Reversal of acetaminophen-generated oxidative stress and concomitant hepatotoxicity by a phytopharmaceutical product
2017-05-22AfolbiAkinmoldunKehindeOguntundeLwreneOwolbiOmotyoIlesnmiJonOgundeleTolulopeOlleyeAfolbiAkindhunsi
Afolbi C.Akinmoldun,Kehinde O.Oguntunde,Lwrene O.Owolbi,Omotyo B.Ilesnmi,b,Jon O.Ogundele,M.Tolulope Olleye,Afolbi A.Akindhunsi
a Phytomedicine,Biochemical and Molecular Pharmacology and Toxicology Laboratories,Department of Biochemistry,School of Sciences,The Federal University of Technology,PMB 704,Akure,Nigeria
b Department of Biological Sciences,Faculty of Science,Federal University Otuoke,Otuoke,Nigeria
c Department of Industrial Chemistry,Faculty of Science,Federal University Oye-Ekiti,PMB 373,Oye-Ekiti,Nigeria
Abstract The increasing popularity of herbal medicine and the well-established health benefit of phytochemicals have spurred the multiplicity of nutraceutical and phytopharmaceutical products.In this study,TrévoTM,a nutraceutical and phytopharmaceutical product,was evaluated for beneficia effects in acetaminophen-induced hepatic toxicity in Wistar rats.Animals received TrévoTM(1.5 mL/kg,3.0 mL/kg or 4.5 mL/kg)orally for 14 days.Hepatotoxicity was induced by the oral administration of acetaminophen(2 g/kg),24 h prior to sacrifice Biochemical liver function tests,oxidative stress indicators and histoarchitectural changes were evaluated.Acetaminophen administration occasioned significan increase(P<0.05)in serum bilirubin level and activities of the aminotransferases,alkaline phosphatase,γ-glutamyltransferase and lactate dehydrogenase accompanied by a significan decrease(P<0.05)in albumin level as well as histopathological alterations in liver sections.Promotion of hepatic oxidative stress by acetaminophen was revealed by significan (P<0.05)increase in lipid peroxidation,depletion of reduced glutathione,and decrease in superoxide dismutase and catalase activities.Administration of TrévoTM remarkably ameliorated acetaminophen-induced histopathological alterations and changes in serum and tissue biochemical markers.The protective effect of TrévoTM(4.5 mL/kg)was at par with that of Silymarin(25 mg/kg).The present study indicates that TrévoTM has notable salubrious effects.
Keywords:Acetaminophen;Antioxidant activity;Hepatoprotection;Nutraceutical;Herbal supplement
1.Introduction
Liver plays a pivotal role in regulating various physiological processes and is the centre of metabolism of nutrients such as carbohydrates,proteins and lipids.It is also involved in the metabolism and excretion of drugs and other xenobiotics and provide protection against foreign substances by detoxifying and eliminating them[1,2].As a result,the liver is exposed to all types of toxic abuse from both endogenous and exogenous sources which may produce liver degeneration.Liver diseases have become one of the major causes of morbidity and mortality in man and hepatotoxicity due to drugs appears to be the most common contributing factor[2,3].
Excessive intake of acetaminophen,also known as paracetamol,can cause severe hepatotoxicity and nephrotoxicity[2].Acetaminophen is activated and converted by cytochrome P450 enzymes to toxic metabolite NAPQI (N-acetyl-pbenzoquinoneimine)that causes oxidative stress and glutathione(GSH)depletion which is associated with its hepatotoxicity[3].In spite of the tremendous advances in modern medicine,there is no effective drug available that stimulates liver function,offers protection to the liver from damage or helps to regenerate hepatic cells[4].
Medicinal plants play a key role in human and animal health care.About 80% of the world population rely on the use of traditional medicine,which is predominantly based on plant material[5].The increasing popularity of herbal medicine and the well-established health benefit of phytochemicals have led to the emergence of several nutraceutical and phytopharmaceutical products which are used as nutritional supplements to enhance general wellbeing or as drugs for therapeutic purposes.Previous studies have shown that hepatoprotective effects are associated with phytoextracts rich in natural antioxidants[6–8].Many bioactive compounds and extracts from plants have thus been investigated for hepatoprotective and antioxidant effects against hepatotoxin-induced liver damage [9–11].The bioactivity of these herbal products are due mainly to constituent phenolics,flvonoids and other phytochemicals[12–15].
TrévoTM,a standardized phytopharmaceutical supplement manufactured in the US is presented as a homogenous mixture of plant-based extracts,water and lipid soluble vitamins,mineral nutrients and other components(Supplementary file) It is touted by users for health-promoting effects and as a remedy for certain ailments.However,there is scanty published evidence for the beneficia and the health-promoting effects of TrévoTMdespite its widespread use in some countries including Nigeria where it is approved by the National Agency for Food and Drug Administration and Control(NAFDAC)(Registration number:A7-1020L;MCC No:150821).Therefore,in this study,we have investigated the ameliorative effect of the nutraceutical against acetaminophen-induced oxidative stress and hepatotoxicity in rats.Silymarin,a f avonoid complex extracted from the seeds ofSilybum marianumserved as a reference standard.
2.Materials and methods
2.1.Chemicals
Acetaminophen,Silymarin,thiobarbituric acid (TBA),trichloroacetic acid(TCA),5,5′-dithiobis-(2-nitrobenzoic acid)(DTNB),sulphosalicylic acid (SSA),reduced glutathione(GSH) and epinephrine were obtained from Sigma–Aldrich,USA.TrévoTMwas a product of TrévoTMLLC,Oklahoma City,USA and was obtained from a store in Akure,Nigeria.Chemical composition and nutritional information on TrévoTMcan be found in the Supplementary file Other chemicals and reagents were of analytical grade.
2.2.Animals and treatment
Male Wistar rats(200–230 g)bred and housed at the animal house of Department of Animal Production and Health,The Federal University of Technology,Akure,Nigeria were used for the study.Animals were fed with laboratory chow(Vital Feeds,Lagos,Nigeria)and water ad libitum.All animal experimental protocols conformed to the guidelines of National Institute of Health(NIH publication 85-23,1985)for laboratory animal care and use.
Forty-two animals were randomly divided into seven groups with six rats apiece.Group 1 (control) and Group 2(hepatotoxicity-induced group)received normal saline throughout the duration of the experiment.Groups 3–5 were orally administered 1.5 mL/kg,3.0 mL/kg,4.5 mL/kg of TrévoTMrespectively,based on the manufacturer’s recommended daily intake for adults,while group 6 received Silymarin(25 mg/kg).Groups3–6weretreatedat24 hintervalsfor14days.Groups2–6 were additionally administered acetaminophen (2 g/kg) orally on day 14 and all animals were sacrifice 24 h later.
2.3.Preparation of sera and liver homogenates
Blood was collected from all animals by cardiac puncture.Serum was separated by centrifugation at 3000 g for 15 min and used for the evaluation of activities of alanine transaminase(ALT),aspartate transaminase(AST),alkaline phosphatase(ALP),γ-glutamyltransferase (GGT),lactate dehydrogenase(LDH) and the levels of albumin (ALB) and bilirubin (BIL).Livers were excised washed in ice cold 1.15%potassium chloride solution,blotted with filte paper and weighed.They were then homogenized in ten volumes of the homogenizing buffer(50 mM tris–HCl,1.15% KCl; pH 7.4) using a Teflo homogenizer.The resulting homogenate was centrifuged at 6000 g at 4◦C for 30 min to obtain the supernatant which was used to evaluate hepatic ALT and AST activities,superoxide dismutase(SOD)activity,catalase activity,reduced glutathione(GSH)concentration,extent oflipid peroxidation and total protein(TP)concentration.Assay for TP was performed using kits obtained from Randox Ltd.,Antrim,UK.
2.4.Biochemical analyses
2.4.1.Markers of hepatic injury
Activities of the transaminases,ALP,GGT and LDH were evaluated using kits obtained from Randox Ltd.,Antrim,UK while levels of ALB and BIL were determined using kits obtained from Vital Development Corporation,St.Petersburg,Russia following the instructions of the manufacturers.
2.4.2.Oxidative stress markers
2.4.2.1.SOD.Evaluation of SOD activity was performed following the method described by Misra and Fridovich [16].Sample (1 mL) was diluted in 9 mL of distilled water to make a 1 in 10 dilution.An aliquot of the diluted sample was added to 2.5 mL of 0.05 M carbonate buffer (pH 10.2) to equilibrate in the spectrophotometer and the reaction was started by the addition of 0.3 mL of freshly prepared 0.3 mM adrenaline to the mixture which was quickly mixed by inversion.The reference cuvettecontained2.5 mLbuffer,0.3 mLofsubstrate(adrenaline)and 0.2 mL of water.The increase in absorbance at 480 nm was monitored every 30 s for 150 s.1 unit of SOD activity was given as the amount of SOD necessary to cause 50%inhibition of the oxidation of adrenaline to adrenochrome during 1 min.
2.4.2.2.Catalase.Catalase activity was evaluated according to the method of Sinha[17].The assay mixture contained 2 mL of H2O2solution (800 μmol) and 2.5 mL of phosphate buffer in a test tube.0.5 mL of properly diluted enzyme preparation was rapidly mixed with the reaction mixture by a gentle swirling motion.The reaction was run at room temperature.A 1 mL portion of the reaction mixture was withdrawn and blown into 2 mL dichromate/acetic acid reagent at 60 s intervals.Addition of the reagent instantaneously produced an unstable blue precipitate of perchromic acid.Subsequent heating for 10 min in a boiling water bath changed the colour of the solution to stable green due to formation of chromic acetate.After cooling at room temperature,the optical density was measured with a spectrophotometer at 570 nm.The catalase contents of the enzyme preparation were expressed in term of Kat.f.
2.4.2.3.GSH.Estimation of GSH level was carried out as described by Jollow et al.[18].Sample(0.2 mL)was added to 1.8 mL distilled water and 3 mL 4% sulphosalicylic acid and centrifuged at 3000 g.The supernatant was added to 0.4 mg/mL DTNB in 0.1 mol/L phosphate buffer.The absorbance of the reaction mixture was read at 412 nm.

Fig.1.Serum enzymic indices ofliver toxicity in rats intoxicated with acetaminophen and administered TrévoTM.Results are presented as mean±SD(n=6).(a)Alanine transaminase activity in all groups.***P<0.001 versus acetaminophen; ###P<0.001 versus control;*P<0.05 versus control; ≈P>0.05 versus Silymarin.(b)Aspartate transaminase activity in all groups.***P<0.001 versus acetaminophen;###P<0.001 versus control;≈P>0.05 versus control.(c)Alkaline phosphatase activity in experimental animals.***P<0.001 versus acetaminophen;###P<0.001 versus control.(d)(-glutamyltransferase activity in all groups.***P<0.001 versus acetaminophen; ###P<0.001 versus control; ≈P>n 0.05 versus control.(e)LDH activity in experimental animals.***P<0.001 versus acetaminophen; ###P<0.001 versus control; ≈P>0.05 versus control.

Fig.2.Activities of hepatic transaminases in controls and TrévoTM administered groups.Results are presented as mean±SD(n=6).***P<0.001 versus acetaminophen; ###P<0.001 versus control.
2.4.2.4.Lipid peroxidation.Lipid peroxidation was assessed according to the method of Varshney and Kale [19].Sample(0.4 mL) was mixed with 1.6 mL of Tris-KCl buffer to which 0.5 mL of 30%TCA was added.This was followed by the addition of 0.5 mL of TBA and the mixture was boiled at 80◦C.After cooling and centrifugation,absorbance of the clear supernatant was measured at 532 nm.Lipid peroxidation was calculated using a molar extinction coefficien of 1.56×105M−1cm−1.
2.5.Histopathological evaluation
Part of the liver of animals in each group was carefully isolated,weighed,washed in buffered saline and fied in 10%formalin.Liver sections (5–6 μm) were processed according to standard histological techniques,stained with hematoxylin and eosin(H and E),and examined under a light microscope to assess histopathological changes in liver sections of control and experimental animals.
2.6.Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software Inc.,San Diego,USA).Data were presented as mean±standard deviation (SD).Statistical differences were determined using one-way analysis of variance(ANOVA).P<0.05 was considered statistically significant
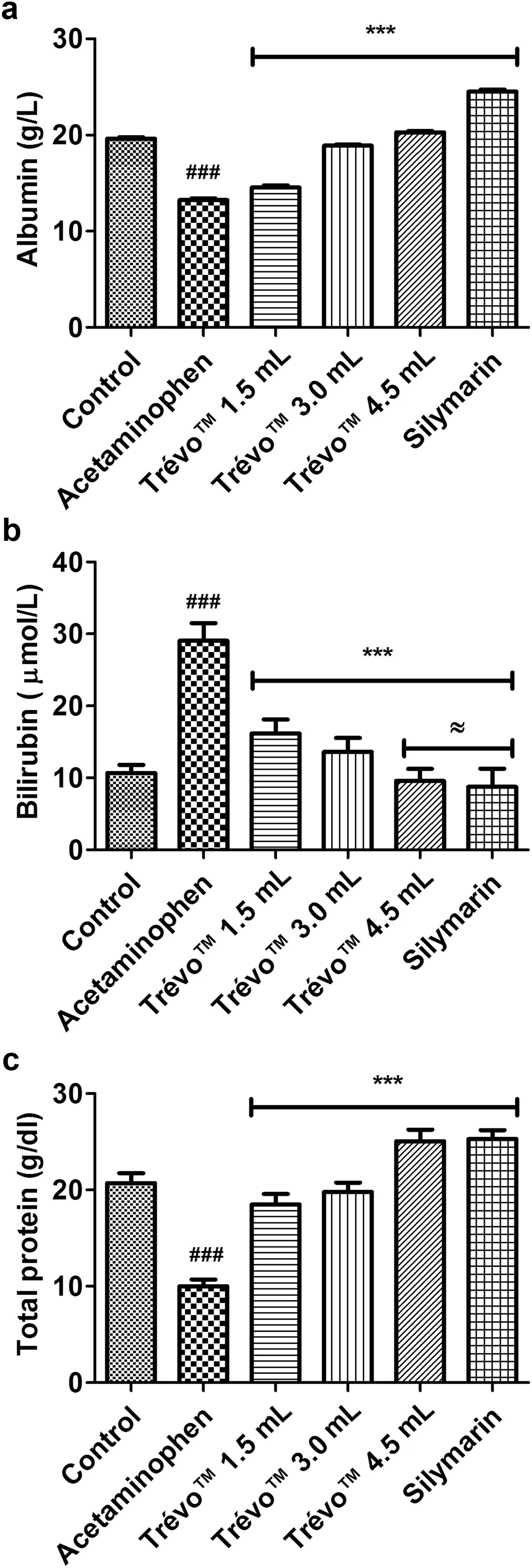
Fig.3.Bilirubin and protein levels in rats subjected to acetaminophen toxicity and administered TrévoTM.Results are presented as mean±SD (n=6).(a) Serum albumin level in all groups.***P<0.001 versus acetaminophen;###P<0.001 versus control.(b)Serum bilirubin level in all groups.***P<0.001 versus acetaminophen;###P<0.001 versus control;≈P>0.05 versus control.(c)Hepatic total protein content in controls and treated groups.***P<0.001 versus acetaminophen; ###P<0.001 versus control.
3.Results
In the acetaminophen intoxicated group (administered acetaminophen but not treated with TrévoTM) there was significan increase in the serum activities (U/L) of ALT(18.50±0.67),AST (56.56±0.94),ALP (75.90±1.27),GGT (30.50±0.63) and LDH (2036.75±39.14) (Fig.1)but significan decrease in the hepatic activity of ALT(27.90±2.13)and AST(40.50±4.40)(Fig.2)compared with the control group 10.42±0.41,32.00±0.93,35.46±1.92,10.05±0.47,454.63±12.68,137.88±6.88 and 127.6±6.02 respectively (P<0.001).These changes were significantl ameliorated in the TrévoTMtreated groups,compared with the acetaminophen intoxicated group (P<0.05).The respective values for these indices in the TrévoTMtreated group were 12.13±0.62,33.51±1.38,38.19±2.08,9.52±0.27,560.49±26.87,100.77±6.30 and 110.00±9.76 respectively.In addition,decrease in serum ALB level in the acetaminophen intoxicated group compared with the control group(P<0.001)was reversed in the TrévoTMtreated groups while serum BIL level which was increased in the acetaminophen intoxicated group compared with the control group (P<0.001) was also reversed in the TrévoTMtreated animals(Fig.3).
Compared with the control group,there was a decrease of 47% (13.37±0.52 to 7.14±0.62 mmol/mg protein),60%(80.58±2.92 to 21.31±2.07 units/mg protein) and 74%(8.96±0.41 to 3.58±0.33 μmol/min/mgprotein)coupled with an increase of 75% (14.77±0.64 to 58.76±2.75 nmol/mg protein) in GSH concentration,SOD activity,catalase activity and MDA concentration respectively (P<0.001) in the acetaminophen-intoxicated group.These alterations were ameliorated by TrévoTMwith values for the highest dose often showing no significan difference compared to the control value.In general,TrévoTMat the highest dose demonstrated comparable activity to Silymarin(Fig.4).
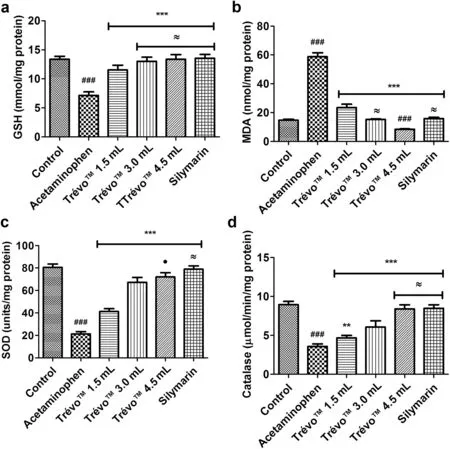
Fig.4.Enzymic and non-enzymic oxidative stress markers in acetaminophen intoxicated rats administered TrévoTM.Results are presented as mean±SD(n=6).(a)Level of reduced glutathione in animals.***P<0.001 versus acetaminophen;###P<0.001 versus control;≈P>0.05 versus control.(b)Extent oflipid peroxidation in all groups.***P<0.001 versus acetaminophen;###P<0.001 versus control;≈P>0.05 versus control;(c)Superoxide dismutase activity in controls and experimental groups.***P<0.001 versus acetaminophen;###P<0.001 versus control;≈P>0.05 versus control;●P>0.05 versus TrévoTM 3 mL.(d)Catalase activity in all groups.***P<0.001 versus acetaminophen;**P<0.01 versus acetaminophen; ###P<0.001 versus control; ≈P>0.05 versus control.
Histopathological examination ofliver sections of control animals revealed normal cellular architecture while liver sections from acetaminophen-intoxicated rats showed severe,multiple foci of vacuolar degeneration in the hepatocytes and severe diffuse coagulative necrosis of hepatocytes with a moderate cellular infiltratio of the liver by polymorponuclear cells.Histoarchitectural alterations were reduced in the treated groups especially groups treated with 3 and 4.5 mL/kg TrévoTMand Silymarin(Fig.5).
4.Discussion
Protection against acetaminophen-induced liver damage and oxidative stress is an accepted method for assessing potential hepatoprotective agents[20–22].The results of the present study showed that TrévoTMdemonstrated excellent protection against acetaminophen provoked hepatic toxicity and oxidative stress in rats.
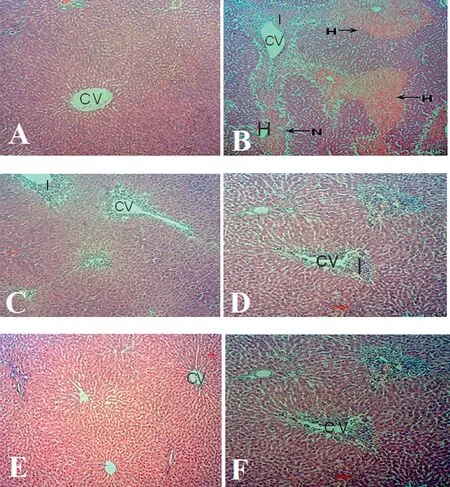
Fig.5.Microscopic observations of H & E stained liver sections (100× magnification of rats subjected to acetaminophen toxicity and administered various doses of TrévoTM and reference standard(Silymarin).(A)Section ofliver tissue is normal in control animals.(B)Section ofliver tissue of 2 g/kg acetaminophen treated group showing massive necrosis,haemorrhage,and inflammation (C)Section ofliver tissue of group pre-treated with 1.5 mL/kg of TrévoTM followed by 2 g/kg acetaminophen showing mild inflammatio (D) Section ofliver tissue of group pre-treated with 3.0 mL/kg of TrévoTM followed by 2 g/kg acetaminophen showing preservation of normal hepatocytes with mild inflammation (E)Section ofliver tissue of group pre-treated with 4.5 mL/kg of TrévoTM followed by 2 g/kg acetaminophen showing normal histology with mild inflammatio (F) Section ofliver tissue of group pre-treated with 25 mg/kg Silymarin followed by 2 g/kg acetaminophen showing preservation of normal hepatocytes.CV:central vein;N:necrosis;I:inflammation H:haemorrhage.
The rise in serum levels of AST and ALT was attributable to the damaged structural integrity of the liver[23–25]because these enzymes are cytoplasmic in location and are released into circulation after cellular damage[26,27]in conformity with the extent ofliver damage[28].GGT and ALP are membrane bound enzymes,which are released unequally depending on the pathological phenomenon[29,30].The elevation of ALP indicates the disturbed excretory function ofliver [31,32].The elevation of serum GGT is regarded as one of the most sensitive indices of hepatic damage[33,34].Increased LDH activity in serum may be due to leakage of the enzyme from the tissues into the blood on account of cellular injury.The remarkable reversal of the increase in serum activity of these enzymes by TrévoTMshowed that it protected hepatic cells from lysis and could have inhibited factors that orchestrate necrotic death in liver tissue.
Bilirubin is one of the most important clinical clues to the severity of necrosis and its accumulation is a measure of binding,conjugation and excretory capacity of hepatocyte[35].Albumin is the most abundant serum protein(representing 55.65%of total protein)produced in the liver and transferred to the blood.Any injury or damage to the liver will reduce albumin production and hence,its serum quantity.These would indicate that the synthetic function of the liver to make albumin and transfer it to the blood has been disturbed[36].The modulatory effect of TrévoTMon these important liver function indices further demonstrated its hepatoprotective property.The decrease in serum bilirubin in the pre-treated groups in liver damage induced by acetaminophen,indicated the effectiveness of the nutraceutical in normalizing the functional status of the liver.
The results of the oxidative stress markers revealed that TrévoTMis a potent antioxidant product.This is a reflectio of thenumerousphytochemicalcomponentsintheproduct.NAPQI is initially detoxifie by conjugation with GSH to form mercapturic acid[37,38].However,when the rate of NAPQI formation exceeds the rate of detoxificatio by GSH,it oxidizes tissue macromolecules such as lipid or –SH group of proteins and alters the homeostasis of calcium after depleting GSH.This implies that the non-enzymic antioxidant,GSH is one of the firs line of defense in acetaminophen toxicity.Its functions,mainly the removal of free radical species such as hydrogen peroxide(H2O2),superoxide radicals and maintenance of membrane protein thiols and as a substrate for glutathione peroxidase(GPx)and glutathione-s-transferase(GST)[39],are very important and provide a hub for the effective functioning of other antioxidant systems.The reversal of the decline in GSH level occasioned by acetaminophen toxicity is therefore a strong indicator of the beneficia effect of TrévoTMagainst oxidative stress and hepatotoxicity.
This is further confirme by the very significan decrease in MDA level by TrévoTMand its positive effect on catalase and SOD.In the present study,the decreased level of GSH has been associated with elevations in the levels of end products(MDA)oflipid peroxidation in acetaminophen control.The increase in MDA level in liver suggests enhanced lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms to prevent formation of excessive free radicals.SOD has been reported as one of the most important enzymes in the enzymatic antioxidant defense system[40].Catalase is widely distributed in all animal tissue and the highest activity is found in the red cells and in liver[41].SOD offers protection from highly reactive superoxide radical and converts them to form H2O2and O2with H2O2being further decomposed to H2O by catalase and GPx [42].Supplements could enhance and augment the coordinate actions of various cellular antioxidants in mammalian cells,which are critical for effectively detoxifying free radicals,by contributing components that possess intrinsic antioxidant activity or those that potentiate the activity of other antioxidative factors in the antioxidant network.
The hepatoprotective effect of TrévoTMwas further substantiated by histopathological assessment which showed preservation of normal histology in TrévoTMtreated acetaminophen intoxicated groups in comparison with acetaminophen control.
The liver is central to the wellbeing of the entire organism as it is the metabolic factory of the body systems.This investigation revealed that TrévoTMremarkably reversed acetaminophengenerated oxidative stress and the concomitant hepatic injury.The very strong antioxidant effect of TrévoTMalongside its hepatoprotective activity may be part of the basis for the touted salubrious effect of the product on general wellbeing.However,detailed studies involving assessment of possible side effects and elucidation of precise mechanisms of action of the product are warranted.
Conflict ofinterest
The authors declare that there is no conflic ofinterest.
Appendix A.Supplementary data
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.fshw.2016.11.001.
杂志排行
食品科学与人类健康(英文)的其它文章
- About the Beijing Academy of Food Sciences
- Toxicological studies of Caesalpinia sappan wood derived dye in Wister albino rats
- Phenolic extract from Ocimum basilicum restores lipid metabolism in Triton WR-1339-induced hyperlipidemic mice and prevents lipoprotein-rich plasma oxidation
- Quantificatio of phenolic compounds and antioxidant capacity of an underutilized Indian fruit:Rayan[Manilkara hexandra(Roxb.)Dubard]
- GUIDE FOR AUTHORS
- Ketogenic diets and Alzheimer’s disease
