Diffusion tensor imaging assesses white matter injury in neonates with hypoxic-ischemic encephalopathy
2017-05-03hongxinLiXingFengQianwangXuanDongMinYuwenjuanTu
hong-xin Li, Xing Feng, Qian wang, Xuan Dong, Min Yu, wen-juan Tu
1 Department of Neonatology, Children’s Hospital of Soochow University, Suzhou, Jiangsu Province, China
2 Department of Neonatology, Changzhou Children’s Hospital, Changzhou, Jiangsu Province, China
3 School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
4 Department of Children’s Health Research Center, Changzhou Children’s Hospital, Changzhou, Jiangsu Province, China
5 Nantong University, Nantong, Jiangsu Province, China
Diffusion tensor imaging assesses white matter injury in neonates with hypoxic-ischemic encephalopathy
hong-xin Li1,2, Xing Feng1,*, Qian wang3, Xuan Dong4, Min Yu5, wen-juan Tu2
1 Department of Neonatology, Children’s Hospital of Soochow University, Suzhou, Jiangsu Province, China
2 Department of Neonatology, Changzhou Children’s Hospital, Changzhou, Jiangsu Province, China
3 School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
4 Department of Children’s Health Research Center, Changzhou Children’s Hospital, Changzhou, Jiangsu Province, China
5 Nantong University, Nantong, Jiangsu Province, China
How to cite this article:Li HX, Feng X, Wang Q, Dong X, Yu M, Tu WJ (2017) diffusion tensor imaging assesses white matter injury in neonates with hypoxic-ischemic encephalopathy. Neural Regen Res 12(4):603-609.
Open access statement:Tis is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:Tis study was supported by a grant from the Clinical Medicine Science and Technology Projects in Jiangsu Province of China, No. BL2014037; a grant from the Changzhou City Science and Technology Support Plan in China, No. CE20165027; a grant from the Changzhou Health Development Planning Commission Major Projects in China, No. ZD201515; the Changzhou High-Level Health Personnel Training Project Funding.
Graphical Abstract
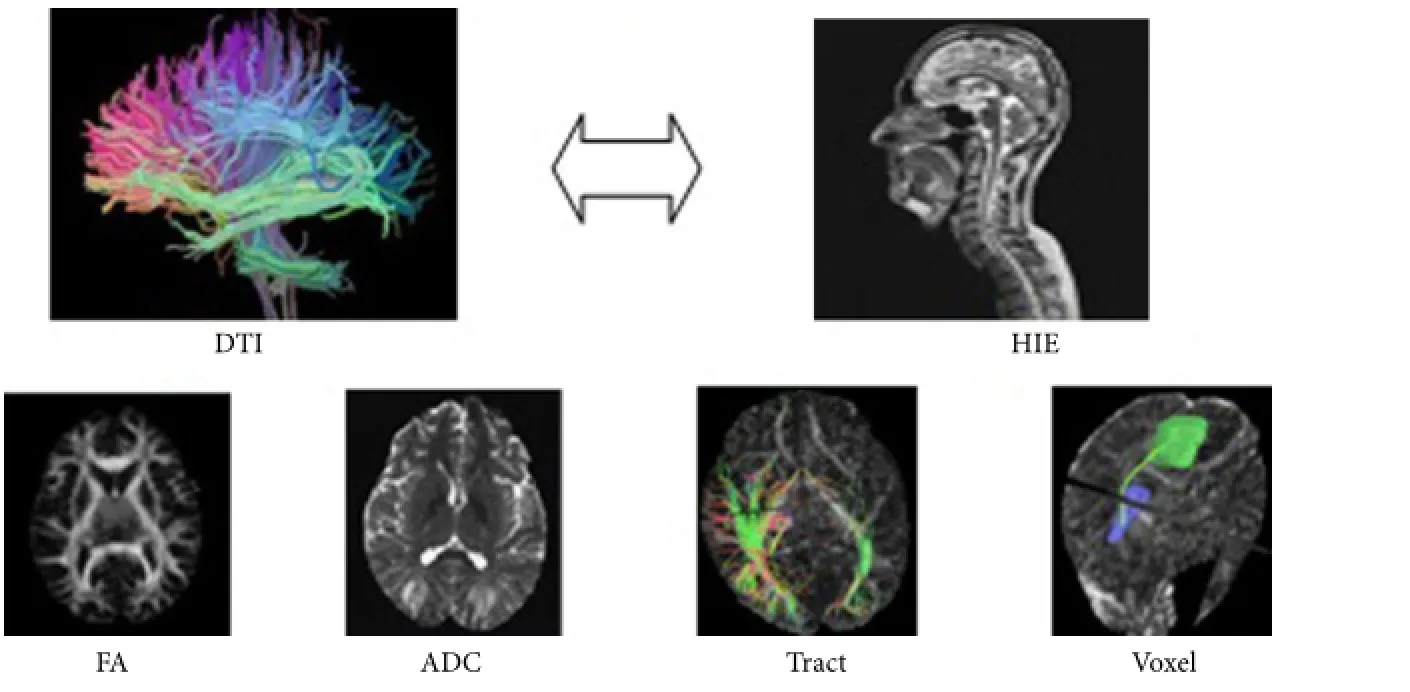
A diffusion tensor imaging (DTI) study
With improvements in care of at-risk neonates, more and more children survive.is makes it increasingly important to assess, soon aer birth, the prognosis of children with hypoxic-ischemic encephalopathy. Computed tomography, ultrasound, and conventional magnetic resonance imaging are helpful to diagnose brain injury, but cannot quantify white matter damage. In this study, ten full-term infants without brain injury and twenty-two full-term neonates with hypoxic-ischemic encephalopathy (14 moderate cases and 8 severe cases) underwent diffusion tensor imaging to assess its feasibility in evaluating white matter damage in this condition. Results demonstrated that fractional anisotropy, voxel volume, and number of fi ber bundles were different in some brain areas between infants with brain injury and those without brain injury.e correlation between fractional anisotropy values and neonatal behavioral neurological assessment scores was closest in the posterior limbs of the internal capsule. We conclude that diffusion tensor imaging can quantify white matter injury in neonates with hypoxic-ischemic encephalopathy.
nerve regeneration; fractional anisotropy; diffusion tensor imaging; apparent diffusion coefficient; voxel volume; neonatal behavioral neurological assessment; brain injury; white matter; neuroimaging; neural regeneration
Introduction
For many years, much attention has been given to improving the assessment of the prognosis of brain injury in hypoxic-ischemic encephalopathy (HIE) children. The incidence of HIE is still high (AbdelAziz, et al., 2017). The success rate of rescue in moderate and severe HIE has signif i cantly improved (Pf i ster and Soll, 2010). Objective and sensitive indicators are needed to judge the prognosis. Head computed tomography (CT), ultrasound, and conventional magnetic resonance imaging (MRI) are helpful to diagnose brain injury, but cannot quantify the degree of white matter damage to arrive at a prognosis (Coleman et al., 2013; Jose et al., 2013; Duong and Watts, 2016). Conventional MRI reveals that the two kinds of brain injury in HIE children are basal ganglia and thalamic abnormalities and watershed damage, which are strongly associated with late motor and cognitive deficits (Okumura et al., 2008). However, children with obvious motor and cognitive abnormalities oen have unremarkable scans (Aridas et al., 2014; Krishnan and Shrof f, 2016). It is vital to fi nd methodology capable of detecting white matter abnormalities in HIE children, who have a high risk of neurological sequelae. We explored the combined use of MRI and diffusion tensor imaging (DTI) to improve identif i cation of CNS abnormalities.
DTI measures water molecule diffusion. It has great advantages in studying the integrity and orientation of white matter in normal and pathological states (Baldoli et al., 2015). Fractional anisotropy (FA) values and apparent diffusion coefficient (ADC) values in different brain regions can quantify the number and integrity of white matter fibers, detect white matter damage, and have been shown to dynamically visualize white matter repair (Pfef f erbaum et al., 2014). DTI is a refinement of diffusion-weighted magnetic resonance imaging (DWI) (de Vries et al., 2011). A previous study showed that DWI in the acute stage of HIE (≤ 7 days) predicted the degree of brain damage; in the subacute stage (1–3 weeks), DWI showed pseudo-normalization, so DWI might underestimate the extent of damage to the basal ganglia and thalamus (Cavalleri et al., 2014). DTI is capable of applying a diffusion gradient magnetic fi eld in at least six directions, can accurately determine the distribution of nerve fiber bundles and anisotropic characteristics of tissue, and is the best imaging method to ref l ect the structural integrity and directionality of white matter (Hüppi and Dubois, 2006). DTI has been used to study neuroregeneration and neurodegeneration, which have been confirmed by pathology or experimental results (Kim et al., 2015). It is thought that DTI can reflect HIE-induced white matter damage, and is certainly correlated with the severity of disease and prognosis (Lee et al., 2012). We also sought to assess the prognosis of neonatal HIE.
Subjects and Methods
Subjects
MRI scan
All subjects were given 10% chloral hydrate 0.25–0.50 mL/kg by nasal feeding or enema, and subjected to scan aer sleeping soundly. Scans were performed on a 3.0 T MRI scanner (Philips Achieva, Philips Inc., Rotterdam, the Netherlands) with a head matrix coil. Sequences were as follows: Fast low-angle shot (FLASH) T1-weighted images (T1WI), (repetition time 9.3 milliseconds, echo time 4.4 milliseconds, fi eld of view 180 mm × 180 mm, matrix 0.9 mm × 0.9 mm, section thickness/intersection gap 4.5 mm/0.5 mm, number of excitations 2), turbo spin echo (TSE) T2-weighted images (T2WI) (repetition time 2,651 milliseconds, echo time 105 milliseconds × 0.74 mm, section thickness/intersection gap 4.5 mm/0.5 mm, number of excitations 2), turbo inversion recovery magnitude (T2 tirm) dark-fluid (repetition time 7,800 milliseconds, echo time 89 milliseconds, fi eld of view 180 mm × 180 mm, matrix 0.9 mm × 1.1 mm, section thickness/intersection gap 4.5 mm/0.5 mm, number of excitations 2). DTI used scanning with a single-shot, spin-echo, echo-planar sequence (repetition time 4,104 milliseconds, echo time 60 milliseconds, fi eld of view 180 mm × 180 mm, matrix 1.8 mm × 1.8 mm, b value 1,000 seconds/mm2, diffusion sensitive gradient direction (diffusion directions) for 12, section thickness/intersection gap 2 mm/0 mm, incentive times 4). The scan baseline and the number of scan layers were the same for all sequences.
Image processing
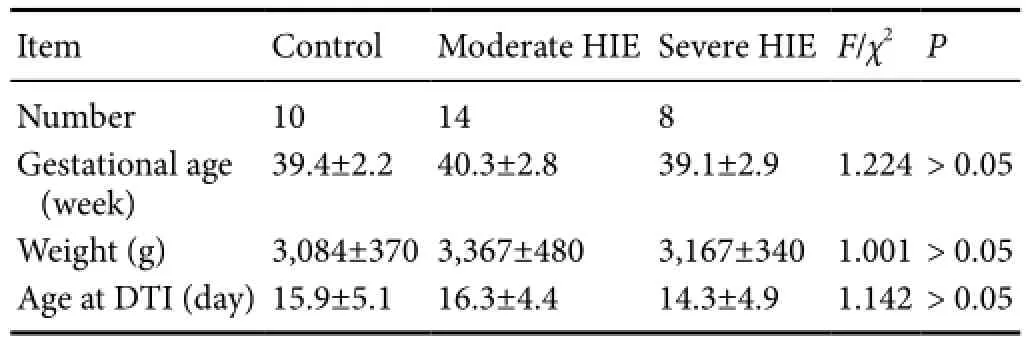
Table 1 Baseline characteristics of HIE and control participants

Table 2 ADC values (× 10–3mm2/s) in different brain areas of normal, moderate, and severe HIE patients
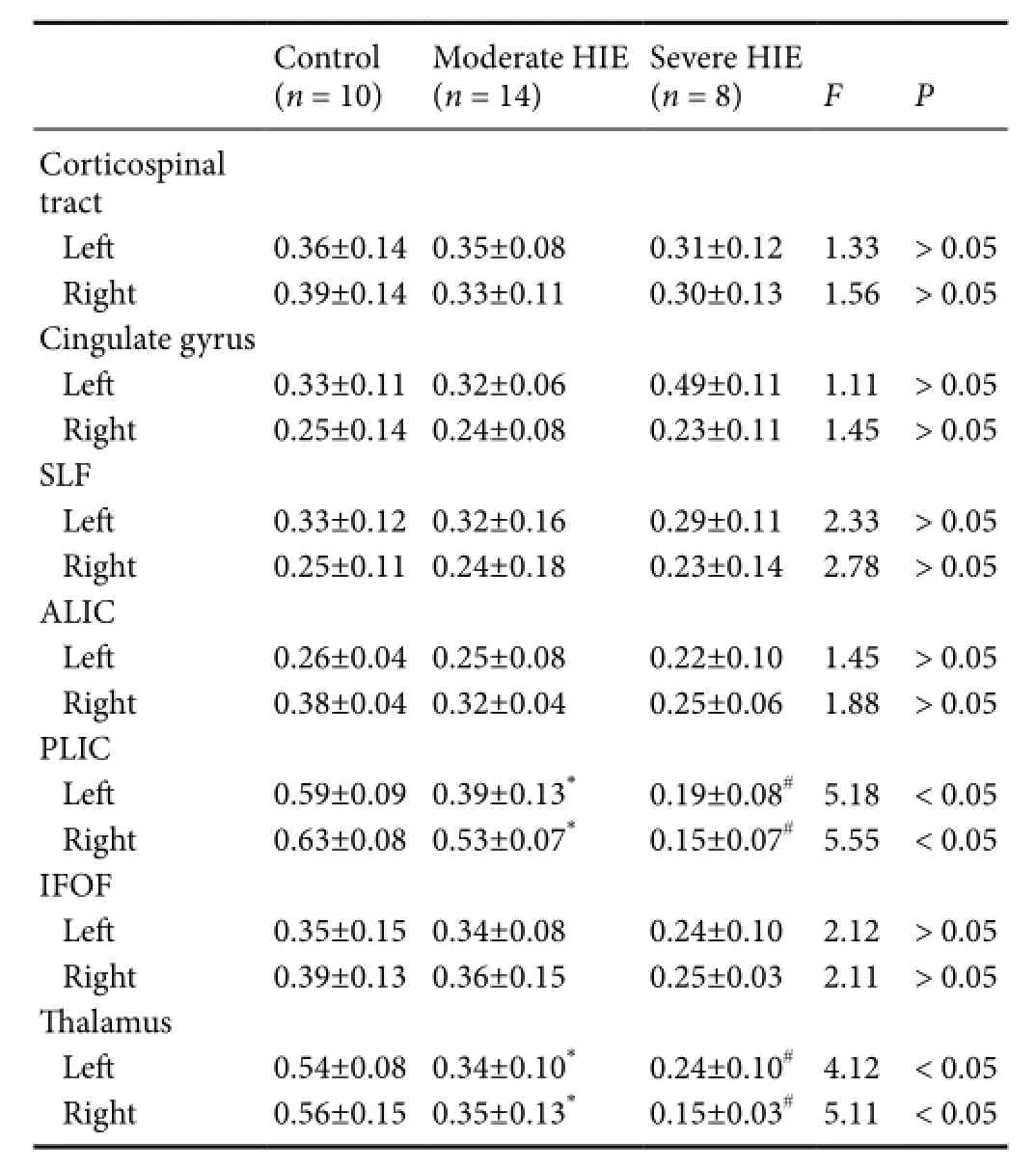
Table 3 FA values in different brain areas of normal, moderate, and severe HIE patients
Prognostication
The neonatal behavioral neurological assessment (NBNA) score (Bao, 1995) was calculated at age 15 days by a doctor. The content of NBNA (5 parts 20 items) includes behavioral ability (6), active and passive muscle tension (8 and 4, respectively), primitive ref l ex (3) and evaluation of general condition (3) for a total of 20 items, each item being scored 0, 1, or 2. Infants scoring > 35 were graded as “normal,”and those with a score < 35 were graded as “abnormal.” All patients were assessed at an ambient temperature of 24–28 °C in dim light and in a quiet environment. Each check was completed within 10 minutes.
Statistical analysis
The data, expressed as the mean ± SD, were analyzed with SPSS 17.0 soware (SPSS, Chicago, IL, USA).eF-test was used to analyze DTI parameters, including ADC and FA values in the ROIs, and voxel volume, as well as the integrity and quantity of white matter fi bers in DTT images, among the three groups. FA values and NBNA scores were analyzedwith Pearson correlation analysis. Receiver operating characteristic (ROC) curves were used to evaluate FA values in predicting the sensitivity and specif i city of the prognosis in HIE children. A value ofP< 0.05 was considered statistically signif i cant.

Table 4 Voxel volume in different brain areas of normal, moderate, and severe HIE patients

Table 5 Tract numbers in different brain areas of normal, moderate, and severe HIE patients
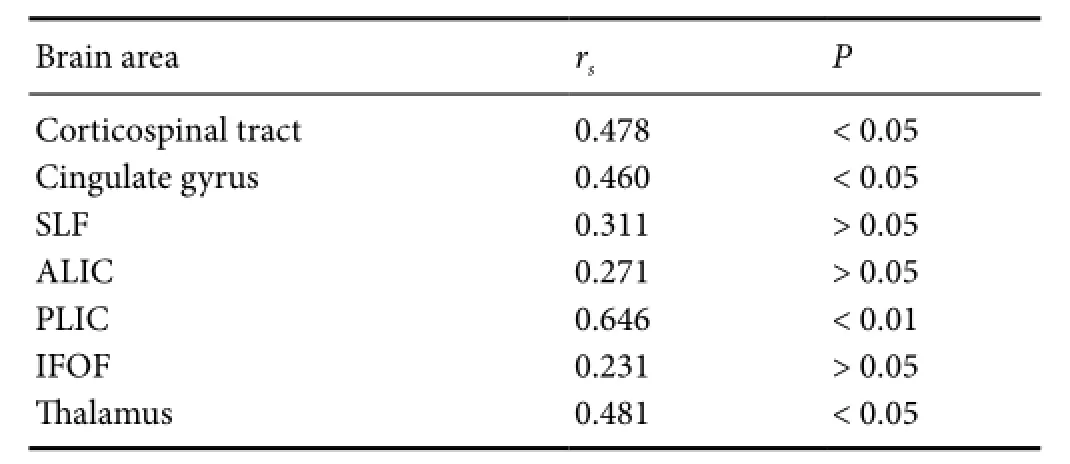
Table 6 Correlation between FA value and NBNA scores in different brain areas
Results
DTI parameters in normal, moderate and severe HIE patients
The ADC values in normal, moderate and severe HIE patients were not signif i cantly different (P> 0.05; Table 2).
FA values in the posterior limbs of the internal capsules and in the thalami showed statistically signif i cant differences between the moderate and severe HIE groups (Fvalue respectively 5.18/5.55 4.12/5.11P< 0.05), and were signif icantly different between the control and moderate groups (P< 0.05; Table 3).
Voxel volumes in the superior longitudinal fasciculi, posterior limbs of the internal capsules and anterior limbs of the internal capsules showed statistically signif i cant differences between the moderate and severe HIE groups (P< 0.05).ere was no statistically signif i cant difference between the control and moderate HIE groups (P> 0.05; Table 4).
DTT parameters in normal, moderate, and severe HIE patients
The fiber numbers in the posterior limbs of the internal capsules, cingulate gyri, superior longitudinal fasciculi and inferior fronto-occipital fasciculi were signif i cantly different between the moderate and severe HIE groups (P< 0.05), but not signif i cantly different between the control and moderate HIE groups (P> 0.05; Table 5, Figure 1).
Correlation between FA value and NBNA scores

Figure 1 diffusion tensor tractography of a normal infant, a moderate HIE infant and a severe HIE infant.

Figure 2 Receiver operating characteristic curve of diagnosis in posterior limb of internal capsule fractional anisotropy behavioral values and neonatal neurological assessment scores.
All 10 cases of the control group had an NBNA score ≥ 35; the moderate HIE group included 3 cases scoring < 35, and 11 cases scoring ≥ 35; in the severe HIE group, all NBNA scores were < 35 (31.1 ± 1.6).F-test results showed that NBNA scores were significantly different among the three groups, with the severe HIE group having the lowest scores (P< 0.01).e correlation coefficient in the posterior limbs of the internal capsules was 0.646, higher than in other areas by Pearson relative analysis (Table 6).
Discussion
The fiber numbers in the corticospinal tracts, inferior fronto-occipital fasciculi, anterior limbs of the internal capsules, and thalami were signif i cantly different between the moderate and severe HIE groups, but were not signif icantly different between the control and moderate HIE groups. The injury to the integrity of white matter fibers means that the corresponding function may be affected. For example, corticospinal tract damage will af f ect motor function of the limbs.e superior longitudinal fasciculi, inferior longitudinal fasciculi, and frontooccipital fasciculi belong to long association fi bers in the cerebral cortex and connect various areas of the cerebral cortex, so their injuries may af f ect cognitive, sensory, and motor functions.e inferior longitudinal fasciculi and frontooccipital fasciculi were damaged in the mild and moderate HIE groups, mainly af f ecting the temporal lobe and occipital lobe, and auditory, olfactory, taste, and language centers (Li et al., 2016a). Nevertheless, the corticospinal tract was the chief site of damage in the severe HIE group, affecting motor function. This is matched with deductible item of NBNA scores and has some guiding signif i cance for the evaluation of prognosis.
Correlation between FA values and NBNA scores in the moderate and severe HIE groups demonstrated that the rank correlation coef ficientrsof FA values and NBNA scores in the posterior limbs of the internal capsules was higher than that in the other regions.is may be associated with closely arranged white matter fi bers, white matter fi bers arranged in parallel, myelinization at birth, and are easily detected by DTI aer injury (Li et al., 2015). Moreover, these locations contain important white matter projection fibers associated with neuromotor function, and the anatomical location is easy to identify and measure. However, we did not obtain a threshold with a high sensitivity and specif i city, which is possibly associated with the small sample size.
In summary, FA values, voxel volume, and number of fi ber bundles in some ROIs quantitatively ref l ected white matter injury in neonates with HIE. The changes in DTI parameters were most obvious in the posterior limbs of the internal capsules, and may allow accurate and objective assessment of the degree of white matter injury in children with HIE. Among these parameters, the FA values in the posterior limbs of the internal capsules closely correlated with NBNA scores. DTI can be carried out in a single individual, so it has important clinical significance, and can accurately and objectively assess the prognosis.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. Te patients understand that their names and initials will not be published and due ef f orts will be made to conceal their identity, but anonymity cannot be guaranteed.
Author contributions:XF contributed to defnition of intellectual content of this topic and paper review. HXL was responsible for literature retrieval and designed the study. QW prepared and edited the paper. HXL, XD, MY and WJT participated in data acquisition. HXL, XF and QW were in charge of data analysis. MY did statistical analysis. All authors approved the fnal version of the paper.
Conf l icts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:Tis paper was double-blinded and stringently reviewed by international expert reviewers.
AbdelAziz NH, AbdelAzeem HG, Monazea EM, Sherif T (2017) Impact of thrombophilia on the risk of hypoxic-ischemic encephalopathy in term neonates. Clin Applromb Hemost 23:266-273.
Aridas JD, Yawno T, Sutherland AE, Nitsos I, Ditchf i eld M, Wong FY, Fahey MC, Malhotra A, Wallace EM, Jenkin G, Miller SL (2014) Detecting brain injury in neonatal hypoxic ischemic encephalopathy: Closing the gap between experimental and clinical research. Exp Neurol 261:281-290.
Baldoli C, Scola E, Della Rosa PA, Pontesilli S, Longaretti R, Poloniato A, Scotti R, Blasi V, Cirillo S, Iadanza A, Rovelli R, Barera G, Scifo P (2015) Maturation of preterm newborn brains: a fMRI–DTI study of auditory processing of linguistic stimuli and white matter development. Brain Struct Funct 220:3733-3751.
Bao XL (1995) Neonatal Behavior and Education of 0–3 Years Old. Beijing: China Children Publishing Press.
Cavalleri F, Lugli L, Pugliese M, D’Amico R, Todeschini A, Della Casa E, Gallo C, Frassoldati R, Ferrari F (2014) Prognostic value of diffusion-weighted imaging summation scores or apparent diffusion coef ficient maps in newborns with hypoxic-ischemic encephalopathy. Pediatr Radiol 44:1141-1154.
Coleman MB, Glass P, Brown J, Kadom N, Tsuchida T, Scaf i di J, Chang T, Vezina G, Massaro AN (2013) Neonatal neurobehavioral abnormalities and MRI brain injury in encephalopathic newborns treated with hypothermia. Early Hum Dev 89:733-737.
de Vries LS, van Haastert IC, Benders MJ, Groenendaal F (2011) Myth: cerebral palsy cannot be predicted by neonatal brain imaging. Semin Fetal Neonatal Med 16:279-287.
Dopwell F, Maypole J, Sinha B, Currier H, DeBassio W, Augustyn M (2017) “More than Meets the Eye”: When the neonatal course may impact several years out. J Dev Behav Pediatr 38 Suppl 1:S44-S46.
Duong TQ, Watts LT (2016) A brief report on MRI investigation of experimental traumatic brain injury. Neural Regen Res 11:15-17.
Guo L, Wang D, Bo G, Zhang H, Tao W, Shi Y (2016) Early identif i cation of hypoxic-ischemic encephalopathy by combination of magnetic resonance (MR) imaging and proton MR spectroscopy. Exper Med 12:2835-2842.
Hüppi PS, Dubois J (2006) diffusion tensor imaging of brain development. Semin Fetal Neonatal Med 11:489-497.
Jose A, Matthai J, Paul S (2013) Correlation of EEG, CT, and MRI brain with neurological outcome at 12 months in term newborns with hypoxic ischemic encephalopathy. J Clin Neonatol 2:125-130.
Kim JH, Kwon YM, Son SM (2015) Motor function outcomes of pediatric patients with hemiplegic cerebral palsy aer rehabilitation treatment: a diffusion tensor imaging study. Neural Regen Res 10:624-630.
Krishnan P, Shrof f M (2016) Neuroimaging in neonatal hypoxic ischemic encephalopathy. Indian J Pediatr 83:995-1002.
Lee AY, Shin DG, Park JS, Hong GR, Chang PH, Seo JP, Jang SH (2012) Neural tracts injuries in patients with hypoxic ischemic brain injury: diffusion tensor imaging study. Neurosci Lett 528:16-21.
Li HX, Tu WJ, Gao M, Jiang KH, Dong X (2014) Application research of resting state functional magnetic resonance imaging in newborn brain damage. Zhonghua Shiyong Erke Linchuang Zazhi 29:386-389.
Li HX, Feng X, Tu WJ, Gao M, Jiang KH, Dong X (2016a) Application of resting state functional MRI in children with central nervous and psycholoical diseases. Zhongguo Shiyong Erke Zazhi 31:150-154.
Li HX, Wang Q, Tu WJ, Gao M, Jiang KH, Lu YJ, Dong X (2015) Application of magnetic resonance diffusion tensor imaging technology in neonates with hypoxic-ischemic encephalopathy in the subacute stage. Zhonghua Shiyong Erke Linchuang Zazhi 30:1868-1872.
Li XY, Tang ZC, Sun Y, Tian J, Liu ZY, Han Y (2016b) White matter degeneration in subjective cognitive decline: a diffusion tensor imaging study. Oncotarget 7:54405-54414.
Okumura A, Hayakawa M, Tsuji T, Naganawa S, Watanabe K (2008) diffusion tensor imaging in infants with basal ganglia-thalamic lesions. Eur J Paediatr Neurol 12:412-416.
Pfef f erbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlf i ng T, Pohl KM, Zahr NM, Sullivan EV (2014) White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry 1:202-212.
Pfister RH, Soll RF (2010) Hypothermia for the treatment of infants with hypoxic-ischemic encephalopathy. J Perinatol 30 Suppl:S82-87.
Rutherford MA, Pennock JM, Counsell SJ, Mercuri E, Cowan FM, Dubowitz LM, Edwards AD (1998) Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 102:323-328.
The Subspecialty Group of Neonatology Pediatric Society Chinese Medical Association (2005) Diagnostic criteria for neonatal hypoxic-ischemic encephalopathy. Zhonghua Erke Zazhi 43:584.
van Laerhoven H, de Haan TR, Offringa M, Post B, van der Lee JH (2013) Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: a systematic review. Pediatrics 131:88-98.
Winter JD, Lee DS, Hung RM, Levin SD, Rogers JM, Thompson RT, Gelman N (2007) Apparent diffusion coefficient pseudonormalization time in neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol 37:255-262.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Xing Feng, M.D., xing_feng66@hotmail.com.
Xing Feng, M.D., xing_feng66@hotmail.com.
orcid: 0000-0003-0519-3780 (Xing Feng)
10.4103/1673-5374.205102
Accepted: 2017-02-13
杂志排行
中国神经再生研究(英文版)的其它文章
- RhoA as a target to promote neuronal survival and axon regeneration
- The complexities underlying age-related macular degeneration: could amyloid beta play an important role?
- The reasons for end-to-side coaptation: how does lateral axon sprouting work?
- Axon degeneration: make the Schwann cell great again
- Recovery of multiply injured ascending reticular activating systems in a stroke patient
- Phosphatidylserine improves axonal transport by inhibition of HDAC and has potential in treatment of neurodegenerative diseases
