椎动脉狭窄血管成形术后支架内再狭窄危险因素相关性研究
2017-05-09林永娟俞婷婷黄明敏郭爱斌尹震宇
林永娟,冯 ,俞婷婷,谢 宇,黄明敏,郭爱斌,尹震宇
·论 著·
椎动脉狭窄血管成形术后支架内再狭窄危险因素相关性研究
目的 探讨椎动脉粥样硬化性狭窄患者血管内支架置入术后再狭窄的危险因素,研究再狭窄患者预后。方法 选择南京鼓楼医院2004-2013年间收治的椎动脉粥样硬化性狭窄患者173例,均行血管内支架置入术,术后随访(55.7±17.31)个月,记录其性别、年龄、合并症、吸烟史、病变部位、术前术后椎动脉直径、支架类型、支架直径、支架长度等,分析血管内支架置入术后再狭窄的危险因素以及再狭窄预后。 结果 所有患者顺利完成血管内支架置入术,随访期间28例出现再狭窄,145例未出现再狭窄,再狭窄发生率为16.2%。2组患者性别、年龄、随访时间、糖尿病、高尿酸血症、冠心病、血管直径、支架类型差异均无统计学意义(P>0.05);而再狭窄组高血压、高脂血症、吸烟、和颅外V1段、颅内V4段支架植入位置均明显高于非再狭窄组(P<0.05)。多因素logistics回归分析显示,高脂血症(HR:4.31,95%CI: 2.99~18.76,P=0.042)为再狭窄危险因素。再狭窄组卒中发生风险明显高于非再狭窄,Kaplan-Meier分析结果显示,再狭窄组卒中终点事件风险高(OR: 0.141, 95%CI: 0.016~1.221,P=0.029),差异有统计学意义。 结论 椎动脉起始部粥样硬化性狭窄患者血管内支架置入术后再狭窄发生率较高,高脂血症是再狭窄独立危险因素,再狭窄患者发生卒中事件风险高。
椎动脉粥样硬化;血管成形术;支架内再狭窄;终点事件
缺血性脑卒中是最常见心脑血管疾病之一,死亡率和致残率高,约25%~30%的缺血性脑卒中由椎动脉粥样硬化性狭窄或闭塞引起[1-3]。对于症状性重度椎动脉粥样硬化性狭窄或闭塞(狭窄程度≥50%),血管内支架置入术(vertebral artery stenting, VAS)已成为最重要的治疗手段。临床研究表明,VAS成功率较高,可有效减轻患者血管狭窄程度,改善颅内血流[1, 4-5]。但支架置入术后再狭窄(instent restenosis, ISR)发生率高,文献报道高达11%~45%(药物涂层支架发生率11%左右,裸金属支架发生率>30%),是血管内支架置入术面临的最大挑战[6-7]。目前关于ISR发生机制仍不清楚,多数学者认为可能和支架置入术中球囊扩张、血管内皮细胞受损、椎动脉变异、支架类型等相关[7-8]。同时支架内再狭窄和再发脑血管事件是否相关目前尚未完全明确[9-10]。因此了解支架内再狭窄的相关因素,对预测、预防ISR、再狭窄患者预后具有重要意义。本研究回顾性分析在我院成功行椎动脉支架置入术,并于1年后复查脑血管造影(DSA) 的患者的临床资料、病变血管特征,探讨可能预测VAS支架内再狭窄的危险因素,研究再狭窄患者预后,为防治椎动脉支架植入术后支架内再狭窄、脑血管事件二级预防提供客观依据。
1 资料与方法
1.1 一般资料 选择南京鼓楼医院2004-2013年间收治的椎动脉粥样硬化性狭窄行VAS患者173例,均符合北美症状性颈动脉内膜切除术试验协作组制定的椎动脉起始部粥样硬化性狭窄诊断标准[11]。平均年龄(66.8±8.2)岁,随访(55.7±17.31)个月,男占79.2%;其中因短暂性脑缺血行VAS患者116例,因卒中行VAS患者57例;支架位置:其中横突孔前段(颅外V1段)共121例,硬膜下段(颅内V4段)共52例;支架类型:裸金属支架(bare metal stents,BMS)98例,药物涂层支架(drug-eluting stents,DES)75例;合并症:高血压137例,糖尿病36例,高脂血症52例,高尿酸血症34例;有吸烟史者55例。所有患者VAS术后造影均未见动脉夹层、支架破裂、以及远端闭塞;术后30 d也无卒中或死亡事件的发生。
1.2 纳入标准和排除标准 纳入标准:①满足因症状性椎动脉狭窄≥50%或非症状性椎动脉狭窄≥70%行血管内支架置入术,②血管内支架置入术成功且残余狭窄率<30%;③无其他术后严重并发症;④临床资料完整且随访时间≥6个月。排除标准: ①支架内夹层;②血管蜷缩;③随访时间<6个月或病历资料不完整;④椎动脉病变远端或近端多发性重度闭塞或狭窄;⑤脑出血患者;⑥有抗血小板药物使用禁忌;⑦严重的肝肾疾病或心功能Ⅲ级以上,肿瘤,血液透析,凝血功能异常者。
1.3 方法 所有VAS均由有经验的神经介入科医生完成,患者术前3 d口服阿司匹林(300 mg/d)+波立维(75 mg/d),支架置入术后1年至少联合抗血小板药物(阿司匹林100 mg/d+波立维75 mg/d)1年。结合文献报道筛选椎动脉粥样硬化性狭窄患者血管内支架置入术后再狭窄的相关因素;原病变部位血管狭窄度≥50%为术后再狭窄。
1.4 随访 所有支架置入术后3个月、6个月、1年以及每年均进行随访,由临床医师完成,包括神经系统体格检查、椎动脉超声,计算机断层扫描血管造影(CTA),术后1年或CTA高度可疑再狭窄时复查DSA进一步明确病情。终点事件包括同侧后循环急性缺血性卒中,持续事件>24 h。血管内支架置入术后30 d内急性卒中考虑为围术期并发症,不记入终点事件。
1.5 统计学分析 采用 SPSS17.0 软件进行统计分析,计量资料以均数±标准差表示,采用独立t检验;计数资料以百分数表示,采用χ2检验;不符合正态分布的定量资料采用秩和检验,若样本较小(n<40)或理论频数太小(T<1),采用Fisher确切概率法。再狭窄的危险因素分析采用多因素Logistic回归分析,长期随访后终点事件分析采用kaplan-meier分析。以P<0. 05为差异有统计学意义。
2 结 果
2.1 再狭窄分析 所有患者顺利完成VAS,随访期间28例发生ISR,发生率为16.2%;随访第1年、第2年ISR均为7例,第3年发现ISR为6例,第4年ISR为5例,第5年ISR为3例。单因素分析显示无再狭窄组和再狭窄组的性别、年龄、随访时间、糖尿病、高尿酸血症、冠心病、血管直径、支架类型均无统计学意义(P>0.05);而再狭窄组高血压、高脂血症、吸烟、和颅外V1段、颅内V4段支架植入位置均明显高于非再狭窄组,且有统计学意义(P<0.05),见表1。将高血压、高脂血症、吸烟和支架部位作为因变量进行多因素Logistics回归分析显示,高脂血症(HR:4.31,95%CI: 2.99~18.76,P=0.042)为ISR独立危险因素,见表2。
2.2 终点事件 随访期间非再狭窄、再狭窄组发生非致死性卒中分别为4例和3例,具有统计学差异,2组间致死性卒中和其他原因的死亡的发生率无明显差异,见表3。将2组卒中进行Kaplan-Meier分析显示,ISR发生卒中终点事件风险高(OR: 0.141, 95%CI: 0.016~1.221,P=0.029),见图1。
表1 椎动脉支架术后再狭窄与非再狭窄组危险因素的比较

指标非再狭窄组(n=145)再狭窄组(n=28)P值年龄(岁)66.8±7.666.7±9.10.109性别[n(%)] 男114(78.6)23(82.1)0.674 女31(21.4)5(17.9)随访时间(月)54.9±0.655.7±17.30.773入院诊断[n(%)] 短暂性脑缺血发作97(66.9)19(67.9)0.921 梗死48(33.1)9(32.1)高血压[n(%)]112(77.2)25(89.2)0.038糖尿病[n(%)]33(22.8)3(10.7)0.151高脂血症[n(%)]39(26.9)13(46.4)0.039高尿酸血症[n(%)]29(20)5(17.9)0.794吸烟[n(%)]41(28.3)14(50)0.024冠心病[n(%)]26(17.9)3(10.7)0.349外周血管疾病[n(%)]4(2.8)2(7.1)0.246合并脑动脉狭窄[n(%)]58(40)13(46.4)0.527血管直径(mm)3.9±0.73.8±0.70.702支架前 最小管腔直径(mm)0.7±0.50.7±0.50.874 狭窄程度(%)70.8±14.972.1±13.20.224 狭窄长度(mm)15.2±5.11.7±4.80.636支架后 最小管腔直径(mm)3.6±0.53.5±0.20.947 狭窄程度(%)9.1±5.110.2±4.70.647 支架长度(mm)19.8±6.220.1±5.30.705椎动脉狭窄部位[n(%)] 单侧128(88.3)24(85.7)0.704 双侧17(11.7)4(14.3)对侧闭塞[n(%)]18(12.4)5(17.9)0.437支架类型[n(%)] BMS82(56.6)16(57.1)0.954 DES63(43.4)12(42.9)狭窄/支架位置[n(%)] V1108(74.5)13(46.4)0.003 V437(25.5)15(53.6)合并用药[n(%)] 质子泵抑制剂21(14.5)2(7.1)0.295 CYP3A4代谢的他汀类58(40.0)11(39.3)0.944 胰岛素28(19.3)6(21.4)0.796
表2 椎动脉支架术后再狭窄独立危险因素的logistic回归分析[n(%)]
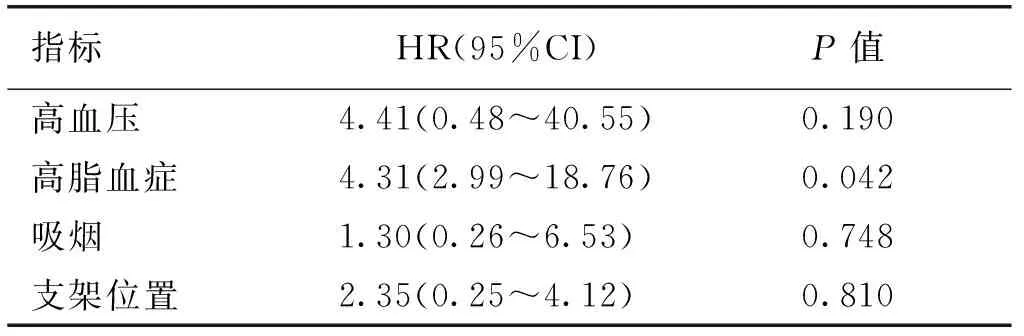
指标HR(95%CI)P值高血压4.41(0.48~40.55)0.190高脂血症4.31(2.99~18.76)0.042吸烟1.30(0.26~6.53)0.748支架位置2.35(0.25~4.12)0.810
表3 椎动脉支架术后再狭窄组与非再狭窄组不良事件的分析[n(%)]
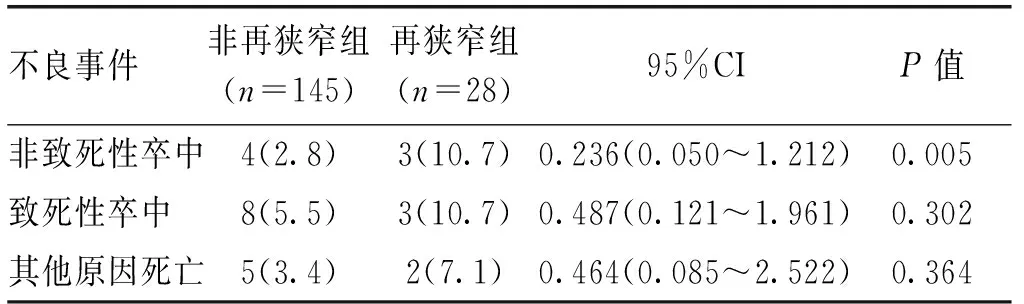
不良事件非再狭窄组(n=145)再狭窄组(n=28)95%CIP值非致死性卒中4(2.8)3(10.7)0.236(0.050~1.212)0.005致死性卒中8(5.5)3(10.7)0.487(0.121~1.961)0.302其他原因死亡5(3.4)2(7.1)0.464(0.085~2.522)0.364
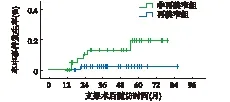
再狭窄组发生卒中终点事件风险明显高于非再狭窄组(P=0.029)
图1 再狭窄组与非再狭窄组卒中的比较(Kaplan-Meier分析)
3 讨 论
目前研究报道,20%左右的后循环卒中是由椎动脉狭窄引起,椎动脉狭窄可发生颅内或颅外,但其中以锁骨下动脉发出的椎动脉起始段最为常见[1,12-13]。动脉粥样硬化是椎动脉狭窄最主要原因[14-15]。目前治疗无症状性的椎动脉起始段狭窄是备受争议的[16-19],虽然大多数无症状性椎动脉狭窄并不需要血管内治疗,但是研究认为优势或单支椎动脉起始段重度椎动脉狭窄(>70%),为了减少卒中的发生应该积极给予积极血管内治疗[20-21]。本研究中随访的患者,有临床症状同时椎动脉狭窄程度≥50%或者无临床症状但狭窄程度≥70%均给予积极的支架治疗,支架置入术后给予口服抗血小板治疗。目前关于椎动脉粥样硬化性狭窄最佳治疗方案目前还不清楚,需要临床进一步研究论证。
临床中椎动脉支架置入术后ISR发生率仍不明确[22],研究报道,ISR的发生率为11%~45%[6],文献报道中再狭窄发生率波动大,和样本量少、支架后抗血小板药物不统一、ISR的定义区别以及支架类型不同相关[7,23]。药物涂层支架能够抑制血管内膜增生,与裸金属支架相比,能够显著降低ISR的发生率(11%vs30%)[14, 24]。本研究结果显示,173例椎动脉支架置入术后再狭窄发生率为16.2%,与文献报道一致,但药物涂层支架与裸金属ISR未见明显差异,可能与样本量少相关。本研究随访中发现第1年、第2年ISR均为7例,第3年发现ISR为6例,第4年ISR为5例, 第5年ISR为3例,提示血管内支架置入术后早期再狭窄发生率高。
目前,关于椎动脉支架置入术后再狭窄危险因素的研究报道较多,但研究结果却不尽相同[23,25-27]。Edgell等[8]研究表明,吸烟、年龄是ISR独立危险因素。Stayman等[28]报道椎动脉粥样硬化斑块长度、支架长度和ISR相关。本研究中再狭窄组高血压、高脂血症、吸烟、支架位置均高于非再狭窄组,其中高脂血症是ISR的独立危险因素。目前研究表明[29-31],再狭窄发病机制可能为支架置入过程中血管内膜受损,脂质易于沉积,此外,血脂代谢异常时血液粘稠度增加,血流减慢,血液中血小板和单核-巨噬细胞等物质易于沉积血管内膜,促进平滑肌细胞生长因子的释放,引起平滑肌细胞的迁移、增殖,进一步加速支架内再狭窄的发生。Sun等[32]研究发现高脂血症和椎动脉支架术后再次卒中事件相关。因此临床需加强支架术后血脂的控制和管理。有研究发现颅内椎动脉支架术后再狭窄发生率高于颅外[9],提示椎动脉支架位置可能是椎动脉支架术后再狭窄危险因素,本研究中支架位置主要位于横突孔前段V1段和硬膜下段(V4段),单因素分析提示支架位置是再狭窄危险因素,而多因素Logistics回归分析无明显统计学意义,考虑与椎动脉支架颅内段病例数较少相关,其统计结果不能完全说明其并非再狭窄好发部位的相关问题。
本研究结合文献报道筛选椎动脉粥样硬化性狭窄患者VAS再狭窄的相关因素,但并未发现年龄、糖尿病、高尿酸血症、吸烟、血管直径、支架类型与ISR的相关性,这可能与本研究样本量小、随访时间有限有关,有待在今后的研究中扩大样本量、进一步延长随访时间或采用多中心联合研究等进一步验证。
本研究发现再发卒中事件和ISR相关,且有统计学意义。文献中亦有报道,椎动脉起始段支架后ISR不能忽视,约10%的ISR会发生卒中[20,33]。Hatano等[34]随访了117例椎动脉起始段支架置入术患者,ISR发生率为23%同时9%需要再次行血管内介入治疗。冠脉支架置入术后也有类似发现,支架置入术后再狭窄和继发缺血性事件相关。
本研究尚有不足之处,本研究为单中心回顾性研究,样本量少,随访时间有限,随访过程中部分样本不能坚持随访或服用抗血小板药物被排除。有待在今后的研究中扩大样本量、进一步延长随访时间或采用多中心联合研究等进一步验证。
[1] Borhani Haghighi A, Edgell RC, Cruz-Flores S,etal. Vertebral artery origin stenosis and its treatment[J]. J Stroke Cerebrovasc Dis, 2011,20(4):369-376.
[2] Brott TG, Halperin JL, Abbara S,etal. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Developed in collaboration with the American Academy of Neurology and Society of Cardiovascular Computed Tomography[J].Catheter Cardiovasc Interv, 2013,81(1):E76-123.
[3] Gupta R, Sivapatham T, Moskowitz SI,etal. Stenting of a symptomatic long-segment extracranial vertebral artery occlusion[J]. J Neurointerv Surg, 2011,3(1):54-56.
[4] Annette C,Bart VW,Wouter JS,etal.Stenting versus medical treatment in patients with symptomatic vertebral artery stenosis: a randomised open-label phase 2 trail[J].Lancet Neurol, 2015,14(6): 606-614.
[5] Broussalis E,Kun NZ, Luthring SH,etal. Treatment of vertebral artery origin stenosis with a Pharos stent device: a single .center experience[J].Interv Neuroradiol, 2011,17(3): 316-322.
[6] Kocak B.Endovascular treatment of extracranial vertebral artery stenosis[J]. World J Radiol, 2012,4(9):391.
[7] Mousa AY, AbuRahma AF, Bozzay J,etal.Anatomic and clinical predictors of reintervention after subclavian artery stenting[J]. J Vasc Surg,2015,62(1):106-114.
[8] Edgell RC, Zaidat OO, Gupta R,etal. Multicenter study of safety in stenting for symptomatic vertebral artery origin stenosis: results from the Society of Vascular and Interventional Neurology Research Consortium[J].J Neuroimaging, 2013,23(2):170-174.
[9] Gao P, Wang D, Zhao Z,etal. Multicenter prospective trial of stent placement in patients with symptomatic high-grade intracranial stenosis[J]. Am J Neuroradiol, 2016,37(7):1275-1280.
[10] Mohammadian R, Sharifipour E, Mansourizadeh R,etal. Angioplasty and stenting of symptomatic vertebral artery stenosis. Clinical and angiographic follow-up of 206 cases from Northwest Iran[J].Neuroradiol J, 2013,26(4):454-463.
[11] North American Symptomatic Carotid Endarterectomy Collaborators,Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis[J].N Engl J Med,1991,325(7):445-453.
[12] Mokin M, Dumont TM, Kass-Hout T,etal. Carotid and vertebral artery disease[J].Primary care, 2013,40(3):135-151.
[13] Musialek P, Langwieser N. Commentary: vertebral artery ostial stenosis stenting technique: the concept reversed?[J] J Endovasc Ther, 2015,22(3):445-448.
[14] Chen X, Huang Q, Hong B,etal. Drug-eluting stent for the treatment of symptomatic vertebral origin stenosis: Long-term results[J].J Clin Neurosci, 2011,18(1):47-51.
[15] Chaturvedi S, Bhattacharya P. Large artery atherosclerosis: carotid stenosis, vertebral artery disease, and intracranial atherosclerosis[J]. Continuum, 2014,20(2):323-334.
[16] Wang L, Shi W, Su Z,etal. Endovascular treatment of severe acute basilar artery occlusion[J].J Clin Neurosci,2015,22(1):195-198.
[17] Feng H, Xie Y, Mei B,etal. Endovascular vs. medical therapy in symptomatic vertebral artery stenosis: a meta-analysis[J].J Neurol, 2016,20(1):415-326.
[18] Zhong C. Influence of stent-assisted angioplasty on cognitive function and affective disorder in elderly patients with symptomatic vertebrobasilar artery stenosis[J].Med Sci Monit,2014,20(1):1129-1136.
[19] Baek JH, Kim BM. Stenting for symptomatic vertebral artery stenosis associated with bilateral carotid rete mirabile: the long-term clinical and angiographic outcome[J].Korean J Radiol, 2015,16(3):678-681.
[20] Antoniou GA, Murray D, Georgiadis GS,etal. Percutaneous transluminal angioplasty and stenting in patients with proximal vertebral artery stenosis[J]. J Vasc Surg,2012,55(4):1167-1177.
[21] Gross BA, Albuquerque FC. Stenting Versus Aggressive Medical Management for Symptomatic Vertebral Artery Stenosis[J].World Neurosurg,2015,84(3):613-615.
[22] Rangel-Castilla L, Gandhi S, Munich SA,etal. Experience with vertebral artery origin stenting and ostium dilatation: results of treatment and clinical outcomes[J].J Neurointerv Surg, 2016,8(5):476-480.
[23] Jiang Y, Xu X, Wen Z,etal. In-stent restenosis after vertebral artery stenting[J].Int J Cardiol, 2015,187:430-433.
[24] Lu H, Zheng P, Zhang W. Long-term outcome of drug-eluting stenting for stenoses of the intracranial vertebrobasilar artery and vertebral ostium[J]. J Neurointerv Surg, 2013,5(5):435-439.
[25] 杨建军,宋雪丹,陈海英,等.血管内支架成形术治疗颅内血管狭窄临床分析[J].东南国防医药,2009,11(2):33-37.
[26] Li Z, Zhang Y, Hong B,etal. Stenting of symptomatic vertebral artery ostium stenosis with self-expanding stents[J].J Clin Neurosci, 2014,21(2):274-277.
[27] Lin YJ, Li JW, Zhang MJ,etal. The association between CYP2C19 genotype and of in-stent restenosis among patients with vertebral artery stent treatment[J].CNS Neurosci Ther, 2014,20(2):125-130.
[28] Stayman AN, Nogueira RG, Gupta R. A Systematic Review of Stenting and Angioplasty of Symptomatic Extracranial Vertebral Artery Stenosis[J]. Stroke, 2011,42(8):2212-2216.
[29] Tank VH, Ghosh R, Gupta V,etal. Drug eluting stents versus bare metal stents for the treatment of extracranial vertebral artery disease: a meta-analysis[J].J Neurointerv Surg, 2016,8(8):770-774.
[30] Ogilvy CS, Yang X, Natarajan SK,etal. Restenosis rates following vertebral artery origin stenting: does stent type make a difference?[J]J Invasive Cardiol, 2010,22(3):119-124.
[31] 林 敏,范 进,陈光辉.动脉粥样硬化性椎动脉狭窄介入治疗的实践与指南[J].医学研究生学报,2011,24(7):758-762.
[32] Sun X, Ma N, Wang B,etal. The long term results of vertebral artery ostium stenting in a single center: Table 1[J].J Neurointerv Surg, 2015,7(12):888-891.
[33] Langwieser N, Prothmann S, Buyer D,etal. Safety and efficacy of different stent types for the endovascular therapy of extracranial vertebral artery disease[J].Clin Res Cardiol, 2014,103(5):353-362.
[34] Hatano T, Tsukahara T, Miyakoshi A,etal. Stent placement for atherosclerotic stenosis of the vertebral artery ostium: angiographic and clinical outcomes in 117 consecutive patients[J]. Neurosurgery, 2011,68(1):108-116.
(本文编辑:叶华珍; 英文编辑:王建东)
Risk factors ofrestenosis in postoperative patients with vertebral artery stenosis treated by endovascular stent implantation
LIN Yong-juan,FENG Jie, YU Ting-ting,XIE Yu, HUANG Ming-min, GUO Ai-bin, YIN Zhen-yu
(DepartmentofGeriatrics,NanjingDrumTowerHospital,TheAffiliatedHospitalofNanjingUniversityMedicalSchool,Nanjing210009,Jiangsu,China)
Objective To investigate the risk factors and restenosis prognosis of restenosis in postoperative patients with atherosclerotic vertebral artery stenosis treated by endovascular stent implantation. Methods A total of 173 patients with atherosclerotic vertebral artery stenosis were selected in the Nanjing Drum Tower Hospital from 2004 to 2013, and all of them were treated by endovascular stent implantation and were followed up for (55.7±17.31). Clinical data including gender, age, complications, smoking history, stent region, preoperative and postoperative diameter of vertebral artery, stent types, stent diameter, stent length and postoperative stenosis length were collected, and the risk factors and restenosis prognosis of restenosis in postoperative patients with atherosclerotic vertebral artery stenosis treated by endovascular stent implantation were analyzed. Results All of the 173 patients successfully completed the endovascular stent implantation, including 28 cases with restenosis, and other 145 cases without restenosis, and the incidence of restenosis was 16.2%. No statistically significant difference of gender, age, time of follow up, incidence of hyperuricemia, diabetes, coronary artey disease, stent diameter,stent types was found between two groups (P>0.05) ; positive rate of smoking history, high blood pressure and hyperlipemia and the stent region of restenosis group was statistically significantly higher than that of the control group (P<0.05); Multivariate logistic regression analysis results showed that hyperlipemia (HR:4.31, 95% CI: 2.99-18.76,P=0.042) was the only risk factor of restenosis in postoperative patients with atherosclerotic vertebral artery stenosis treated by endovascular stent implantation. The results of Kaplan-Meier showed that recurrent ischemic events were associated with in-stent restenosis (OR: 0.141, 95% CI: 0.016-1.221,P=0.029). Conclusion The incidence of restenosis is relatively high in postoperative patients with atherosclerotic vertebral artery stenosis treated by endovascular stent implantation, and hyperlipemia is the risk factor, while recur- rent ischemic events are associated with restenosis. [Key words] Atherosclerosis; Vertebral artery stents; Postoperative restenosis; Risk factors
210009南京,南京大学附属鼓楼医院老年科
尹震宇,E-mail:506474559@qq.com
林永娟,冯,俞婷婷,等.椎动脉狭窄血管成形术后支架内再狭窄危险因素相关性研究[J].东南国防医药,2017,19(2):136-140.
R743
A
1672-271X(2017)02-0136-05
10.3969/j.issn.1672-271X.2017.02.006
2017-01-12;
2017-02-25)
