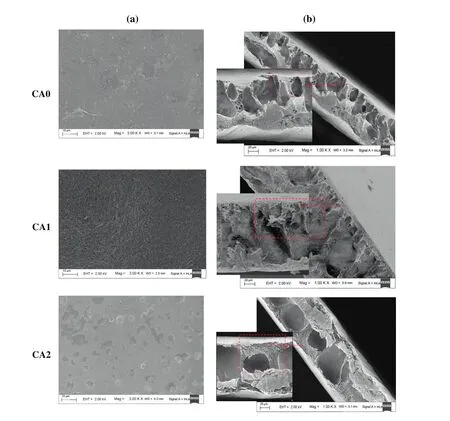
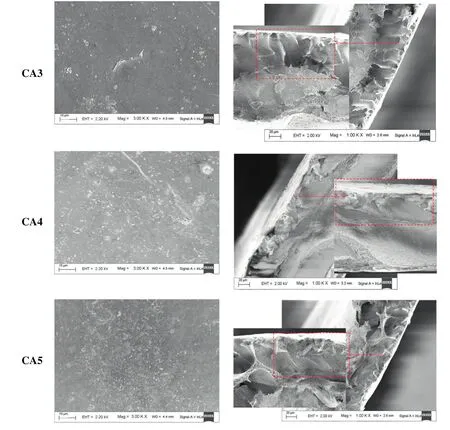
Table 2Compaction factor,hydraulic characteristics,EWC and porosity of all prepared membranes at 300 kPa
3.2.Pure water flux and hydraulic permeability
3.2.1.Effect of additives
The study of compaction factor(CF)is very important to understand the pore structures of the membranes specifically the membrane sublayer structure.Membranes with high CF implies that they are highly compacted which may,in turn,indicate the availability of a large number of macro-voids or finger-like arrangements in the membrane's sublayer structure.Therefore,the impacts of compaction time on PWF of the prepared membranes are depicted in Fig.5.All the membranes prepared using both solvents(Ac:DMAc and DMF),and both additives(PEG and PVP)are presented in Fig.5(a)and(c),respectively.The PWF of allthe membranes was observed to decline slowly with time because of the compaction and finally the steady state fluxes were reached after 80 min.This gradualdecrease in PWF results can be explained due to the compaction of pore walls(attain a uniform and denser structures)causing the pore size and the flux to decrease[1].Therefore it is important to investigate the effect of additives on the compaction process,where the effects of the additives were significantly noticed from this study.Another point which is observed from this study was that the membranes without additives showed lesser PWF than membranes with additives for both the solvents.This result is apparent that the introduction of hydrophilic additives to the membrane casting solution makes the membrane porous and more hydrophilic[32].The result evidently shows thatthe introduction ofPEGand PVP impactthe development of porous structure in the membrane,where the permeability properties of the ultra filtration membranes were significantly affected[33,34].Moreover,the whole removal of polymeric additive from the membrane-forming polymeric medium may not be achieved during the phase separation process in the coagulation bath and even after rinsing with DI water.Consequently,the residual additives(i.e.PEG and PVP),are forced to remain within the membrane matrix permanently,thus,forming more hydrophilic membrane[34-37].In the current study,due to the introduction of the hydrophilic additives,a small quantity of PVP and PEG may permanently stay entangled in the membrane's matrix.These results can improve the hydrophilic nature of the CA membranes(Fig.6).Therefore,the polymeric additives can play an important role in the formation of porous membrane with improving its hydrophilic nature which is again directly related with its water permeability performance.Comparing both the additives,the membranes with PVP gained higher flux than membranes with PEG for both solvents.It is clearly explained from the FESEM images(Fig.4)that the membranes formed using PVP additive(i.e.CA2 and CA5)showed the development of greater macro-voids when compared with membranes formed using PEG additive(CA1 and CA4).The decrease in water flux for membranes prepared from casting solutions containing PEG additive is due to the development of a denser top layer as the phase separation is delayed.These results are also supported by the porosity and pore size measurements reported in Table 2,where it clearly shows that a relatively lower affinity of PEG to the membrane forming polymer interferes the de-mixing process and prevents the formation of large pores.Therefore,regardless of the effect of solvents,the membranes with PVP additive are more porous than the membranes with PEG additive,which in fluences their water flux performance.
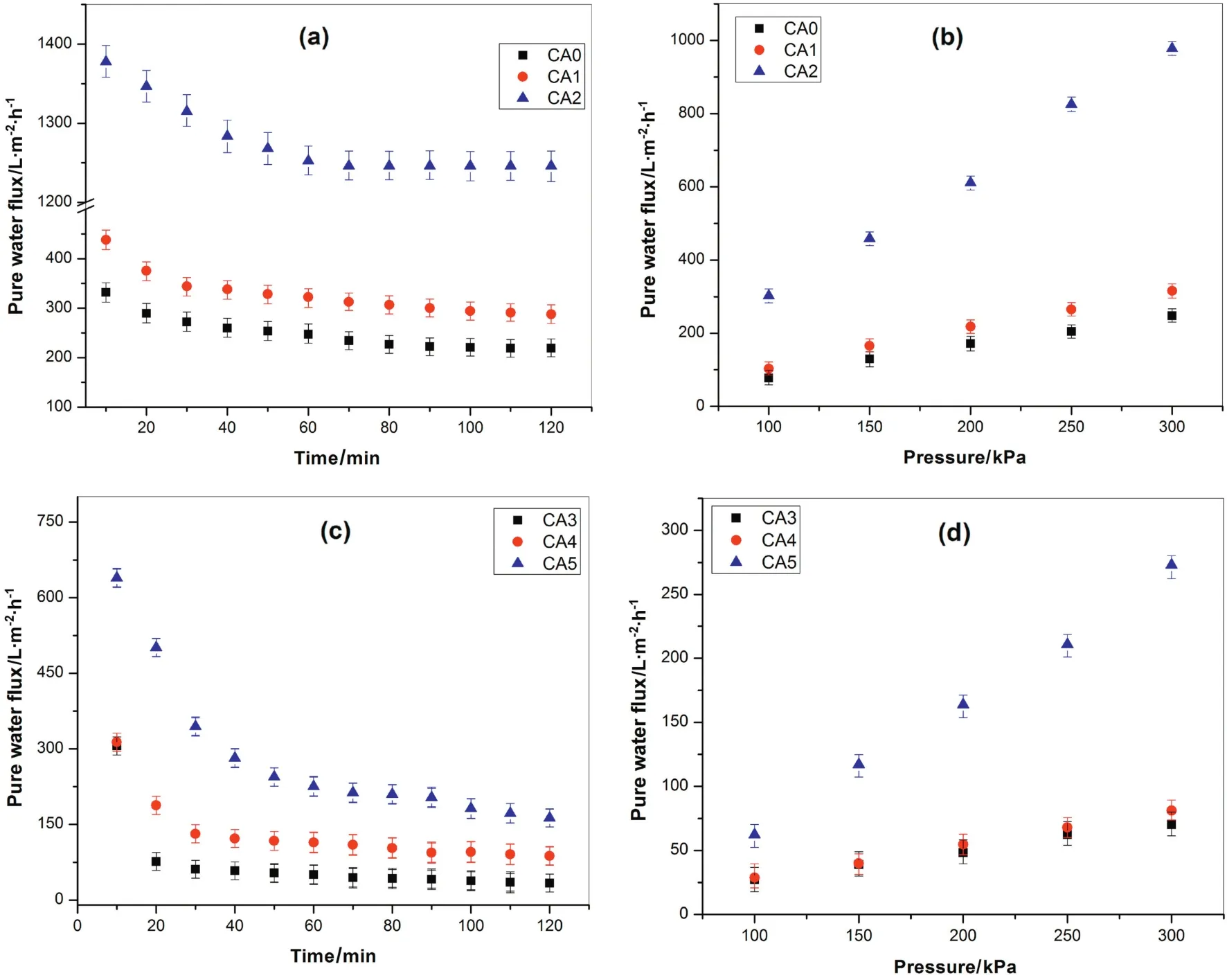
Fig.5.(a,c)PWF profile during compaction study and(b,d)effect of operating pressure on PWF.
3.2.2.Effect of solvents
Generally,to develop the membrane skin layer with sharp polymeric concentration difference(lower concentration atthe lowestsurface and a higher concentration at the upper surface)should be attained.The polymeric concentration difference will be made because of the solvent evaporation before the immersion and due to the immersion of the casting solution into the non-solvent.Therefore,the investigation of the effects of organic solvents on preparation of membranes by phaseseparation is an important matter[38].On the other hand,the membrane characteristics,which are evidently interrelated to the membrane morphological structures,can be strongly impacted due to the level of interaction between the membrane forming polymers and solvents during the preparation ofsolution casting.An additional interesting pointis that the residual solvents in the membrane after completing the phase separation may also alter the final membrane properties[39].Normally,the morphological structure variation of the membranes can be associated with the solvent and polymer interaction.In a solventand polymer interaction scheme,three kinds of interactionsviz.polymer:polymer,polymer:solvent and solvent:non-solvent are applied.When we use good polymer solvents,the degree of polymer stretching reaches to its highest level and more favorable polymer/solvent interactions can occur[40,41].Furthermore,the affinity between the solvent and polymer can be predicted by presenting the “solubility parameter”, δ,which is described as the square root of the cohesive energy density and expresses the strengths of attractive forces among the molecules.Solvent:polymer interaction in a polymer solution has been estimated based on the solubility parameter difference(Δ)between polymer and solvent as already clearly mentioned by Hansen[42].The Hansen solubility parameters of the solvents,additives,and polymer used in this study with their boiling points are presented in Table 3.According to Hansen,the permanent dipole-dipole interactions(δp),dispersive(δd)and hydrogen bonding forces(δh)should be taken intoconsideration.Consequently,the solubility parameters(δ)can be calculated as follows[42]:
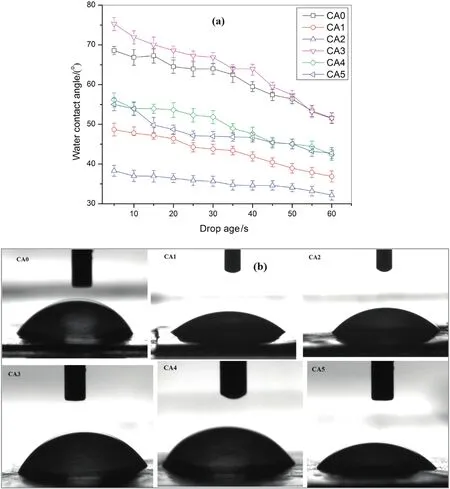

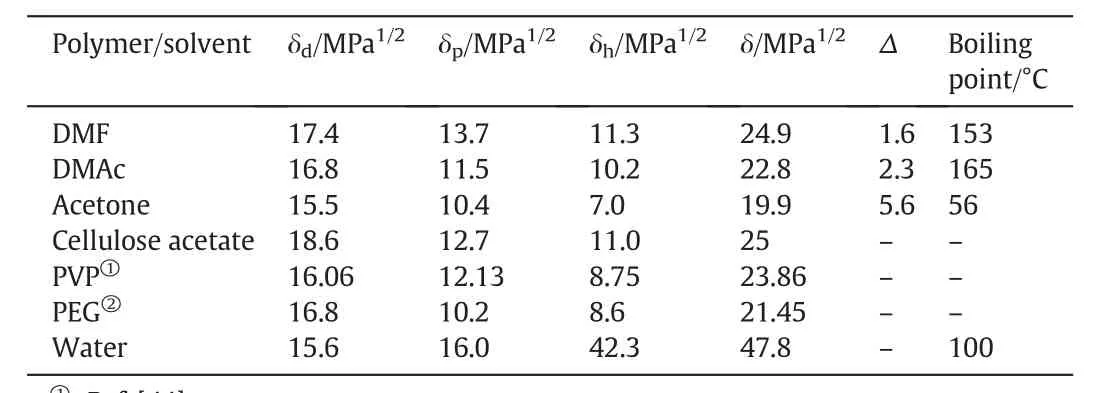
Table 3Hansen solubility parameters for selected solvents,non-solvent,additives,and cellulose acetate[39,43]
The Hansen solubility parameter differences(Δ)among the solvents and the membrane forming polymers were computed using the following equation:
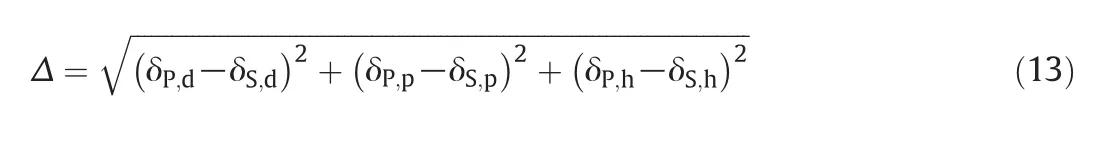
where,S and P denote the solventand polymer respectively;and p,d,and h represent for polar,dispersive,and hydrogen bonding elements of Hansen solubility parameter.The polymer-solvent interaction parameter is inversely proportional with Hansen solubility parameters(Δ),i.e.the smaller the difference between the solubility parameters of polymer and solvent,the stronger the polymer-solvent interaction.The results of the solubility parameter difference are listed in Table 3 and show that the Δsof the solvents are in decreasing order(i.e.DMF< DMAc< Acetone).Therefore,the solvent:polymerinteraction ofthe three solvents increases in the order of DMF>DMAc>Acetone.From these results,it can be suggested that a relatively strong hydrogen bonding interaction was happened between remaining DMF and CA when compared with CA/Ac/DMAc.Therefore,the strong interaction between DMF and CA was suggested during the preparation of solution casting process for membrane forming.
The effectofsolvents on the PWF was also observed clearly fromthis study.Membranes prepared using Ac:DMAc(CA0,CA1,and CA2)showed higher fluxes than membrane fabricated with DMF(CA3,CA4,and CA5)regardless of the effect of additives.Moreover,the initial sharp decrease in PWF for CA/DMF membranes was significantly improved for CA/Ac:DMAc membranes.This result is evidently related with the effect of solvent on the morphological/sublayer structures of the membranes.All the membranes prepared with CA/DMF system depicted large flux decline due to non-uniformsublayer pore structures.The percentage flux decline were found to be as 89%,72%and 75%for CA3,CA4,and CA5,respectively.Secondly,the formation of larger macro-voids due to an instantanoues de-mixing may have resulted in the formation of non-uniform pore distributions within the membrane matrix.However,for the membranes prepared from CA/Ac:DMAc system,the percentage flux decline for CA0,CA1,and CA2 were 34%,32%and 33%,respectively.Unlike the CA/DMF membranes,the uniformly interconnected pore structuresofthe sublayerwith uniformpore distribution of CA/Ac:DMAc membranes showed better permeability characteristics.The difference in flux between the CA-DMF and CA-Ac:DMAc membranes can also be associated with the effect of the solvents on the hydrophilicity nature of the membrane.As it is believed that there are some residual solvents within the final membrane matrix[39,46],they may affect the water flux performance of the membrane.Guanetal.[39]also reported thatthe membranes with DMAc solventshowed a higher hydrophilic nature than membranes with DMF.Similar results were found in our study.Regardless of the effects the polymeric additives the CA/AC:DMAc showed more hydrophilicity nature than the CA/DMF membranes.It is clearly shown from the membranes prepared without additives(i.e.CA0 and CA3).The effects of the residual solvents are not significant as compared to the effect of additives,however,it is believed to have some in fluences on the membrane performance.
Table 2 presents the compaction factor and hydraulic characteristics of all prepared membranes.It was observed that for both CA/Ac:DMAc and CA/DMF membranes,the CF decreases with introduction of the additives.However,for membranes prepared using both solvents the CF for CA/PEG was less than CA/PVP.This can be described that an introduction of additive into the membrane forming solutions may suppress or increase formation of macro-voids in the membrane sublayer based on the type of additive and solvent[27].In the current study,for both the solvents less formation of macro-voids was observed for CA/PEG than CA/PVP.Due to these reasons,membranes with PVP additives have resulted in highly porous sublayer structure because of the occurrence of more numbers of macro-voids for both the solvents.These results are also depicted from the FESEM images(Fig.4).
The effects ofpolymeradditives and solvents on PWF atdifferentoperating pressures are presented in Fig.5(b)and(d).The results of PWF for all the membranes were increased almost uniformly with increasing in an operating pressure from 100 to 300 kPa.It is also shown that the PWF for all the CA/DMF membranes is lesser than that of CA/Ac:DMAc membranes.Nevertheless of the effect of the solvent,the membranes with PVP additives showed higher PWF than that of membranes with PEG additives for both the solvents.These results are in good agreement with the conclusions of the compaction study in Fig.5(a,c).The hydraulic resistance(Rm)of CA/DMF membranes was greater than that of CA/AC:DMAc membranes.In addition,theRmfor the membranes without additives was higher than all the membranes with additives for both solvents.On the other hand,theRmfor CA/PEG was higher than that of CA/PVP for both the solvents.Therefore,an increase in hydraulic resistance and decrease in flux with varying additives and solvents may be accredited to the decline in pore size during compaction.In general,both the additive namely,PVP and PEG are more hydrophilic than the CA polymer.Therefore the CA membranes with these additives showed improved flux results.Comparing the two additives the CA/PVP showed higher flux and lowerRmthan membrane of CA/PEG.These results can be explained because of higher solubility and diffusivity of PVP than PEG,where most of the PVP can be washed out more easily and quickly(as compared to PEG)with the non-solvent(i.e.water)during the membrane fabrication period[10].This result may also be explained due to the formation some macrovoids because of the rapid penetration of non-solvent at certain weak spots in the top layer of the membrane;the PVP rich phase might act as the weak spots during the precipitation process and result in the development of macro-voids[17].As a result,the suppressed sublayers of the PEG membranes are possible to give additional resistances to water permeability than PVP membranes resulting in low flux.The effects of additives as a pore former as well as hydrophilicity enhancer were also investigated,and the results were calculated using Eqs.(4)and(5).The wettability of the membranes was studied by measuring the water contact angle and the drop age,de fined as the duration of the water droplet on the surface of the membrane and spreading and/or permeating through the membrane cross-section[47].The variation of water contact angle of all the prepared membranes is presented in Fig.6(a).The images of the water droplets with a volume of about 2 μl at 0.16 ml·min-1on the membrane surface after 60 s are shown in Fig.6(b).CA1,CA2,CA4,and CA5 membranes have taken about 15 s,10 s,25 s,and 20 s,respectively,and show best water wettability,where mostpartofthe membrane surface were almostcompletely wetted and smaller spread radius of the water drops on the top side of the membrane were observed after 60 s.On the other hand,CA0 and CA3 membranes have taken about 34 s and 36 s to initiate the surface wetting and big water drops spread radius on the top side of the membrane were observed after60 s(Fig.6b).The smallerthe water drop spread radius wetting area between top and bottom surface and on the top membrane side,the better the water permeability is.As clearly observed form the graph the contact angle results of the pristine CA0 and CA3 membranes show water contact angle about(61.3 ± 1.6)°and(64.1 ± 1.5)°,respectively.In contrast,after the introduction of hydrophilic additives,all the CA1,CA2,CA4,and CA5 membranes showed greatly reduced water contact angle results(i.e.(43.3 ± 2.2)°,(35.1 ± 3.1)°,(49.6 ± 1.8)°,and(47.6 ± 2.0)°,respectively).Membranes having smaller contact angle results are considered as more hydrophilic membranes.Therefore,an increase in the hydrophilicity,EWC,and porosity of the membrane after adding of additives is due to the pore forming and hydrophilicity properties of PEG and PVP.Besides,as already discussed above using of different solvents also in fluenced the hydrophilicity,EWC,and porosity of the membrane in some way.Thus,the CA/Ac:DMAc membranes showed better hydrophilicity nature than that of CA/DMF membranes irrespective of the effect of additives.Moreover,it is con firmed that all the membranes are in ultra filtration range as seen from the pore radius(rm)measurement results in Table 2.
3.3.Membrane fouling and rejection experiments
Membrane fouling is de fined as the deposition of retained particles colloids,macromolecules,salts,etc.at the membrane surface,pore mouth or pore wall causing flux decline.Fouling can be caused by broadly three kinds of substances:organic(macromolecules,biological substances like protein,enzyme,etc.),inorganic(metal hydroxides,calcium salts,etc.),and particulates.Proteins are strongly adsorbed on hydrophobic or less hydrophilic surfaces but less on hydrophilic surfaces[1,48].In this study,the fouling and rejection experiments of all the membranes were done in the ultra filtration stirred batch cell to examine the consequence of PVP and PEG additives and effect of DMF and Ac:DMAc solvents on BSArejection and permeate flux.The concentration of bovine serum albumin(BSA)protein and pH of the solutions were kept at(1 g·L-1)and at 7.9,respectively,using deionized water as solvent.To examine the fouling performance of the prepared membranes,three steps BSA filtration processes were done.The flux results of pure water and BSA solutions of the prepared membranes are presented in Fig.7.
3.3.1.Effect of additives and solvents
It is clearthat the fouling and rejection behavior of the ultra filtration membranes are strongly dependent on morphological structures of the membrane(i.e.both top layer and sublayer).The effects of additives have their potential role on the variation in morphological structure of the membranes.Therefore,the introduction of PVP and PEG into the membrane matrix observed to in fluence the flux and BSA rejection performance of the prepared membranes.Generally,the fluxes and rejections of protein in ultra filtration membrane can be described using the theory of resultant pore narrowing and protein adsorption;due to electrostatic and hydrophobic interaction between BSA molecule and the membrane's surface[49].Regardless of the effect of solvent,the effect of PEG and PVP were clearly observed from the fouling experiments.Therefore,the fouling experiment results of CA/Ac:DMAc membrane system and CA/DMF membrane system are presented in Fig.7(a)and(b),respectively.The results showed that initially the CA/PVP membranes showed higher PWF fluxes than the CA/PEG membranes for both the solvents.However,a relatively higher BSA fluxes results were observed for CA/PEG membranes.It is clear from the results that the membranes without additives(i.e.CA0 and CA3)showed less PWF as well as the BSA fluxes due to their less hydrophilic nature and less pore formation.The improvement of pure water and BSA fluxes for membranes with additives(i.e.CA1,CA2,CA4,and CA5)were attributed to the pore-forming effect and hydrophilic nature of PEG and PVP additives.On the other hand,whole removal of additives may not be attained from the matrix of the membrane[50].
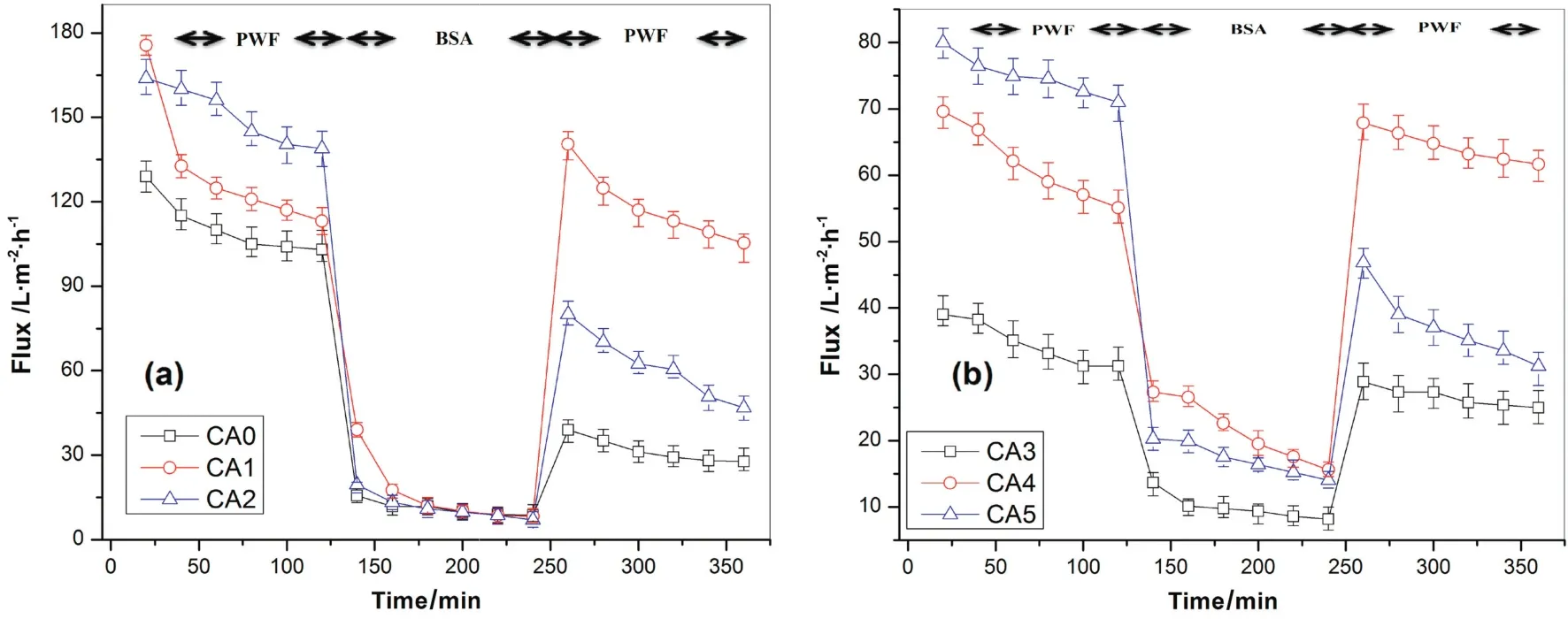
Fig.7.Permeate flux versus filtration time for(a)CA/Ac:DMAc and(b)CA/DMF membranes:effect of solvents and additives on fouling performance(25°C,150 kPa).
As clearly depicted in Fig.7,in the second 2 h BSA filtration operation,it is observed that with increasing time,the BSA fluxes gradually decreases for both CA/DMF and CA/Ac:DMAc membranes.The decrease in BSA fluxes with increasing time could be due to susceptible pore blocking of the membranes because of BSA protein adsorption and deposition on membrane surface,where the effect of concentration polarization was reduced by using a high molecular weight of BSA(66000)molecules and rigorous stirring(200 r·min-1)on the surface of the membrane.Moreover,the rapid drop in the primary fluxes are realized,and the ending fluxes are slowly dropped which is credited to the porosity lossofthe membrane due to an interioradsorption ofBSA protein which further lead to pore blocking.After membrane cleaning operation,the third step pure water filtration operation result showed that greater relative fluxes were recovered for both CA/PEG and CA/PVP membranes for both solvents.Normally,the orders of flux recovery ratios forthe examined membranes were consistentwith theirhydrophilic and porosity nature.Thus,the additive free membrane is more likely to prone to pore-blockage and fouling because of protein adsorption than the membrane with additives.In the literature,it is mentioned that both PEG and PVP[51]have the potential to minimize membrane fouling due to protein adsorption.Moreover,the PEG among various polymers is believed to be an excellent material to resist nonspecific protein adsorption contact with the surfaces due to the hydrated,neutral,highly mobile,and flexible chains since they provide maximum entropic repulsion between the proteins and surfaces[52,53].Therefore,the anti-fouling capacity of CA/PEG membranes was better than that of CA/PVP membranes irrespective of the effect of the solvents.The desorption of the adsorbed BSA proteins was performed by soaking the samples in DI water for 2 h and followed by rinsing using DI water.Moreover,the PWFs of CA-PEG-DMF and CA-PEG-Ac:DMAc were slightly increased after desorption of the BSA by washing the membranes for 2 h.Such phenomenon is not common in the conventional trends for fouling ultra filtration experiments.These results could be caused by the inherentinteractionsbetween foulant(BSA),and PEG additive entangled in the membrane matrixes and the membrane top layer[20,54,55].It was also con firmed that PEG could efficiently avoid the irreversible adsorption of the protein on the surfaces.Therefore,due to the hydrophobic interaction between PEG and BSA,the proteins might be wrapped by PEG chains,forming a protective layer[53].Generally,membrane fouling is consisted ofreversible and irreversible fouling.In the case of reversible fouling deposited protein could be eliminated easily using hydraulic cleaning(i.e.back washing and cross flushing)[56].Conversely,the irreversible fouling occurs due to an irreversible adsorption of proteins which can only be removed using chemical cleaning[57].To further examine the anti-fouling properties of the prepared membranes and to study the effect ofadditives,the flux losses viz.total fouling(Ft),reversible fouling(Fr)and irreversible fouling(Fir)were calculated,and the results are presented in Table 4.From these results,it can be shown that theFirfor the CA/PEG membranes is less than CA/PVP membranes.However,the reversible fouling(Fr)for CA/PEG membranes are higher than CA/PVP membranes.This may be credited due to extra BSA protein buildup on membrane surfaces because of the better BSA rejection.In spite of the greater reversible fouling,total fouling(Ft)of CA/PEG membranes showed lesser results than CA/PVP membranes due to the decreasing of irreversible fouling.From this result,it can be suggested that the fouling resistances,particularly resistances due to irreversible fouling,of CA/PVP membranes are significantly higher than CA/PEG membranes irrespective of the solvent effects.
The effect of solvents on the membrane morphological structures and PWFs were already explained in detail in previous discussions(Sections 3.1.2 and 3.2.2).Therefore,from these experiments it was clearly observed that both the PWF and BSA flux of CA/Ac:DMAc membranes(Fig.7a)are higher than the CA/DMF membranes(Fig.7b).Similarly,the BSA protein rejection of CA/Ac:DMAc membranes are higher than CA/DMF membranes irrespective to the effects of additives.
3.3.2.Rejection performance
The rejection performances of the prepared membranes are showed in Fig.8.The maximum BSA rejection results for CA/Ac:DMAc and CA/DMF system(i.e.94.4%and 82.9%rejections were attained for CA0 and CA3 membranes,respectively).These results can be explained due to the additive free membranes have less porous structures,where a better resistance to protein molecules was observed.On the other hand,the higher BSA rejection(91.9%and 69.9%)for CA/PEG(i.e.CA1 and CA4)membranes than CA/PVP(CA2 and CA5)membranes(77.9%and 60.7%),respectively,were achieved irrespective of the effect of the solvents.The characteristic of the BSA rejection can be described using the protein adsorption phenomenon as we have already explained above.The increase in BSA rejection for CA/PEG membranes is understood to be an indicator of substantial protein adsorption inside and on the membrane surfaces.Therefore,the surface depositions of BSA proteins provide an extra hindrance to solute transportation.Another reason for CA/PEG membranes to have higher rejections than CA/PVP membranes may be due to the adsorption of protein on pore-wall of CA/PVP membrane has lesser in fluence on pore-narrowing because of their highly porous structure and higher macro-voids.However,the rejection result for CA3 is higher than CA2 but less than CA0 and CA1 membranes.This resultcan be explained due to high resistance capacity of the membranes without additives having less porous properties.The high rejections and comparatively lower fluxes of CA/PEG membranes than CA/PVP for both the solvents can be assumed from the morphological study of the membranes as explained in Section 3.1.1.The reasonably dense asymmetrical layer possibly describes the enhancement in the rejection rate whereas the sublayer with inhibited macro-voids gives resistance ensuing in moderately lower fluxes and higher BSA rejections.From the above discussions,it is clear that the type of additives and type of solvents showed significant effects on the membrane flux and BSA rejection performances.The results attained in this specific study seem to be reasonably fascinating that the membranes with PEG additives gained higher rejection results and moderate fluxes.On the other hand,the membranes prepared using Ac:DMAc solvent showed better characteristics(i.e.hydrophilicity, flux and rejection)than the membranes prepared using DMF as solvent.
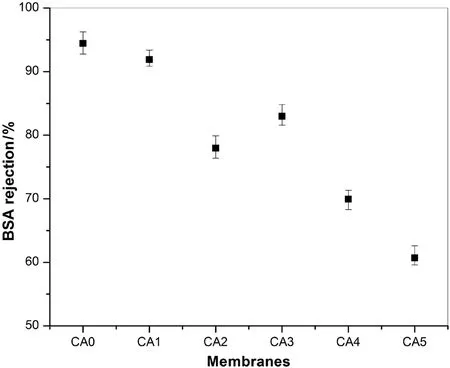
Fig.8.Rejection performance of different CA membranes:effect of solvents and additives(25°C,150 kPa).
4.Conclusions
Phase inverted CAmembranes were prepared from casting solutions comprising of two different solvents,namely Ac:DMAc and DMF individually using immersion precipitation process.Two different hydrophilic additivesviz.PEG and PVP were used as membrane pore formers and hydrophilic enhancers.The effects ofadditives and solvents on the morphological structures(i.e.top surface and a cross-sectional structure of the membranes)were studied in detail.The permeabilityproperties ofthe prepared membranes using differentadditives and solvents were also estimated using CF,hydraulic permeability,hydraulic resistance,hydrophilicity,porosity,EWC,PWF,and BSA rejection performance.The outcomes from this study presented the following concluding points:
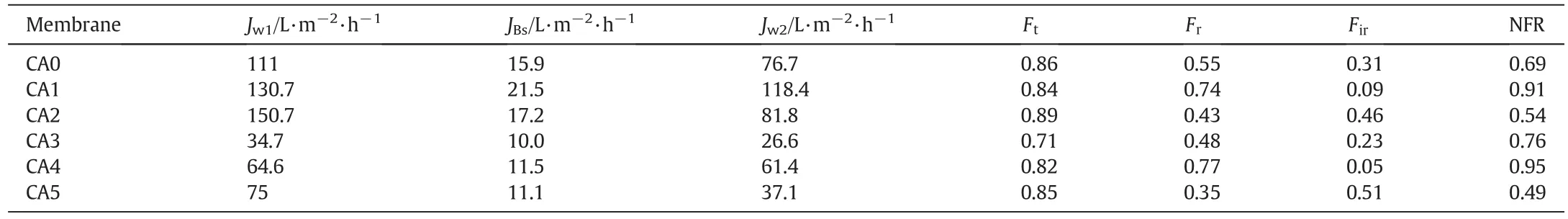
Table 4Fouling study data of the prepared membranes
(1)The membranes prepared using PVP additive showed the development of greater macro-voids and finger-like structure when compared with membranes formed using PEG additive.
(2)The introduction of hydrophilic additives to the membrane casting solution makes the membrane highly porous and more hydrophilic where the permeability properties of the CA ultra filtration membrane were significantly in fluenced.
(3)The difference in flux between the CA/DMF and CA/Ac:DMAc membranes are associated with the effect of the solvents on the hydrophilic nature of the membrane.As it is believed that there are some residual solvents within the final membrane matrix.
(4)The fouling resistances,particularly resistances due to irreversible fouling,of CA/PVP membranes are significantly higher than CA/PEG membranes irrespective of the solvent effects.
(5)Higher BSA rejection and relatively moderate flux of CA/PEG membranes than CA/PVP for both the solvents were obtained and the comparatively dense asymmetric layer of CA/PEG membrane possibly describes the enhancement in the rejection rate whereas the sublayer with inhibited macro-voids gives resistance resulting in moderately lower fluxes and higher BSA rejections.
Nomenclature
Aeffective membrane area,m2
Cfconcentration in the feed,mg·L-1
Cpconcentration in permeate,mg·L-1
Firirreversible fouling
Frreversible fouling
Fttotal fouling
JBBSA solution flux,L·m-2·h-1
JBSsteady state BSA flux,L·m-2·h-1
Jw0pure water flux,L·m-2·h-1
Jw1initial water flux,L·m-2·h-1
Jw2water flux in second run,L·m-2·h-1
NFR normalized flux ratio,%
Ptransmembrane pressure,kPa
Pmhydraulic permeability,L·m2·h-1·kPa-1
Qvolume of water permeated,L
Rfresistance due to fouled membrane,m-1
Rmresistance due to membrane,m-1
RPresistance due to concentration polarization,m-1rmmean pore radius,nm
Wdmass of dry membrane,g
Wwmass of wet membrane,g
W0initial mass of membrane,g
Acknowledgements
Central Instruments Facility(CIF),IIT Guwahati,India is gratefully acknowledged by the authors for allowing us to use FESEM,and Prof.Bishnupada Mandal,Department of Chemical Engineering,IIT Guwahati for providing us his casting knife.
[1]M.Mulder,Basic Principles of Membrane Technology, first ed.Kluwer Academic Publishers,Dordrecht,1996.
[2]T.-H.Young,L.-W.Chen,Pore formation mechanism of membranes from phase inversion process,Desalination103(1995)233-247.
[3]T.Mohammadi,E.Saljoughi,Effect of production conditions on morphology and permeability of asymmetric cellulose acetate membranes,Desalination243(2009)1-7.
[4]H.Strathmann,K.Kock,The formation mechanism of phase inversion membranes,Desalination21(1977)241-255.
[5]B.Chakrabarty,A.K.Ghoshal,M.K.Purkait,Preparation,characterization and performance studies of polysulfone membranes using PVP as an additive,J.Membr.Sci.315(2008)36-47.
[6]Z.Zhu,J.Xiao,W.He,T.Wang,Z.Wei,Y.Dong,A phase-inversion casting process for preparation of tubular porous alumina ceramic membranes,J.Eur.Ceram.Soc.35(2015)3187-3194.
[7]M.Algarra,M.I.Vázquez,B.Alonso,C.M.Casado,J.Casado,J.Benavente,Characterization of an engineered cellulose based membrane by thiol dendrimer for heavy metals removal,Chem.Eng.J.253(2014)472-477.
[8]R.Konwarh,N.Karak,M.Misra,Electrospun cellulose acetate nanofibers:The present status and gamut of biotechnological applications,Biotechnol.Adv.31(2013)421-437.
[9]R.Abedini,S.M.Mousavi,R.Aminzadeh,A novel cellulose acetate(CA)membrane using TiO2nanoparticles:Preparation,characterization and permeation study,Desalination277(2011)40-45.
[10]E.Saljoughi,T.Mohammadi,Cellulose acetate(CA)/polyvinylpyrrolidone(PVP)blend asymmetric membranes:Preparation,morphology and performance,Desalination249(2009)850-854.
[11]B.Chakrabarty,A.K.Ghoshal,M.K.Purkait,Effect of molecular weight of PEG on membrane morphology and transport properties,J.Membr.Sci.309(2008)209-221.
[12]J.-H.Kim,K.-H.Lee,Effect of PEG additive on membrane formation by phase inversion,J.Membr.Sci.138(1998)153-163.
[13]E.Saljoughi,M.Amirilargani,T.Mohammadi,Effect of PEG additive and coagulation bath temperature on the morphology,permeability and thermal/chemical stability of asymmetric CA membranes,Desalination262(2010)72-78.
[14]S.Wongchitphimon,R.Wang,R.Jiraratananon,L.Shi,C.H.Loh,Effect of polyethylene glycol(PEG)as an additive on the fabrication of polyvinylidene fluoride-cohexa fluropropylene(PVDF-HFP)asymmetric microporous hollow fiber membranes,J.Membr.Sci.369(2011)329-338.
[15]M.Yang,S.Xie,Q.Li,Y.Wang,X.Chang,L.Shan,L.Sun,X.Huang,C.Gao,Effects of polyvinylpyrrolidone both as a binder and pore-former on the release of sparingly water-soluble topiramate from ethylcellulose coated pellets,Int.J.Pharm.465(2014)187-196.
[16]S.H.Yoo,J.H.Kim,J.Y.Jho,J.Won,Y.S.Kang,In fluence of the addition of PVP on the morphology of asymmetric polyimide phase inversion membranes:Effect of PVP molecular weight,J.Membr.Sci.236(2004)203-207.
[17]S.Zhao,Z.Wang,X.Wei,X.Tian,J.Wang,S.Yang,S.Wang,Comparison study of the effect of PVP and PANI nanofibers additives on membrane formation mechanism,structure and performance,J.Membr.Sci.385-386(2011)110-122.
[18]H.Liu,Y.-L.Hsieh,Ultra fine fibrous cellulose membranes from electrospinning of cellulose acetate,J.Polym.Sci.40(2002)2119-2129.
[19]S.Tungprapa,T.Puangparn,M.Weerasombut,I.Jangchud,P.Fakum,S.Semongkhol,C.Meechaisue,P.Supaphol,Electrospun cellulose acetate fibers:Effect of solvent system on morphology and fiber diameter,Cell14(2007)563-575.
[20]J.Garcia-Ivars,M.-I.Alcaina-Miranda,M.-I.Iborra-Clar,J.-A.Mendoza-Roca,L.Pastor-Alcañiz,Enhancement in hydrophilicity of different polymer phaseinversion ultra filtration membranes by introducing PEG/Al2O3nanoparticles,Sep.Purif.Technol.128(2014)45-57.
[21]E.Yuliwati,A.F.Ismail,T.Matsuura,M.A.Kassim,M.S.Abdullah,Effect of modified PVDF hollow fiber submerged ultra filtration membrane for re finery wastewater treatment,Desalination283(2011)214-220.
[22]L.Luo,G.Han,T.-S.Chung,M.Weber,C.Staudt,C.Maletzko,Oil/water separation via ultra filtration by novel triangle-shape tri-bore hollow fiber membranes from sulfonated polyphenylenesulfone,J.Membr.Sci.476(2015)162-170.
[23]Y.-F.Zhao,L.-P.Zhu,Z.Yi,B.-K.Zhu,Y.-Y.Xu,Improving the hydrophilicity and fouling-resistance of polysulfone ultra filtration membranesviasurface zwitterionicalization mediated by polysulfone-based triblock copolymera dditive,J.Membr.Sci.440(2013)40-47.
[24]Y.-Q.Wang,T.Wang,Y.-L.Su,F.-b.Peng,H.Wu,Z.-Y.Jiang,Remarkable reduction of irreversible fouling and improvement of the permeation properties of poly(ether sulfone)ultra filtration membranes by blending with Pluronic F127,Langmuir21(2005)11856-11862.
[25]G.Wu,S.Gan,L.Cui,Y.Xu,Preparation and characterization of PES/TiO2 composite membranes,Appl.Surf.Sci.254(2008)7080-7086.
[26]F.G.Paulsen,S.S.Shojaie,W.B.Krantz,Effect of evaporation step on macrovoid formation in wet-cast polymeric membranes,J.Membr.Sci.91(1994)265-282.
[27]P.S.T.Machado,A.C.Habert,C.P.Borges,Membrane formation mechanism based on precipitation kinetics and membrane morphology:Flat and hollow fiber polysulfone membranes,J.Membr.Sci.155(1999)171-183.
[28]R.M.Boom,I.M.Wlenk,T.V.Boomgaard,C.A.Smolders,Microstructures in phase inversion membranes.Part 2.The role of a polymeric additive,J.Membr.Sci.73(1992)277-292.
[29]J.-J.Qin,Y.Li,L.-S.Lee,H.Lee,Cellulose acetate hollow fiber ultra filtration membranes made from CA/PVP 360 K/NMP/water,J.Membr.Sci.218(2003)173-183.
[30]C.A.Smolders,A.J.Reuvers,R.M.Boom,I.M.Wienk,Microstructures in phaseinversion membranes.Part 1.Formation of macrovoids,J.Membr.Sci.73(1992)259-275.
[31]B.Reuvers,Membrane Formation Mechanism:Diffusion Indused de-Mixing Processes in Ternary Polymeric System(Phd thesis)University of Twente,1987.
[32]J.Marchese,M.Ponce,N.A.Ochoa,P.Prádanos,L.Palacio,A.Hernández,Fouling behaviour of polyethersulfone UF membranes made with different PVP,J.Membr.Sci.211(2003)1-11.
[33]D.B.Mosqueda-Jimenez,R.M.Narbaitz,T.Matsuura,G.Chowdhury,G.Pleizier,J.P.Santerre,In fluence of processing conditions on the properties of ultra filtration membranes,J.Membr.Sci.231(2004)209-224.
[34]A.G.Fane,C.J.D.Fell,A.G.Waters,The relationship between membrane surface pore characteristics and flux for ultra filtration membranes,J.Membr.Sci.9(1981)245-262.
[35]C.J.D.Fell,K.J.Kim,V.Chen,D.E.Wiley,A.G.Fane,Factors determining flux and rejection of ultra filtration membranes,Chem.Eng.Process.27(1990)165-173.
[36]A.Idris,L.K.Yet,The effect of different molecular weight PEG additives on cellulose acetate asymmetric dialysis membrane performance,J.Membr.Sci.280(2006)920-927.
[37]W.-L.Choua,D.-G.Yu,M.-C.Yang,C.-H.Jou,Effect of molecular weight and concentration of PEG additives on morphology and permeation performance of cellulose acetate hollow fibers,Sep.Purif.Technol.57(2007)209-219.
[38]H.Matsuyama,A.Yamamoto,H.Yano,T.Maki,M.Teramoto,K.Mishima,K.Matsuyama,Effect of organic solvents on membrane formation by phase separation with supercritical CO2,J.Membr.Sci.204(2002)81-87.
[39]R.Guan,H.Dai,C.Li,J.Liu,J.Xu,Effect of casting solventon the morphology and performance of sulfonated polyethersulfone membranes,J.Membr.Sci.277(2006)148-156.
[40]D.Lu,H.Zou,R.Guan,H.Dai,L.Lu,Sulfonation of polyethersulfone by chlorosulfonic acid,Polym.Bull.54(2005)21-28.
[41]B.Kruczek,Develepment and Characterization of Dense Membranes for Gas Separation Made from High Molecular Weight Sufonated poly(Phenylene Oxide).Effect of Cating Conditionon the Morphology and Performances of the Membranes(Phd thesis)University of Ottawa,1998.
[42]C.M.Hansen,The three Dimensional Solubility Parameter and Solvent Diffusion Coefficient, first ed.Copenhagen Danish Technical Press,Copenhagen,1967.
[43]G.C.Vebber,P.Pranke,C.N.Pereira,Calculating Hansen solubility parameters of polymers with genetic algorithms,J.Appl.Polym.Sci.131(2013)39696.
[44]R.Nair,N.Nyamweya,S.Gonen,L.J.M.Miranda,S.W.Hoag,In fluence of various drugs on the glass transition temperature of poly(vinylpyrrolidone):A thermodynamic and spectroscopic investigation,Int.J.Pharm.225(2001)83-96.
[45]C.Özdemir,A.Güner,Solubility profiles of poly(ethylene glycol)/solvent systems,I:Qualitative comparison of solubility parameter approaches,Eur.Polym.J.43(2007)3068-3093.
[46]R.W.Kopitzke,C.A.Linkous,H.R.Anderson,G.L.Nelson,Conductivity and water uptake of aromatic-based proton exchange membrane electrolytes,J.Electrochem.Soc.147(2000)1677-1681.
[47]H.Shi,Y.He,Y.Pan,H.Di,G.Zeng,L.Zhang,C.Zhang,A modified mussel-inspired method to fabricate TiO2decorated superhydrophilic PVDF membrane for oil/water separation,J.Membr.Sci.506(2016)60-70.
[48]B.K.Dutta,Principles of Mass Transfer and Separation Process, first ed.PHI learning Pvt.Ltd.,New Delhi,2007.
[49]J.-H.Jiang,L.-P.Zhu,X.-L.Li,Y.-Y.Xu,B.-K.Zhu,Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin,J.Membr.Sci.364(2010)194-202.
[50]G.Arthanareeswaran,P.Thanikaivelan,K.Srinivasn,D.Mohan,M.Rajendran,Synthesis,characterization and thermal studies on cellulose acetate membranes with additive,Eur.Polym.J.40(2004)2153-2159.
[51]S.Robinson,P.A.Williams,Inhibition of protein adsorption onto silica by polyvinylpyrrolidone,Langmuir18(2002)8743-8748.
[52]J.Wu,Z.Wang,W.Lin,S.Chen,Investigation ofthe interaction between poly(ethylene glycol)and protein molecules using low field nuclear magnetic resonance,Acta Biomater.9(2013)6414-6420.
[53]Z.Yang,J.A.Galloway,H.Yu,Protein interactions with poly(ethylene glycol)self-assembled monolayers on glass substrates:Diffusion and adsorption,Langmuir15(1999)8405-8411.
[54]N.Maximous,G.Nakhla,W.Wan,K.Wong,Performance of a novel ZrO2/PES membrane for wastewater filtration,J.Membr.Sci.352(2010)222-230.
[55]Q.Shi,Y.Su,W.Zhao,C.Li,Y.Hu,Z.Jiang,S.Zhu,Zwitterionic polyethersulfone ultra filtration membrane with superior antifouling property,J.Membr.Sci.319(2008)271-278.
[56]Y.P.Tang,J.X.Chan,T.S.Chung,M.Weber,C.Staudt,C.Maletzko,Simultaneously covalent and ionic bridging towards antifouling of GO-imbedded nanocomposite hollow fiber membranes,J.Mater.Chem.A3(2015)10573-10584.
[57]Y.Feng,G.Han,L.Zhang,S.-B.Chen,T.-S.Chung,M.Weber,C.Staudt,C.Maletzko,Rheology and phase inversion behavior of polyphenylenesulfone(PPSU)and sulfonated PPSU for membrane formation,Polymer99(2016)72-82.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Suppression of gold nanoparticle agglomeration and its separation via nylon membranes☆
- Implications from protein uptake kinetics onto dextran-grafted Sepharose FF coupled with ion exchange and affinity ligands☆
- Experimental detection of bubble-wall interactions in a vertical gas-liquid flow☆
- Horizontal gas mixing in rectangular fluidized bed:A novel method for gas dispersion coefficients in various conditions and distributor designs
- Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions
- Hydrodynamic dispersion ofreactive solute in a Hagen-Poiseuille flow of a layered liquid
