Low frequency repetitive transcranial magnetic stimulation improves motor dysfunction after cerebral infarction
2017-05-03ZhiyongMengWeiqunSong
Zhi-yong Meng, Wei-qun Song
Department of Rehabilitation, Xuanwu Hospital, Capital Medical University, Beijing, China
Low frequency repetitive transcranial magnetic stimulation improves motor dysfunction after cerebral infarction
Zhi-yong Meng, Wei-qun Song*
Department of Rehabilitation, Xuanwu Hospital, Capital Medical University, Beijing, China
How to cite this article:Meng ZY, Song WQ (2017) Low frequency repetitive transcranial magnetic stimulation improves motor dysfunction after cerebral infarction. Neural Regen Res 12(4):610-613.
Open access statement:Tis is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:Tis project was supported by the National Natural Science Foundation of China, No. 30540058, 30770714; the Natural Science Foundation of Beijing of China, No. 7052030; the Talents Foundation of Organization Department of the Beijing Municipal Committee in China; the Beijing Science Plan Project Fund of China, No. Z0005187040191-1; the Research Foundation of Capital Medical Development of China, No. 2007-2068.
Graphical Abstract
Low frequency (1 Hz) repetitive transcranial magnetic stimulation helps improve motor function aer cerebral infarction
Low frequency (≤ 1 Hz) repetitive transcranial magnetic stimulation (rTMS) can af f ect the excitability of the cerebral cortex and synaptic plasticity. Although this is a common method for clinical treatment of cerebral infarction, whether it promotes the recovery of motor function remains controversial. Twenty patients with cerebral infarction combined with hemiparalysis were equally and randomly divided into a low frequency rTMS group and a control group.e patients in the low frequency rTMS group were given 1-Hz rTMS to the contralateral primary motor cortex with a stimulus intensity of 90% motor threshold, 30 minutes/day.e patients in the control group were given sham stimulation. Aer 14 days of treatment, clinical function scores (National Institute of Health Stroke Scale, Barthel Index, and Fugl-Meyer Assessment) improved signif i cantly in the low frequency rTMS group, and the ef f ects were better than that in the control group. We conclude that low frequency (1 Hz) rTMS for 14 days can help improve motor function aer cerebral infarction.
nerve regeneration; brain injury; repetitive transcranial magnetic stimulation; motor dysfunction; cerebral infarction; National Institute of Health Stroke Scale; Barthel Index; Fugl-Meyer Assessment; neural regeneration
Introduction
Cerebral infarction is a common and frequently occurring disease, with high mortality and disability rates. As a consequence, improving motor function in patients with cerebral infarction has become a focus in basic and clinical research. Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive painless treatment that can affect the excitability of the cerebral cortex and ef f ect changes in synaptic plasticity, which in turn enhance the recovery of neurological function (Hendricks et al., 2002; Rodger and Sherrard, 2016).
This study investigated the effect of 1-Hz low frequency rTMS on the recovery of motor function by analyzing neurological function scores in patients with subacute cerebral infarction.
Subjects and Methods
Subjects
Twenty patients with cerebral infarction combined with hemiparalysis were chosen from June 2012 to December 2012 in the Department of Rehabilitation Medicine, Xuanwu Hospital, Capital Medical University, China. Patients were collected in accordance with cerebral infarction standards.e 20 patients were randomly divided into a low frequency rTMS group (n= 10; 9 males; age: 64.8 ± 9.5 years) and a control group (n= 10; 8 males; age: 65.2 ± 9.7 years). Allpatients were right-handed.e study was approved by the Ethical Committee of Capital Medical University Xuanwu Hospital.

Figure 1 Flow chart of the study.
Inclusion criteria
Patients meeting all the following criteria were included in the study: (1) cerebral infarction of the internal carotid artery that was diagnosed based on clinical symptoms and signs, cranial computed tomography, or magnetic resonance imaging; (2) unilateral limb dysfunction resulting from unilateral cortical damage; (3) first-time stroke; (4) age range: 35–80 years; (5) signed informed consent before treatment by the patients and their families.
Exclusion criteria
Patients meeting any of the following criteria were excluded from the study: (1) use of cardiac pacemakers, implantable def i brillator, or other similar equipment; (2) presence of cerebral hemorrhage, subarachnoid hemorrhage, or transient ischemic attack; (3) worsening of the illness or emergence of new infarctions; (4) medical history of epilepsy; (5) failure of the heart, liver, lung, kidney, or other important organs; (6) severe cognitive or communicative barriers.
rTMS
rTMS was delivered with a Magstim Rapid stimulator (e Magstim Company, Ltd., Carmarthenshire, UK).e fi gure-8-shaped stimulating coil had a coil diameter of 70 mm.e site of stimulation, parameters, and motor threshold for the rTMS were set according to a previous study (Rossi et al., 2009). Stimulation frequency was 1 Hz and the intensity was 90% of motor threshold.e stimulation site was the contralateral primary motor cortex (M1) contralateral to the infarction. Continuous stimulation was delivered daily in one 30-minute session for 14 days. A total of 1,800 pulses were administered each day. In the control group, patients were given 30 minutes of sham stimulation once per day. Patients were able to hear magnetic stimulator, but did not receive any real magnetic stimulation. Rehabilitation training (exercise) was conducted twice a day for each group, with each session lasting 40 minutes. Therapeutic responses and adverse reactions were observed during all treatments.
Neurological assessment
Neurological function was scored in each group before the experiment and aer 14 days of treatment. Functions were assessed using the National Institute of Health Stroke Scale (NIHSS; Vanacker et al., 2016). Additionally, the Barthel Index (BI) was utilized to assess daily living ability and the Fugl-Meyer Assessment (FMA) was employed to evaluate motor function of the limbs (Wei et al., 2011; Kaushal et al., 2014).
Statistical analysis
Data were analyzed using SPSS 17.0 soware (SPSS, Chicago, IL, USA). Intergroup comparison was done by independent samplesttest or pairedttest. A mixed-ef f ect model was used to analyze the influence of confounding factors. The dependent variables were NIHSS, BI, and FMA.e fi xed effects were age, sex, course of disease, hypertension, diabetes, coronary heart disease, smoking, and drinking. Signif i cance was set atP< 0.05.
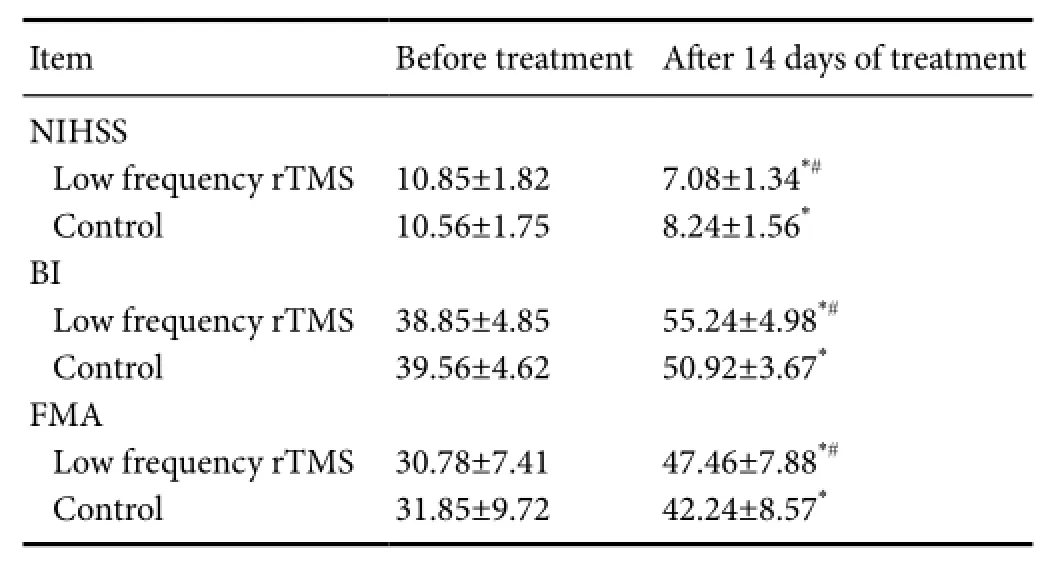
Table 1 Changes in NIHSS, BI, and FMA scores for each group
Results
Baseline data
None of the patients had new clinical symptoms. Before the experiment, NIHSS, BI, and FMA scores did not differ significantly between the two groups (P> 0.05; Figure 1 and Tables 1, 2).
Efect of low frequency rTMS on the recovery of motor function
Fourteen days after treatment, NIHSS scores significantly decreased, BI and FMA scores significantly increased in each group (P< 0.01). Further, NIHSS scores significantly decreased and BI and FM scores signif i canty increased in the low frequency rTMS group than in the control group (allP<0.05; Tables 1, 2).
Multivariate analysis results
The dependent variables were, NIHSS, BI, and FMA. The analysis showed that none of the factors (age, gender, duration, hypertension, diabetes, coronary heart disease, smoking, or drinking) signif i cantly af f ected FMA, NIHSS and BI scores (P> 0.05).
Adverse reaction
None of the patients had any severe adverse reactions such as recurrent stroke or seizures. One individual in the low frequency rTMS group experienced dizziness, but the symptoms disappeared soon aer treatment.
Discussion

Table 2 Multivariate analysis of factors that might infuence post-treatment motor function (Pvalue)
Low frequency stimulation of the contralateral hemisphere not only can reduce the excitability of the contralateral cortex, but also can enhance the effect of functional exercise.us, all kinds of exercise training may improve motor function in patients with cerebral infarction. Kakuda et al. (2011) used low frequency rTMS (1 Hz) combined with occupational therapy in post stroke hemiplegic patients with spastic upper extremity, and found that the therapy promoted sports recovery and improved limb spasticity. In the current study, we combined low frequency (1 Hz) rTMS, delivered at 90% of motor threshold (1,800 pulses) to contralateral M1 with daily rehabilitation training. Our results showed that aer 14 days of treatment, neurological function improved significantly in rTMS group than in the control group. However, Nichols-Larsen et al. (2005) reported that rTMS did not enhance the therapeutic ef f ect of exercise therapy, possibly because of severe movement disorders in patients; in the shortterm, the protocol was not enough to signif i cantly improve motor function.
Lefaucheur (2006) believed that rTMS could promote the recovery of motor function in patients with cerebral infarction, but the duration of improvement was short and was only observed during stimulation or a few minutes afterward. A relationship between the duration and dose of stimulation and the therapeutic ef f ect of rTMS could explain this result. However, many other studies have conf i rmed that rTMS also produces signif i cant long-term ef f ects (Fitzgerald et al., 2006; Chang et al., 2010). Khedr et al. (2010) studied the long-term ef f ects of two different rTMS frequencies on motor stroke and found that rTMS stimulation signif i cantly improved motor function.
Here, the long-term efficacy of rTMS has been conf i rmed. Because the brain is a functional network, the absence of motor function after cerebral infarction is not only associated with the local impairments that are directly related with the affected side or its related corticospinal tract, but it is also associated with corticospinal tract integrity and the whole brain. When local brain damage occurs, the network of non-damaged areas is activated, and compensation can gradually occur to alleviate the def i cits. different brain regions, such as dorsal premotor cortex, ventral premotor cortex, supplementary motor area, and top posterior cortex, contribute to the recovery of motor function. Moreover, rTMS promotes the functional reconstruction of the brain neural network, and plays a lasting regulatory role in modulating cortical excitability at the stimulation site and remote areas (Gilio et al., 2003; Quartarone et al., 2005).
In conclusion, low frequency rTMS is useful because it is painless, safe to use, convenient, and because it facilitates motor function recovery in patients with ischemic stroke.
Declaration of patient consent:Te authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. Te patients understand that their names and initials will not be published and due ef f orts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgments:we would kike to thank all the doctors from the Rehabilitation Department of Xuanwu Hospital of China for their help in this publication.
Author contributions:WQS designed this study and revised the paper. ZYM performed experiments, analyzed data and wrote the paper. Both authors approved the fnal version of the paper.
Conficts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:Tis paper was double-blinded and stringently reviewed by international expert reviewers.
Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PK (2010) Longterm effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med 42:758-764.
Feng JY, Shan L, Wang B, Jia FY (2016) Ef f ects of ultra-low frequency transcranial magnetic stimulation on motor function and intelligence of children with spastic cerebral palsy: study protocol for a randomized parallel-cohort controlled trial. Asia Pac J Clin Trials Nerv Syst Dis 1:25-30.
Fitzgerald PB, Fountain S, Daskalakis ZJ (2006) A comprehensive review of the ef f ects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117:2584-2596.
Gao F, Wang S, Guo Y, Wang J, Lou M, Wu J, Ding M, Tian M, Zhang H (2010) Protective ef f ects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: a microPET study. Eur J Nucl Med Mol Imaging 37:954-961.
Gilio F, Rizzo V, Siebner HR, Rothwell JC (2003) Ef f ects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol 551:563-573.
Hara T, Abo M, Kakita K, Masuda T, Yamazaki R (2016) Does a combined intervention program of repetitive transcranial magnetic stimulation and intensive occupational therapy af f ect cognitive function in patients with post-stroke upper limb hemiparesis? Neural Regen Res 11:1932-1939.
Hendricks HT, Zwarts MJ, Plat EF, van Limbeek J (2002) Systematic review for the early prediction of motor and functional outcome after stroke by using motor-evoked potentials. Arch Phys Med Rehabil 83:1303-1308.
Hiscock A, Miller S, Rothwell J, Tallis RC, Pomeroy VM (2008) Informing dose-finding studies of repetitive transcranial magnetic stimulation to enhance motor function: a qualitative systematic review. Neurorehabil Neural Repair 22:228-249.
Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, Ito H, Tominaga A, Umemori T, Kameda Y (2011) Anti-spastic effect of low-frequency rTMS applied with occupational therapy in poststroke patients with upper limb hemiparesis. Brain Inj 25:496-502.
Kaushal K (2014) Using Barthel index for assessment of disability: a comment on functional disability among elderly persons in a rural area of Haryana. Indian J Public Health 58:284.
Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AA (2010) Longterm ef f ect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand 121:30-37.
Lefaucheur JP (2006) Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS). Neurophysiol Clin 36:105-115.
Liu P, Liu BB (2015) Repetitive transcranial magnetic stimulation in a rat model of middle cerebral artery occlusion: variation of nerve regeneration microenvironment in infarcted brain areas and recovery of rat neurological function. Zhongguo Zuzhi Gongcheng Yanjiu 19:4333-4338.
Murase N, Duque J, Mazzocchio R, Cohen LG (2004) Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55:400-409.
Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S (2005) Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke 36:1480-1484.
Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant’angelo A, Battaglia F, Messina C, Siebner HR, Girlanda P (2005) Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res 161:114-124.
Ramakrishna R, Kim L (2010) Transcranial magnetic stimulation may provide benef i t in acute ischemic stroke. World Neurosurg 73:427-428.
Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG (2008) Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586:325-351.
Rodger J, Sherrard RM (2015) Optimising repetitive transcranial magnetic stimulation for neural circuit repair following traumatic brain injury. Neural Regen Res 10:357-359.
Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008-2039.
Vanacker P, Heldner MR, Amiguet M, Faouzi M, Cras P, Ntaios G, Arnold M, Mattle HP, Gralla J, Fischer U, Michel P (2016) Prediction of large vessel occlusions in acute stroke: national institute of health stroke scale is hard to beat. Crit Care Med 44:e336-343.
Wei XJ, Tong KY, Hu XL (2011) The responsiveness and correlation between Fugl-Meyer Assessment, Motor Status Scale, and the Action Research Arm Test in chronic stroke with upper-extremity rehabilitation robotic training. Int J Rehabil Res 34:349-356.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Wei-qun Song, songwq666@163.com.
Wei-qun Song, songwq666@163.com.
orcid: 0000-0002-5053-5084 (Wei-qun Song)
10.4103/1673-5374.205100
Accepted: 2017-03-07
杂志排行
中国神经再生研究(英文版)的其它文章
- RhoA as a target to promote neuronal survival and axon regeneration
- The complexities underlying age-related macular degeneration: could amyloid beta play an important role?
- The reasons for end-to-side coaptation: how does lateral axon sprouting work?
- Axon degeneration: make the Schwann cell great again
- Recovery of multiply injured ascending reticular activating systems in a stroke patient
- Phosphatidylserine improves axonal transport by inhibition of HDAC and has potential in treatment of neurodegenerative diseases
