膨润土/褐铁矿改性白云石催化剂改善松木蒸汽富氢气化性能
2017-05-16牛永红韩枫涛张雪峰陈义胜
牛永红,韩枫涛,张雪峰,陈义胜,王 丽,许 嘉
(1.内蒙古科技大学矿业研究院,包头 014010; 2.内蒙古科技大学能源与环境学院,包头 014010;3.白云鄂博矿多金属资源综合利用重点实验室,包头 014010; 4.内蒙古科技大学分析测试中心,包头 014010)
膨润土/褐铁矿改性白云石催化剂改善松木蒸汽富氢气化性能
牛永红1,2,韩枫涛2,张雪峰1,3,陈义胜4,王 丽4,许 嘉4
(1.内蒙古科技大学矿业研究院,包头 014010; 2.内蒙古科技大学能源与环境学院,包头 014010;3.白云鄂博矿多金属资源综合利用重点实验室,包头 014010; 4.内蒙古科技大学分析测试中心,包头 014010)
采用蒸汽气化,以松木燃料棒为试验对象,分析气化气及焦油组分变化,研究改性白云石(膨润土为载体,白云石为活性组分,并负载少量褐铁矿)催化性能随温度升高的变化规律。试验表明:750 ℃时,在改性白云石催化条件下,H2的体积分数为45.77%±0.23%,相较无催化和白云石催化条件下的富氢作用,气化所需温度下降100 ℃;富氢程度在较低温度下明显,但随温度升高而消弱;改性白云石不仅促使烃端链上碳碳长链断链,产生氢自由基,进而形成H2,同时促进芳香环开环反应,脱羧基及脱羟基反应,使得裂解后的焦油更易转化为小分子气体;催化剂中活性中心 Fe3+随温度高逐渐减少,使得改性白云石的质量增加在 900 ℃降低至最小值,但相比白云石,改性白云石催化剂在气化前后的色泽和形态变化较轻,反映其表面积碳及机械强度得到优化。项目研究可为生物质蒸汽催化气化及廉价高效改性白云石的应用提供参考。
催化剂;气化;生物质;松木燃料棒;改性白云石;富氢气化气;焦油
牛永红,韩枫涛,张雪峰,陈义胜,王 丽,许 嘉. 膨润土/褐铁矿改性白云石催化剂改善松木蒸汽富氢气化性能[J].农业工程学报,2017,33(7):213-219.doi:10.11975/j.issn.1002-6819.2017.07.028 http://www.tcsae.org
Niu Yonghong, Han Fengtao, Zhang Xuefeng, Chen Yisheng, Wang Li, Xu Jia. Performance improvement of steam gasification of pine for hydrogen-rich gas with dolomite catalyst modified by bentonite/limonite[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2017, 33(7): 213-219. (in Chinese with English abstract)doi:10.11975/j.issn.1002-6819.2017.07.028 http://www.tcsae.org
0 引 言
生物质水蒸汽气化能够显著提高气化气氢气含量和燃料转化率,并具有工艺简单,投资较少及过程控制便捷等优点[1-4]。然而,气化反应生成的焦油难于从气化气中分离,这不但限制了气化气的实际应用,还增加了该技术的投资成本,使系统的运行负担加重,而它的浪费不仅造成了环境的污染,还严重威胁工作人员的身体健康[5]。
镍基催化剂催化重整焦油的活性较高,但其价格昂贵,且容易中毒失活[6]。白云石的催化活性仅次于镍基催化剂,其活性随钙镁比例的增加而增加,且类质同象铁也促进其催化活性,同时其机械强度也相应增加[7]。研究表明,Fe有利于提高催化剂制粒热传导性,增加催化剂的比表面积,提高抗烧结和抗积碳能力,促进催化剂微孔结构的加固,尤其有利于增加催化剂携氧能力,促进催化剂的反应活性和稳定性,并提高其对长链烯的选择性[8-11]。载体是催化剂的不可缺少组成部分,但目前报道中,对生物质气化技术所需载体并没有得到足够的重视。Occelli 等学者[12]对比蒙脱土(膨润土主要成分)与FCC催化剂的催化裂解反应活性时发现,蒙脱土有较好的催化活性,但存在微孔积碳的弊端。Aldersley等[13]在SCR反应中证明膨润土具有很好的SO2抗毒性,通过与活性物质结合达到相互促进的作用。
针对白云石自身机械强度低,适应性较差,采用膨润土为载体,并负载一定量褐铁矿的方法对白云石改性,通过生物质水蒸汽催化气化试验,改变气化温度,分析气相组分及焦油的傅里叶变换红外图谱变化,考察该催化剂富氢作用及其催化性能。
1 材料与方法
1.1 试验原料
试验采用废弃回收的松木屑为原料(产自包头市某木材加工厂),如图1a所示。松木屑需在气化试验前采用KL120型颗粒机造粒成型(图1b所示,河南巩义机械厂制造),该方法有效提高试验材料密度,减少飞灰损失,方便试验过程中的加料及卸料。挑选直径约4 mm,长约20 mm的成型松木燃料棒作为试验对象,其颗粒密度约1 200 kg/m3,如图1c所示,其元素分析和工业分析见表1。
白云石来自唐县鑫磊矿物粉体加工厂,试验选取粒径为(8±2) mm白云石颗粒,在电阻炉内以 800 ℃煅烧3 h,并在干燥瓶中密闭冷却至室温以待备用,图2a为煅烧后白云石催化剂实物,煅烧前的成分分析如表2所示。

图1 生物质气化原料与装备Fig.1 Experimental materials and device for biomass gasification
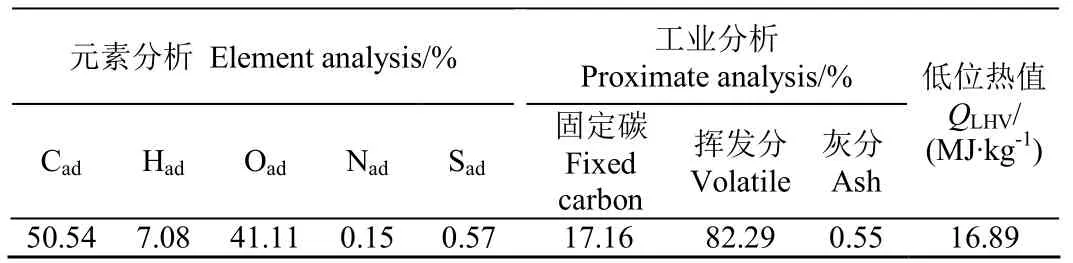
表1 松木燃料棒组分分析Table1 Component analysis of pine fuel rods
改性白云石催化剂采用膨润土为载体,以白云石为主要活性组分,并负载一定量褐铁矿,具体制作方法如下:将白云石研磨成粉末(研磨至 120目左右),与钙基膨润土(河南省巩义市元亨净水材料厂生产)和褐铁矿(研磨至120目左右)充分混合,其质量配比为3:2:0.3,并在其中加入一定浓度的胶黏剂(硅酸钠溶液,硅酸钠为天津市天达净化材料精细化工厂生产),搅拌均匀后挤压,拉条再造粒。将成型催化剂颗粒在 105 ℃的干燥箱烘干1 h,在电阻炉内以800 ℃煅烧3 h,并在干燥瓶中密闭冷却至室温,试验选取粒径为(5±1) mm的催化剂以待备用,图2b为煅烧后改性白云石催化剂实物,钙基膨润土和褐铁矿成分分析如表2所示。

图2 煅烧后的催化剂样品Fig.2 Catalysts calcined samples

表2 催化剂原料组分分析Table2 Component analysis of catalyst materials %
1.2 试验装置及试验方案
试验系统(如图3a)包括:高温水蒸汽发生装置(电热蒸汽发生器、水蒸汽加热装置)、气化反应主体装置(立式管式电加热炉)、温控及电控装置、气化气净化装置及其采集装置。其中,生物质气化反应装置中的加热管长约75 mm,内径约22 mm。

图3 试验系统示意图Fig.3 Experimental system sketch
试验前先通入氮气排空系统内空气并进行气密性检查。打开电炉并设置气化反应温度(750、800、850、900 和 950 ℃),开启水泵,保证系统正压,待温度达到设定值,称取50 g左右成型松木燃料棒和21 g左右的催化剂加入反应器中,试验计时开始,产气稳定后收集气体样品,反应计时开始约30 min后停炉并关闭水泵,收集焦油样品,待管式炉体温度冷却至室温后卸料,并收集称量后的反应残留物。
试验过程中,氮气流量约为500 mL/min,S/B(水蒸汽加入量与生物质加入量的比值)约为1[14],多余的气化气冷却净化后燃烧处理。图3b为催化剂在反应器中的位置,反应前需加入一定量催化剂形成催化床。每组工况进行多次试验,并取 3个平行样的平均值作为该工况的测试结果。
1.3 试验产物样品测试
气化气样品组分通过美国安捷伦公司生产的气相色谱仪(Agilent 7890B型)进行图谱分析。以氩气作载气,利用氢火焰离子化检测器(FID)分析C2H6、C2H4、C2H2、C3H8、C3H6等有机气体,利用热导检测器(TCD)分析H2、CO、CO2等无机气体和CH4。
试验收集的焦油由德国Bruker生产的TENSOR II型傅里叶变换红外光谱仪进行分析,其分辨率为0.5 cm-1。由于生物质水蒸汽气化焦油富含水分,成分复杂,且在静置后分 3层:分别为不溶于水且比水密度大的焦油,溶于水的焦油和不溶于水且比水密度小的焦油(不溶于水且比水密度大的焦油含量极少)。由于焦油成分复杂,试验测试选取最上层不溶于水且比水密度小的焦油做为研究对象,与蒸馏(组分有损失)和萃取(萃取剂干扰,且不能完全萃取)比,该方法采用气化产出焦油原样,通过考察特征官能团分析组分变化,能够反映焦油催化裂解特性,具有代表性,不受萃取剂的影响,减少水分参混,避免焦油组分亲水基团与水分子氢键链接缔合,方便焦油样品的提取及试验数据可靠的分析。
2 结果与分析
2.1 催化剂添加对气相产物的影响
高温使得生物质气化更加充分,使水蒸汽气化重整反应加剧,进而促进气化气组分中H2体积分数在整体上呈上升趋势,碳氢气体体积分数下降。
图4反映改性白云石催化作用下气化气组分随温度升高的变化规律。由于催化剂中铁氧化物催化裂解焦油,使得碳氧化物体积分数迅速增加,并在催化过程中消耗H2,导致H2体积分数从750 ℃时的45.77%±0.23%降低到800 ℃的42.67%±0.25%,后由于催化气化反应随温度升高而加强,使得该值又升高到 950 ℃时的57.19%±0.17%。随温度升高,CO2体积分数由25.99%±0.09%降至900 ℃的16.02%±0.13%,随后表现平稳;CO体积分数先从 750 ℃时 14.03%±0.11%升高到850 ℃时的19.16%±0.15%,后表现出下降的趋势;CH4体积分数与CnHm(n≥2)的体积分数的降低趋势较为微弱。

图4 改性白云石催化的气化气组分随温度变化的关系Fig.4 Gas volume fractions with temperature changing under condition of modified dolomite catalyst
虽然在850 ℃之前,CO与水蒸汽的反应相较其他气化反应更占优势[15-17],且Fe3+氧化CO2的反应消耗CO生成 CO2的程度随温度升高而加强,但煅烧后的白云石富含MgO和CaO,易与CO2结合生成MgCO3和CaCO3,且随温度升高而减弱,而催化剂中铁氧化物催化裂解焦油生成小分子气体(CO2、H2、CO、H2O和CnHm等)的反应及自身氧化性随温度升高而加剧,这造成 CO2体积分数在最开始表现为平缓后呈下降趋势,而CO体积分数在850℃之前呈上升趋势。但Fe2+与H2O的氧化还原反应随温度的升高而消弱,使得催化剂中活性中心 Fe3+减少,造成Fe3+氧化CO和H2的反应消弱,使CO和CO2体积分数表现出下降的趋势。

图5 氢气体积分数随温度变化的关系Fig.5 Volume fraction of H2with temperature changing
如图5所示,750 ℃时,改性白云石催化剂使气化气中富氢程度明显,但随着温度的增加,富氢程度逐渐消弱,主要是由于催化剂的加入使得焦油催化裂解,且随温度升高而加剧,使得气化气中碳氧化物气体的体积分数增加,如图6所示。研究表明[18-19],MgO/CaO引起脂肪烃和环烷烃端链上 π电子体系重新排布,引起碳长链逐步断链,进而产生氢自由基,并形成H2,造成气化气中H2体积分数增加。助剂Fe经H2O活化为Fe3+,促进焦油裂解并转化为Fe2+,裂解后的焦油更易经水蒸汽的重整反应转化为小分子裂解气。
气化气携带气化焦油进入催化层,催化裂解焦油,影响水蒸汽气化重整反应(CnHm与H2O的反应和CO与H2O的反应),进而改变气化气组分变化。图6为气化气组分中碳氧化物气体体积分数随温度的变化。当气化温度大于850 ℃时,如图6a所示,由于Fe3+氧化作用,CO的体积分数低于不加催化剂和白云石催化剂条件下的CO的体积分数,但由于温度升高,Fe3+催化裂解焦油加剧,造成改性白云石催化剂条件下CO的体积分数逐渐大于白云石催化条件下CO的体积分数。图6b反映CO2体积分数随温度变化的关系,该组分变化的原因总结为两点:煅烧后的白云石碱金属氧化物易与 CO2结合生成 MgCO3和CaCO3,且随温度升高而减弱,并在850 ℃后变现为分解大于结合;气化温度上升,使Fe2+与H2O的反应进行程度消弱,造成催化剂中活性中心Fe3+减少,造成Fe3+与CO反应的消弱,产生CO2体积分数表现出下降的趋势。

图6 碳氧化物气体体积分数随温度变化的关系Fig.6 Volume fraction of CO2and CO with temperature changing
图7为气化气组分中CnHm体积分数随温度的变化。白云石催化剂有效的减少了气化气中CnHm含量,但改性白云石催化剂中的Fe使得CnHm含量相对白云石催化条件下CnHm含量高,这是由于活化的Fe3+加速焦油催化裂解成小分子气体,该速率大于CnHm自身催化氧化分解的速率。Fe2+与 H2O的反应随气化温度升高而消弱,使得催化剂中活性中心 Fe3+减少,造成焦油催化裂解效率较低,使得CH4的体积分数在900℃时降低(如图7a),CnHm的体积分数在850 ℃时降低(如图7b)。
2.2 催化剂添加对液相产物的影响
图8以试验反应温度为750℃条件下产出焦油的红外图谱为例(其他温度下图谱的峰值与此出现位置相近)。参考煤化工领域中傅里叶变换红外技术的应用,采用标准图谱与之对照,根据特定基团出现特征峰位置,判断焦油组分(如表 2所示),并在该处附近进行积分,分析气化焦油各组分含量变化规律。
在3 430 cm-1左右出现的特征峰为O-H的对称伸缩振动,此宽缓峰的出现与焦油中所含苯酚有关,该峰受多聚物分子间及分子内间氢键连接程度影响[20-21];在3 007 cm-1左右出现的特征峰为芳香烃的C-H伸缩振动,由于其取代基为脂肪链,其峰值较小,并在较低波数位置出现,且与脂肪烃 C-H伸缩振动引起的峰值产生缔合;在2 333~2 360 cm-1出现的特征峰为C≡N或C≡C的伸缩振动;在3 000~3 100 cm-1的峰值为C-H伸缩振动,对应的化合物为芳香烃;在2 800~3 000 cm-1的峰值为C-H伸缩振动,对应的化合物为脂肪烃;在1 707 cm-1的峰值为C=O伸缩振动,对应的化合物为羧酸和酮类;在1 513 cm-1的峰值为芳香骨架振动,对应的化合物为芳香烃;在1 100~1 300 cm-1的峰值为C-O伸缩振动和O-H伸缩振动,对应的化合物为酚类和醇类;在 1 267和1 034 cm-1的峰值分别为C-O-C对称伸缩振动和C-O-C非对称伸缩振动,对应的化合物为芳香醚。由于生物质气化反应涉及气液固三相反应,过程复杂[22-23]。试验前期针对无催化剂条件[24]及白云石催化条件[25]的试验研究,发现气化性能在800~850 ℃时出现拐点,为方便分析并对后续研究奠定基础,本试验分别选取750、800、850和900 ℃条件下傅里叶红外变换光谱进行对比。

图7 碳氢气体体积分数随温度变化的关系Fig.7 Volume fraction of CnHmwith temperature changing

图8 气化焦油傅里叶红外谱图(750 ℃)Fig.8 FT-IR spectrum of biomass tar(750 ℃)
图9分别反映了焦油中脂肪烃、芳香烃和含氧基团随温度升高的变化规律。Rahman等[26-27]指出,在水蒸汽气氛下,有机大分子更易发生开环反应及断链反应。而开环或断链的结构更易转化为小分子气体[28-30]。所以,在未添加催化剂条件下,芳香烃随温度升高含量迅速下降,由于芳香烃开环反应生成脂肪烃的速率大于脂肪烃断链裂解的速率,脂肪烃含量微弱增加。
添加改性白云石催化剂后,脂肪烃、芳香烃及含氧基团含量小于添加白云石催化剂催化裂解时的含量,这由此说明改性白云石催化剂能够促进焦油中长链有机分子断链,芳香烃开环裂解,脱羧基及脱羟基作用,使焦油更易向小分子有机气体转化,所以该催化剂比白云石催化剂更具催化活性。

图9 特征峰面积随温度变化Fig.9 Characteristic peaks area with temperature changing
2.3 催化剂的性能评价
对比图2与图10,反应后白云石催化剂表面积碳严重,且在反应过程中破碎成更小的颗粒,不易确定其在催化反应后质量增加。但改性白云石催化剂表面积碳较轻,形态基本无变化,说明改性后的白云石催化活性较高,机械强度相较白云石催化剂有了明显改善。
定义Δ为催化剂前后质量增加百分比(如式(1)所示),图11反映,改性白云石催化剂催化速率先升高,当温度达到900 ℃后,改性白云石催化剂催化速率降低,原因是由于气化温度上升,使Fe2+与H2O的反应进行程度消弱,造成催化剂中活性中心Fe3+减少,催化剂空隙积碳量增加,使得改性白云石催化剂催化活性降低。

式中Δ为催化剂前后质量增加量,%;m0为催化剂反应前总质量,g;m为催化剂反应前总质量,g。

图10 反应后催化剂形态(750 ℃)Fig.10 Catalyst characteristics after reaction (750 ℃)
引起催化剂质量增加的主要原因是催化剂空隙积碳。催化剂空隙积碳能够导致催化剂活性降低,催化剂质量增加越多,其活性越低,催化速率约低于积碳速率,所以催化剂质量增加大小与催化剂催化速率及活性呈负相关。

图11 催化剂质量增加量随温度变化的关系Fig.11 Increased percent of catalyst weight with temperature changing
3 结 论
1)在改性白云石催化条件下,气化温度为 750℃时H2的体积分数为45.77%±0.23%,相较无催化和白云石催化条件下的富氢作用,气化温度下降 100 ℃;随着温度升高,改性白云石促使碳氧化物和碳氢化物体积分数增加,使得富氢程度消弱。
2)改性白云石不仅促使烃端链上碳长链断裂,进而形成H2,同时促进芳香环开环反应,脱羧基及脱羟基反应,使得裂解后的焦油更易转化为小分子含碳气体,这有利于减少气化焦油的产生,提高气化效率。
3)催化剂中活性中心Fe3+随温度升高逐渐减少,使得改性催化剂质量增加,在 900 ℃降低至最小值,但相比白云石,改性白云石催化前后的色泽和形态变化较轻,反映其表面积碳及机械强度得到优化。
白云石的催化活性仅次于镍基催化剂,但价格低廉,具有潜在的经济价值。本研究对比白云石催化生物质蒸汽气化,采用膨润土为载体,并负载少量褐铁矿对白云石改性,探索其催化性能,为白云石改性及生物质蒸汽气化研究提供思路。
[1] Skoulou V, Swiderski A, Yang W, et al. Process characteristics and products of olive kernel high temperature steam gasification (HTSG)[J]. Bioresour Technology, 2009, 100(8): 2444-2451.
[2] 边轶,刘石彩,简相坤. 生物质热解焦油的性质与化学利用研究现状[J]. 生物质化学工程,2011,45(2):51-55. Bian Yi, Liu Shicai, Jian Xiangkun. The state art of view of research progress on characteristics and chemical utilization of tar from biomass pyrolysis[J]. Biomass Chemical Engineering, 2011, 45(2): 51-55. (in Chinese with English abstract)
[3] Nimit Nipattummakula, Islam I Ahmeda, Ashwani K Gupta. Hydrogen and syngas yield from residual branches of oilpalm tree using steam gasification[J]. International Journal of Hydrogen Energy, 2011, 36(6): 3835-3843.
[4] Aitziber Erkiaga, Gartzen Lopez, Maider Amutio, et al. Syngas from steam gasification of polyethlene in a conical spouted bed reactor[J]. Fuel, 2013, 109(7): 461-469.
[5] 典平鸽,张乐观,江程程. 裂解温度对生物质热解焦油成分的影响[J]. 可再生能源,2012,30(5):54-58. Dian Pingge, Zhang Leguan, Jiang Chengcheng. The influence of pyrolysis temperature on the component of biomass pyrolytic tar[J]. Renewable Energy Resources, 2012, 30(5): 54-58. (in Chinese with English abstract)
[6] 栾艳春. 铁基催化剂对生物质高温蒸汽气化影响的实验研究[D]. 包头:内蒙古科技大学,2015. Luan Yanchun. Experimental Study on the Influence of Iron-based Catalyst for High Temperature Steam Gasification of Biomass[D]. Baotou: Inner Mongolia University of Science and Technology, 2015. (in Chinese with English abstract)
[7] Marco Baratieri, Elisa Pieratti, Thomas Nordgren, et al. Biomass gasification with dolomite as catalyst in a small fluidized bed experimental and modelling analysis[J]. Waste and Biomass Valorization, 2010, 1(3): 283-291.
[8] Borah B J, Borah S J, Saikia K, et al. Efficient Suzuki-Miyaura coupling reaction in water: Stabilized Pd-montmorillonite clay composites catalyzed reaction[J]. Applied Catalysis A: General, 2014, 469: 350-356.
[9] Yu Q Z, Brage C, Nordgreen T, et al. Effects of Chinese dolomites on tar cracking in gasification of birch[J]. Fuel, 2009, 88(10): 1922-1926.
[10] 邓双辉. 铜渣催化气化生物质的动力学研究[D]. 昆明:昆明理工大学,2013. Deng Shuanghui. An Experimental Study of Steam Gasification of Biomass with Copper Slag Catalysts[D]. Kunming: Kunming University of Science and Technology, 2013. (in Chinese with English abstract)
[11] 陈定千,沈来宏,肖军,等. 水泥改性铁矿石载氧体的煤化学链燃烧实验研究[J]. 中国电机工程学报,2013,33(20):40-45. Chen Dingqian, Shen Laihong, Xiao Jun, et al. Experiments on chemical-looping combustion of coal with cement-decorated iron ore as oxygen carrier[J]. Proceedings of the CSEE, 2013, 33(20): 40-45. (in Chinese with English abstract)
[12] Occelli M L, Tindwa R M. Physicochemical properties of montmorillonite interlayered with cationic oxyaluminum pillars[J]. Clays and Clay Minerals, 1983, 31(1): 22-28.
[13] Aldersley M F, Joshi P C. RNA dimer synthesis using montmorillonite as a catalyst: The role of surface layer charge[J]. Applied Clay Science, 2013, 83/84: 77-82.
[14] 牛永红,韩枫涛,陈胜义. 高温蒸汽松木颗粒富氢气化试验研究[J]. 农业工程学报,2016,32(3):247-252. Niu Yonghong, Han Fengtao, Chen Yisheng. Experimental study of High-temperature steam gasification of pine particles for hydrogen-rich gas[J]. Transactions of the Chinese Society of Agricultural Engineering(Transactions of the CSAE), 2016, 32(3): 247-252. (in Chinese with English abstract)
[15] Soncini R M, Means N C, Weiland N T. Co-pyrolysis of low rank coals and biomass: Product distributions[J]. Fuel, 2013, 112(3): 74-82.
[16] 李琳娜. 生物质高温水蒸气气化制备富氢燃气的研究[D].北京:中国林业科学研究院,2011. Li Linna. Preparation of Hydrogen Rich Gas High Temperature Steam Gasification Biomass Research[D]. Beijing: China Academy of Forestry Science, 2011. (in Chinese with English abstract)
[17] 涂军令. 木屑/木屑炭高温水蒸气气化制备合成气研究[D].北京:中国林业科学研究院,2012. Tu Junling. Sawdust/Sawdust Charcoal High Temperature Steam Gasification and the Preparation of Syngas[D]. Beijing: Chinese Academy of Forestry, 2012. (in Chinese with English abstract)
[18] 孙云娟. 生物质催化气化及焦油的裂解研究[D]. 北京:中国林业科学研究院,2006. Sun Yunjuan. Study on Biomass Catalytic Gasification and Tar Cracking[D]. Beijing: Chinese Academy of Forestry, 2006. (in Chinese with English abstract)
[19] 贾立. 生物质热解气白云石催化重整的实验研究[D]. 武汉:华中科技大学,2007. Jia Li. An Experimental Research on Reforming Pyrolyzation Gas with Dolomite[D]. Wuhan: Huazhong University of Science and Technology, 2007. (in Chinese with English abstract)
[20] 张寰. 秸秆类生物质教育热解动力学及碳纤维制备实验研究[D]. 郑州:河南农业大学,2014. Zhang Huan. Study of Thermal Properties of Biomass Tar Using Straw and Manufacture Technology of Carbon Fiber[D]. Zhengzhou: Henan Agricultural University, 2014. (in Chinese with English abstract)
[21] 秦岭. 生物质热解动力学及反应机理的研究[D]. 北京:清华大学,2012. Qin Ling. Study on Biomass Pyrolysis Kinetics and Mechanisms[D]. Beijing: Tsinghua University, 2012. (in Chinese with English abstract)
[22] Ahmed I, Jangsawang W, Gupta A K. Energy recovery from pyrolysis and gasification of mangrove[J]. Applied Energy, 2012, 91(1): 173-179.
[23] 赖艳华,马春元,施明恒. 生物质燃料层热解过程的传热传质模型研究[J]. 热科学与技术,2005,4(3):219-223. Lai Yanhua, Ma Chunyuan, Shi Mingheng. Heat and mass transfer in pyrolysis processof biomass fuel bed[J]. Journal of Thermal Science and Technology, 2005, 4(3): 219-223. (in Chinese with English abstract)
[24] 牛永红,韩枫涛,李义科,等. 松木成型燃料水蒸气气化反应特性[J]. 化工学报,2017,68(3):1191-1198. Niu Yonghong, Han Fengtao, Li Yike, et al. Steam gasification characteristic of pine briquette fuel[J]. CIESC Journal, 2017, 68(3): 1191-1198. (in Chinese with English abstract)
[25] 牛永红,韩枫涛,张雪峰,等. 白云石催化松木燃料棒水蒸气气化试验[J]. 农业机械学报,2016,47(12):246-252. Niu Yonghong, Han Fengtao, Zhang Xuefeng, et al. Experiment on steam gasification of pine fuel rods withdoldmite catalyst[J]. Transactions of the Chinese Society for Agricultural Machinery, 2016, 47(12): 246-252. (in Chinese with English abstract)
[26] Rahman M S, State diagram of foods: Its potential use in food processing and product stability[J]. Trends in Food Science and Technology, 2006, 17(3): 129-141.
[27] Ahmed I, Gupta A. Sugarcane bagasse gasication: Global reaction mechanism of syngas evolution[J]. Applied Energy, 2012, 91(1): 75-81.
[28] Baldwin R M, Magrini-Bair K A, Nimlos M R, et al. Current research on thermochemical conversion of biomass at the National Renewable Energy Laboratory[J]. Applied Catalysis B: Environmental, 2012(115/116): 320-329.
[29] Irfan M F, Usman M R, Kusakabe K. Coal gasificationin CO2atmosphere and its kinetics since 1948: A brief review[J]. Energy, 2011, 36(1): 12-40.
[30] Guizani N, Al-Saidi G S, Rahman M S, et al. State diagram of dates: Galass transition, freezing curve and maximal freeze concentration condition[J]. Journal of Food Engineering, 2010, 99(1): 92-97.
Performance improvement of steam gasification of pine for hydrogen-rich gas with dolomite catalyst modified by bentonite/limonite
Niu Yonghong1,2, Han Fengtao2, Zhang Xuefeng1,3, Chen Yisheng4, Wang Li4, Xu Jia4
(1. Mining Research Institute, Inner Mongolia University of Science and Technology, Baotou 014010, China; 2. School of Energy and Environment, Inner Mongolia University of Science and Technology, Baotou 014010, China; 3. Key Laboratory of Integrated Exploitation of Bayan Obo Multi-metal Resources, Baotou 014010, China; 4. Analytical Center, Inner Mongolia University of Science and Technology, Baotou 014010, China)
In this study, we adopted steam gasification of biomass, and pine fuel rods as the experimental objects. We used gas chromatography and Fourier transform infrared spectrometer to analyze components of gas and tar change during the process of gasification. We also determined catalytic performance of modified dolomite (bentonite as the carrier, dolomite as the active component and a small amount of load limonite) as temperature rose. Our experiment results showed that, high-temperature made steam gasification of biomass intensified so that steam reforming reaction intensified and thus contributing to H2gas volume fraction in the overall upward trend in the gasification components, but hydrocarbon gas volume fraction in downward trend. Gasification gas carries gasification tar into the catalyst layer, which makes tar catalytic cracked, promotes steam reforming reaction intensified and thereby changes the composition of gasification gas. Under the condition of steam gasification with modified dolomite catalyst at 750 ℃, H2volume fraction was 45.77%±0.23%. Compared to hydrogen-rich action of catalytic and non-catalytic conditions dolomite, gasification temperature dropped to 100 ℃. With the rise of temperature, modified dolomite promoted the increase of carbon oxides and hydrocarbons volume fraction, but hydrogen-rich degree weaken. The calcined dolomite contained MgO/CaO as active ingredients can cause the long carbon chain cracked in aliphatic hydrocarbons, conducive to the production of radical hydrogen ion, and be helpful to generate hydrogen. When Fe as a promoter was used, it was activated to Fe3+by high temperature steam, and converted to Fe2+after promoting tar cracking. Modified dolomite not only promoted the scission of carbon chain length on the hydrocarbon side chain and the formation of H2, but also promoted opening reactions of aromatic ring, decarboxylation and dehydroxylation reactions, thereby making tar after pyrolysis easily to be converted into small molecule gas carbon containing, which helped to reduce the production of tars gasified and improved the gasification efficiency. The catalyst active center Fe3+decreased at higher temperature, which made the gaining weight of homemade catalyst after catalysis reduced to a minimum of 900 ℃, but compared to dolomite, the change of color and shape of modified dolomite between front and rear catalytic was small, which reflected the mechanical strength and surface area carbon optimized. The catalytic activity of dolomite was secondly to nickel-based catalyst, but it had potential economic value with cheaper price. In this study, we compared to steam gasification of biomass with dolomite catalyst, explored the catalytic properties of modified dolomite, which would provide useful information of dolomite modification method and studies of steam gasification of biomass. The purpose of this research was to promote the use of dolomite catalysts, and our results showed that they can be used for the treatment of tailings and to deepen the study of biomass steam gasification.
catalysts; gasification; biomass; pine fuel rods; modified dolomite; rich-hydrogen gas; tar
10.11975/j.issn.1002-6819.2017.07.028
TK6
A
1002-6819(2017)-07-0213-07
2016-07-07
2017-04-05
内蒙古科技创新引导奖励资金项目(01850401);内蒙古自然基金项目(2015MS0106);内蒙古科技大学大学生科技创新项目(2015138;2016065)
牛永红,男(汉族),内蒙古凉城人,博士生,副教授,主要从事生可再生高效清洁利用研究。包头 内蒙古科技大学矿业研究院,014010。
Email:yonghong_niu@126.com
