地上和地下植食者互作对水稻氮分配及土壤活性氮的影响*
2017-04-19郭瑞华罗琌张腾昊2刘满强陈小云
郭瑞华罗 琌张腾昊,2刘满强陈小云†胡 锋
(1 南京农业大学资源与环境科学学院土壤生态实验室,南京 210095)
(2 河北省环保产品质量监督检验院,石家庄 050000)
地上和地下植食者互作对水稻氮分配及土壤活性氮的影响*
郭瑞华1罗 琌1张腾昊1,2刘满强1陈小云1†胡 锋1
(1 南京农业大学资源与环境科学学院土壤生态实验室,南京 210095)
(2 河北省环保产品质量监督检验院,石家庄 050000)
植物茎叶和根系植食者虽然在空间上隔离,但二者的相互作用被认为是联系地上和地下部生态系统的基础。土壤氮素有效性通过植物影响植食者已得到大量研究的证实,但有关地上和地下部植食者互作对土壤氮素影响的研究却鲜有报道。以水稻褐飞虱和潜根线虫分别作为地上和地下的植食者,采用两因素交互试验设计,两个褐飞虱水平(未接种褐飞虱、接种褐飞虱),两个潜根线虫水平(未接种潜根线虫、接种潜根线虫),探讨二者的交互作用对水稻氮吸收和土壤活性氮(微生物生物量氮、可溶性有机氮以及铵态氮和硝态氮)的影响。结果表明,褐飞虱和潜根线虫相互抑制,二者的共存加剧了对水稻茎叶和根系生长的危害。褐飞虱未影响水稻茎叶和根系含氮量,而潜根线虫显著降低了水稻根系含氮量(p<0.05)。褐飞虱和潜根线虫对土壤活性氮的影响表现出强烈的交互作用,在未接潜根线虫的处理中,褐飞虱显著提高了微生物生物量氮含量(p< 0.05),显著降低了硝态氮含量(p< 0.05);潜根线虫显著影响了微生物生物量氮含量和土壤活性氮总量。总之,褐飞虱和潜根线虫的相互抑制关系对土壤活性氮的影响格局较为复杂,相比褐飞虱,潜根线虫趋向于提高土壤活性氮水平,这可能影响与氮转化有关的土壤生态功能。
褐飞虱;潜根线虫;氮分配;微生物生物量氮;可溶性有机氮;矿质氮
在陆地生态系统中,地上部与地下部的生物通过植物作为桥梁而密切关联[1-3]。其中,植物与植食者的关系是联接生态系统地上和地下部的基石[2]。地上部植食者的取食会引起光合作用产物向根系转移,增加土壤中资源有效性;植食者引起的植物系统防御响应也会影响根系分泌物的组成,通过根系输入土壤的资源数量和质量的改变则进一步影响土壤生物群落和功能的发展[4]。虽然对地下部根系植食者关注较晚,但越来越多的证据表明根系植食者同样会通过宿主植物影响地上部的植食者[5-6]。迄今,虽然理论上推测地上部和地下部植食者存在普遍的关系,但是以往的研究往往集中在地上或地下部植食者,二者互作关系的研究仍较少。
植物化学组成和养分含量是影响植食者种群发展的关键因素。大量的研究表明,植物体元素组成尤其是氮素含量对地上部植食者有决定性作用[7-8]。相比之下,有关植食者特别是根系植食者对土壤养分有效性的影响却鲜见报道。理论上,地上部植食者的取食导致植物生长功能受损,因而根系对土壤养分的吸收能力减弱,土壤氮素有效性会相对增加;此外,地上部植食者的取食会促进根系分泌物的分泌,刺激微生物生长发育,加速土壤氮素的周转。因此,在地上部植食者的影响下,土壤氮素有效性增加或减少的可能性均存在[9-10]。对于根系植食者而言,除了有可能影响根系吸收及刺激微生物生长改变土壤氮水平外,根系损伤导致的含氮物质的泄漏势必也会引起土壤氮素有效性增加[11]。迄今对土壤氮素有效性在作物和地上/地下部害虫直接影响下的变化了解甚少。实际上,研究土壤氮素有效性在植食者作用下的改变对于进一步了解植食者互作机制,特别是植食者互作引发的一系列后续效应,如对植物之间竞争、土壤生物群落的影响及对地上部的反馈作用均有重要意义。
水稻是我国重要的粮食作物,褐飞虱(Nilaparvata lugens Stål)和潜根线虫(Hirschmanniella oryzae)分别是水稻地上和地下的重要害虫。虽然褐飞虱与潜根线虫在自然条件下共存,并且均可能对水稻生长及土壤养分产生影响,但目前关于二者交互作用的研究尚未见报道。土壤活性氮(微生物生物量氮、可溶性有机氮以及铵态氮和硝态氮)是土壤生态系统中氮循环最活跃的部分,在土壤氮素转化和生态系统氮循环中具有重要的作用。因此,研究水稻害虫相互作用对水稻氮吸收及土壤氮的影响对于深入了解地上地下部植食者对土壤生态系统结构和功能的影响具有重要意义。
1 材料与方法
1.1 实验材料
盆栽所用土壤采自江西进贤红壤研究所内的双季稻田耕层(0~18 cm),土壤母质为第四纪红黏土,属于潴育型水稻土。鲜土过5 mm筛,剔除其中大中型土壤动物及根茬等,在121℃下灭菌;然后按1%比例接入原土配制的土壤菌悬液(水土比5∶1,孔径0.023 mm筛网滤去线虫),调节至60%田间含水量,室温黑暗培养60 d以恢复和稳定土壤微生物群落[12],获得除去线虫的原位土壤。培养后土壤的理化性质为:有机碳9.7 g kg-1,全氮1.7 g kg-1,有效磷38.3 mg kg-1,速效钾52.0 mg kg-1。
供试水稻品种为TN1(台中1号Taichung Native 1),褐飞虱(N. lugens)在室内以TN1水稻苗饲养数代备用,潜根线虫(H. oryzae)由江西进贤潴育型水稻土中挑选并经历多个世代富集而来[12]。
1.2 盆栽实验
采用两因素(褐飞虱和潜根线虫)完全交互的盆栽实验。褐飞虱处理包括2水平:未接褐飞虱(CK-N)和接种褐飞虱(N);潜根线虫处理2水平:未接潜根线虫(CK-H)和接种潜根线虫(H);每处理设4个重复。
实验用盆钵装入相当于1.0 kg干土的上述除去线虫的培养土壤,待水稻在营养液内育苗30 d后,挑选长势一致的水稻苗,每盆钵移栽8株,定期浇水保持盆钵内水面高度为1cm左右,盆钵在玻璃温室内随机排列并用60目纱网(80 cm×25 cm× 25 cm)罩住防虫。水稻移栽后15 d、25 d时,分别接潜根线虫(每盆钵500条,基于田间调查密度)和褐飞虱(每株10头,即每盆接80头4龄若虫[13]),对照不接虫。接褐飞虱10 d,植株显示出危害症状,则进行破坏性采样。
1.3 采样及分析方法
采样时将所有褐飞虱用吸虫器吸取至50 ml塑料离心管中。沿土表剪去水稻植株地上部分,并迅速分离根系与土壤,褐飞虱、茎叶、根系及土壤样品分别保存。
将采集的褐飞虱样品在60℃下烘48h后称量生物量并计数;对于与根系分离后的鲜土样,用浅盘法分离潜根线虫,并在体视显微镜(Motic SMZ-168,中国)下计数[14];将水稻茎叶和根系清洗干净分别称量鲜重后,放置于烘箱中105℃杀青2 h,60℃下烘72 h,然后取出称重。水稻茎叶和根系全氮用浓H2SO4-H2O2法消化并用半微量凯氏定氮法测定[15];土壤微生物生物量氮用氯仿熏蒸-K2SO4浸提法,滤液中氮含量测定用半微量凯氏定氮法,熏蒸土样与未熏蒸土样的有机氮差值除以转换系数(KN= 0.45)[16]即为微生物生物量氮;可溶性有机氮用超纯水浸提(土∶水=1∶5),过孔径0.45 μm的醋酸纤维素滤膜后,通过连续流动分析仪(Skalar,荷兰)测定;矿质氮用2 mol L-1KCl浸提(土∶水=1∶5)后,通过连续流动分析仪(Skalar,荷兰)测定。
1.4 数据分析
采用SPSS 20.0进行数据分析,分析前检验数据的正态分布及方差齐性,并在必要时利用对数转换。以多因素方差分析评估褐飞虱和潜根线虫对水稻和土壤变量的主效应和交互效应,以独立样本T检验分析有无褐飞虱的差异,均值的比较检验采用最小显著极差法(LSD)(p<0.05)。
2 结 果
2.1 褐飞虱和潜根线虫的变化
接入同等数量的褐飞虱后,与对照相比,潜根线虫显著降低了褐飞虱的生物量(图1A);与对照相比,褐飞虱的存在显著降低了潜根线虫的数量(图1B)。即地上部植食者褐飞虱与地下部植食者潜根线虫之间是互相抑制的作用。
2.2 褐飞虱和潜根线虫互作下水稻生长的变化
与无褐飞虱的对照相比,褐飞虱有降低水稻茎叶生物量的趋势(图2A);与无潜根线虫的对照相比,潜根线虫有降低水稻茎叶和根系生物量的趋势(表1,图2A,图2B)。
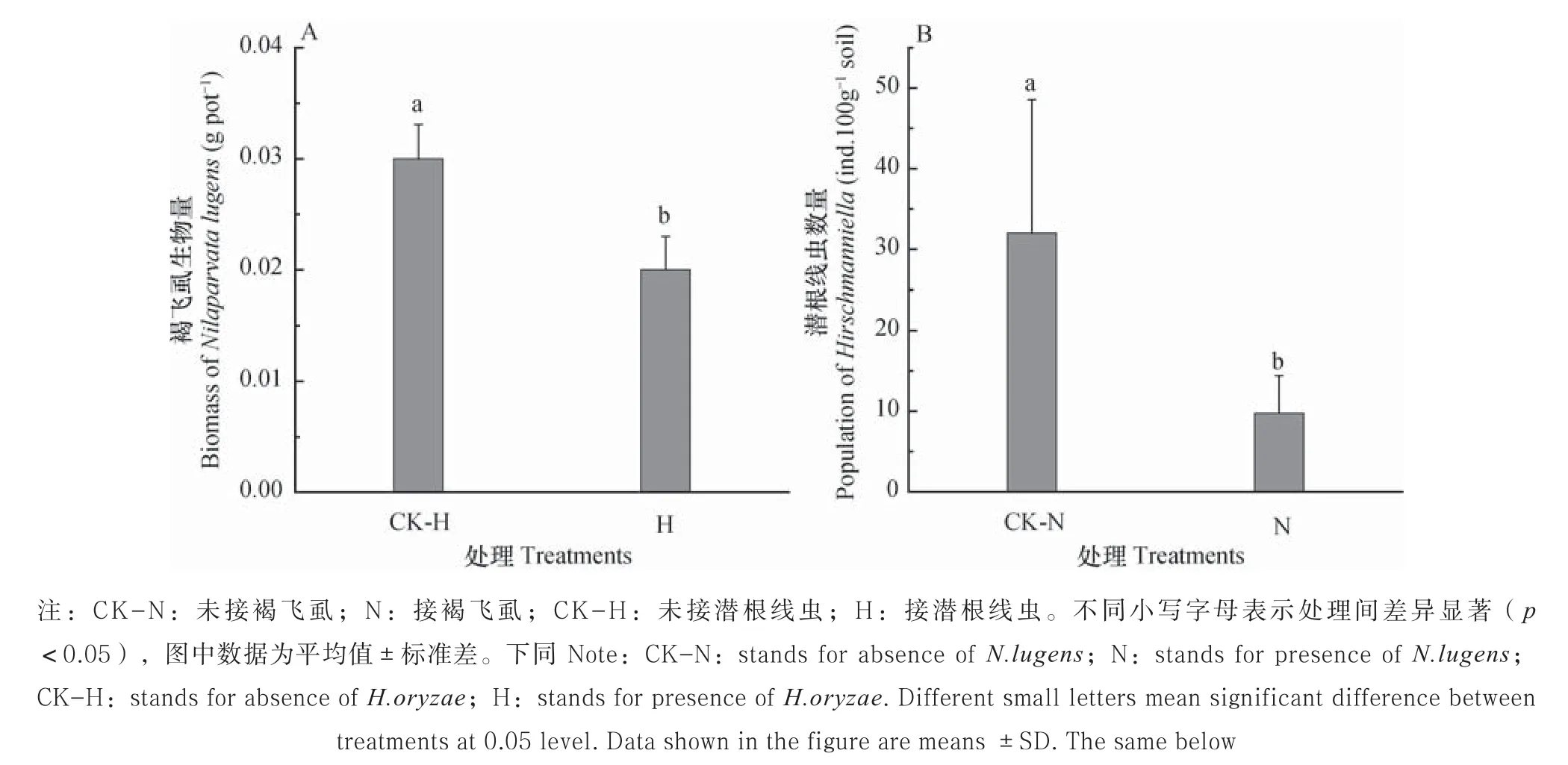
图1 褐飞虱生物量和潜根线虫数量的变化Fig. 1 Biomass of N. lugensand population of H. oryzae

表1 褐飞虱和潜根线虫互作对水稻性状和土壤活性氮影响的方差分析结果(F值)Table 1 ANOVA(F-values)of the effects of N. lugens and H. oryzae on rice performance and soil labile nitrogen

图2 褐飞虱与潜根线虫对水稻茎叶和根系生物量的影响Fig. 2 Effects of N. lugens and H. oryzae on rice shoot and root biomass
2.3 褐飞虱和潜根线虫互作下植株含氮量的变化
与无褐飞虱的对照相比,褐飞虱对水稻地上部和地下部含氮量的影响不显著(图3A,图3B);与无潜根线虫的对照相比,潜根线虫显著降低了水稻地下部含氮量(表1,图3B)。
2.4 褐飞虱和潜根线虫互作下土壤活性氮的变化
土壤微生物生物量氮和可溶性有机氮显著受到两种植食者的交互影响(表1),无潜根线虫时,与对照相比,褐飞虱提高了土壤微生物生物量氮和可溶性有机氮;而有潜根线虫时,褐飞虱对微生物生物量氮和可溶性有机氮的作用不明显(图4A,4B)。与无潜根线虫的对照相比,潜根线虫显著影响了微生物生物量氮(表1,图4A)。潜根线虫与褐飞虱的共同取食有提高土壤铵态氮含量的趋势(图4C)。无潜根线虫时,与无褐飞虱的对照相比,褐飞虱显著降低了土壤硝态氮含量(图4D)。总体上,相比褐飞虱,潜根线虫有提高土壤活性氮总量的趋势(表1,图5)。

图3 褐飞虱与潜根线虫对水稻茎叶和根系含氮量的影响Fig. 3 Effects of N. lugens and H. oryzae on nitrogen content in shoot and root of the rice plant

图4 褐飞虱和潜根线虫对土壤微生物生物量氮、可溶性有机氮、铵态氮和硝态氮的影响Fig. 4 Effects of N. lugens and H. oryzae on microbial biomass nitrogen,dissolved organic nitrogen,ammonium nitrogen and nitrate nitrogen
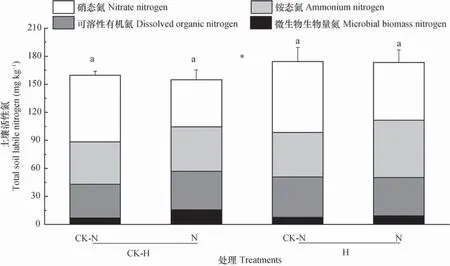
图5 褐飞虱和潜根线虫对土壤活性氮的影响Fig. 5 Effects of N. lugens and H. oryzae on soil labile nitrogen
3 讨 论
3.1 地上和地下植食者互作对水稻生物量和氮分配的影响
越来越多的研究表明地上和地下部生物虽然有距离上的分隔,但可通过宿主植物相互影响[17-18]。本研究结果发现,褐飞虱和潜根线虫存在相互抑制的作用,这与已有研究结果一致[19-20]。水稻受到潜根线虫的侵染后生物量减少,即对于褐飞虱而言,其食物资源减少,因此,与对照相比生长发育受到影响,生物量也随之下降。此外,根系植食者的取食可能提高了宿主植物茎叶内抗性物质如萜类物质和酚类物质的含量,或通过刺激植物挥发物质的释放,吸引地上部植食者的天敌,进而对地上部植食者产生了消极的作用[21]。潜根线虫数量的降低可能由于一方面褐飞虱的取食严重损伤了地上部植株,降低了水稻光合作用,减少了运输到根系的营养物质含量[22],另一方面可能是植食者的取食改变了根系分泌物的数量和质量,从而抑制了根系植食者[21]。
水稻在植株生长发育过程中根系主要吸收水分和养分,茎叶则主要进行光合作用。本研究结果显示,褐飞虱降低了水稻茎叶生物量,而潜根线虫同时降低了水稻茎叶和根系的生物量,这与已有研究结果一致[23-24]。褐飞虱可直接通过刺吸水稻茎叶组织的汁液,阻碍稻株体内水分和养分的运输,降低水稻同化作用,进而影响水稻的生长[25],也可间接通过影响根系分泌物的数量和质量,改变土壤微生物的群落结构和根际土壤有效养分[26],进而影响水稻的生长。潜根线虫的取食能直接危害水稻根系,降低根系对水分和养分的吸收功能,抑制水稻茎叶和根系的生长,也可间接通过根系损伤引起物质泄漏,促进根际土壤有效养分的增加,影响土壤微生物的群落结构和功能[27],进一步通过影响土壤养分的供应和吸收改变水稻的生长。
氮作为生物体必需大量元素之一,是核酸、蛋白质和叶绿体的组成元素。本研究结果显示,褐飞虱对水稻茎叶和根系氮含量的影响不明显,潜根线虫降低了水稻根系含氮量,这与潜根线虫抑制水稻生长的趋势一致,与已有研究结果一致[28]。有关植物对植食者耐受性的实验表明,植物受到侵害后会将新摄取的氮资源运移受害部位以减轻自身损害[29],但是植物对这部分资源的利用方式多样,如将资源储存起来,用于其他部位代谢性增长,产生抗性物质,或通过根系分泌到土壤中[30]。潜根线虫的取食会损伤水稻根系,可能导致含氮物质的泄漏[27]。根系分泌物的增加和含氮物质的泄漏都会刺激土壤微生物的发展[31],从而可能影响土壤微生物参与的矿化和硝化过程,进一步影响植物对养分的吸收。
3.2 褐飞虱和潜根线虫互作对土壤活性氮的影响
土壤微生物生物量氮是土壤中最活跃的氮库之一,转化较快、有效性高[32]。土壤可溶性有机氮代表了土壤生态系统中活跃的有机物组分,来自于外源有机物的分解和土壤有机质的微生物矿化过程,是土壤微生物可直接利用的主要氮素来源,也是土壤微生物代谢活动的中间产物[33]。本研究发现,褐飞虱与潜根线虫均有提高土壤微生物生物量氮的趋势,与已有研究结果一致[34]。这是因为一方面根系植食者的取食能够通过对植物根系的机械性损伤,引起根系内营养物质“渗漏”到土壤中,促进根际土壤有效养分的增加,另一方面植食者的取食可直接增加根系分泌物,促进根际土壤有效养分的增加和土壤生物群落的发展[35],也可诱发植物的耐受性反应,间接地促进其地上部营养物质向根系的分配,进一步促进了根际土壤资源和生物群落的发展[36]。褐飞虱与潜根线虫对土壤微生物生物量氮存在交互作用,这是因为在水稻已经受到潜根线虫侵害的情况下,褐飞虱的取食作用会加剧植食者对植物的负面影响,使其受到抑制,可能会减少根系分泌物数量,土壤生物活性也随之降低。
氮的转化尤其是有机氮向矿质氮的转化是生态系统中养分循环的重要过程。目前,已有研究发现,植食者对矿质氮的作用可以是积极的、中性的,也可以是消极的[9,35,37]。本研究中,褐飞虱和潜根线虫均影响了土壤矿质态氮水平。二者的取食作用有提高土壤铵态氮含量的趋势,这与已有研究结果一致[23],原因可能主要是植食者的取食降低了根系吸收能力。褐飞虱有降低土壤硝态氮含量的趋势,这与已有研究结果一致[35],由于土壤矿质氮受到多种因素的交互影响,特别是与植物吸收以及微生物的竞争和矿化能力有关,所以其中的机制还很难解释。植食者对土壤矿质氮作用的影响存在争议,可能与取食强度、植物种类及土壤生物群落结构等有关。总之,褐飞虱和潜根线虫对土壤活性氮各组分的影响存在差异,但总体上看,潜根线虫对土壤活性氮有促进趋势,其原因涉及复杂的氮素转化和植物吸收过程,今后需要利用氮同位素标记技术及对氮素不同形态的细致分析深入探究。
4 结 论
褐飞虱和潜根线虫之间表现出相互抑制作用,二者共存加剧了对水稻生长的危害。潜根线虫的取食显著降低了植株根系生物量和含氮量。褐飞虱和潜根线虫对土壤活性氮的影响格局较为复杂,相比褐飞虱,潜根线虫有促进土壤活性氮提高的趋势,这可能会促进以氮转化过程为主的土壤生态功能的发挥。今后需要采用同位素示踪技术和质谱技术进一步了解植食者作用下地上和地下部的养分转化与分配的联动机制。
[1]Wardle D A,Bardgett R D,Klironomos J N,et al. Ecological linkages between aboveground andbelowground biota. Science,2004,304(5677):1629—1633
[2]Bardgett R D,Wardle D A. Aboveground-belowground linkages. New York:Oxford University Press,2010
[3]Bardgett R D,van der Putten W H. Belowground biodiversity and ecosystem functioning. Nature,2014,515(7528):505—511
[4]Bardgett R D,Wardle D A. Herbivore-mediated linkages between aboveground and belowground communities. Ecology,2003,84(9):2258—2268
[5]Kafle D,Krähmer A,Naumann A,et al. Genetic variation of the host plant species matters for interactions with above-and belowground herbivores. Insects,2014,5(3):651—667
[6]Huang W,Siemann E,Carrillo J,et al. Below-ground herbivory limits induction of extrafloral nectar by aboveground herbivores. Annals of Botany,2015,115 (5):841—846
[7]Barber N A,Kiers E T,Theis N,et al. Linking agricultural practices,mycorrhizal fungi,and traits mediating plant-insect interactions. Ecological Applications,2013,23(7):1519—1530
[8]栗治,刘小侠,张青文. 不同氮水平对麦二叉蚜生长发育和繁殖的影响. 应用昆虫学报,2014,51(2):353—359
Li Z,Liu X X,Zhang Q W. Effects of nitrogen fertilizer on the development and fecundity of Schizaphis graminum(Rondani)(In Chinese). Chinese Journal of Applied Entomology,2014,51(2):353—359
[9]Bagchi S,Ritchie M E. Herbivore effects on aboveand belowground plant production and soil nitrogen availability in the Trans-Himalayan shrub-steppes. Oecologia,2010,164(4):1075—1082
[10]Noboru K,Alessandro O S,Osamu K,et al. Herbivorous insect decreases plant nutrient uptake:The role of soil nutrient availability and association of below-ground symbionts. Ecological Entomology,2014,39(4):511—518
[11]Treonis A M,Murray P J,Dawson L A. Effects of root feeding,cranefly larvae on soil microorganisms and the composition of rhizosphere solutions collected from grassland plants. Applied Soil Ecology,2005,28 (3):203—215
[12]毛小芳,李辉信,龙梅,等. 不同食细菌线虫取食密度下线虫对细菌数量、活性及土壤氮素矿化的影响. 应用生态学报,2005,16(6):1112—1116
Mao X F,Li H X,Long M,et al. Effects of bacteria-feeding nematode at its different density on bacterial number,bacterial activity and soil nitrogen mineralization(In Chinese). Chinese Journal of Ecology,2005,16(6):1112—1116
[13]Huang J H,Liu M Q,Chen X Y,et al. Effects of intraspecific variation in rice resistance to aboveground herbivore brown planthopper,and rice root nematodes on plant yield,labile pools of plant and rhizosphere soil. Biology and Fertility of Soils,2015,51(4):417—425
[14]Alef K,Nannipieri P. Methods in applied soil microbiology and biochemistry. London:Academic Press,1995
[15]鲁如坤. 土壤农业化学分析方法. 北京:中国农业科技出版社,2000
Lu R K. Analytical methods for soil and agro-chemistry (In Chinese). Beijing:China Agricultural Science and Technology Press,2000
[16]Witt C,Biker U,Galicia C C,et al. Dynamics of soil microbial biomass and nitrogen availability in a flooded rice soil amended with different C and N sources. Biology and Fertility of Soils,2000,30(5):520—527
[17]Johnson S N,Clark K E,Hartley S E,et al. Aboveground-belowground herbivore interactions:Ameta-analysis. Ecology,2012,93(10):2208—2215
[18]Olga K,Patrick P J M,Bezemer T M. Effects ofroot herbivory on pyrrolizidine alkaloid content and aboveground plant-herbivore-parasitoid interactions in Jacobaea vulgaris. Journal of Chemical Ecology,2013,39(1):109—119
[19]Kaplan I,Sardanelli S,Rehill B J,et al. Toward a mechanistic understanding of competition in vascularfeeding herbivores:An empirical test of the sink competition hypothesis. Oecologia,2011,166(3):627—636
[20]Magdalene K,Marcus P,Caroline M. Effects of root herbivory by nematodes on the performance and preference of a leaf-infesting generalist aphid depend on nitrate fertilization. Journal of Chemical Ecology,2014,40(2):118—127
[21]Bezemer T M,van Dam N M. Linking aboveground and belowground interactions via induced plant defenses. Trends in Ecology and Evolution,2005,20(11):617—624
[22]Meldau S,Woldemariam M,Fatangare A,et al. Using 2-deoxy-2-[18F]fluoro-D-glucose([18F]FDG)to study carbon allocation in plants after herbivore attack. BMC Reaearch Notes,2015,8(1):1—9
[23]Huang J H,Liu M Q,Chen X Y,et al. Intermediate herbivory intensity of an aboveground pest promotes soil labile resources and microbial biomass via modifying rice growth. Plant and Soil,2013,367(1/2):437—447
[24]Tsunoda T,Kachi N,Suzuki J I. Effects of belowground vertical distribution of a herbivore on plant biomass and survival in Lolium perenne. Ecological Research,2014,29(2):351—355
[25]Senthil-Nathan S,Choi M Y,Paik C H,et al. Toxicity and physiological effects of neem pesticides applied to rice on the Nilaparvata lugens,Stål,the brown planthopper. Ecotoxicology and Environmental Safety,2009,72(6):1707—1713
[26]Frost C J,Hunter M D. Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytologist,2008,178(4):835—845
[27]Dromph K M,Cook R,Ostle N J,et al. Root parasite induced nitrogen transfer between plants is density dependent. Soil Biology &Biochemistry,2006,38 (8):2495—2498
[28]Kaplan I,Sardanelli S,Denno R F. Field evidence for indirect interactions between foliar-feeding insect and root-feeding nematode communities on Nicotianatabacum. Ecological Entomology,2009,34 (2):262—270
[29]Haase J,Brandl R,Scheu S,et al. Above and belowground interactions are mediated by nutrient availability. Ecology,2008,89(11):3072—3081
[30]Xoaquín M,Rafael Z,Luis S. Genetic variation and phenotypic plasticity of nutrient re-allocation and increased fine root production as putative tolerance mechanisms inducible by methyl jasmonate in pine trees. Journal of Ecology,2012,100(3):810—820
[31]Rogers C D,Evans K A. Wheat bulb fly,Delia coarctata,larval attraction to phenolic components of host-plant root exudates. Entomologia Experimentalis Et Applicata,2014,150(2):166—173
[32]张奇春,王光火,方斌. 不同施肥处理对水稻养分吸收和稻田土壤微生物生态特性的影响. 土壤学报,2005,42(1):116—121
Zhang Q C,Wang G H,Fang B. Influence of fertilization treatment on nutrients uptake by rice and soil ecological characteristics of soil microorganism in paddy field(In Chinese). Acta Pedologica Sinica,2005,42(1):116—121
[33]赵满兴,Kalbitz Karsten,周建斌. 黄土区几种土壤培养过程中可溶性有机碳、氮含量及特性的变化. 土壤学报,2008,45(3):476—484
Zhao M X,Karsten K,Zhou J B. Variation of content and structural characteristics of dissolved organic carbon and nitrogen in soluble organic matter during mineralization of several soils in the loess region(In Chinese).Acta Pedologica Sinica,2008,45(3):476—484
[34]Klumpp K,Fontaine S,Attard E,et al. Grazing triggers soil carbon loss by altering plant roots and their control on soil microbial community. Journal of Ecology,2009,97(5):876—885
[35]Olsen Y S,Dausse A,Garbutt A,et al. Cattle grazing drives nitrogen and carbon cycling in a temperate salt marsh. Soil Biology &Biochemistry,2011,43(3):531—541
[36]Nalam V J,Shah J,Nachappa P. Emerging role of roots in plant responses to aboveground insect herbivory. Insect Science,2013,20(3):286—296
[37]Liu Y S,Pan Q M,Liu H D,et al. Plant responses following grazing removal at different stocking rates in an Inner Mongolia grassland ecosystem. Plant and Soil,2011,340(1/2):199—213
Effects of Interactions of Above- and Below-ground Herbivores on Nitrogen Distribution in Rice Plant and Labile Nitrogen in Soil
GUO Ruihua1LUO Ling1ZHANG Tenghao1,2LIU Manqiang1CHEN Xiaoyun1†HU Feng1
(1 Soil Ecology Laboratory,College of Resources and Environmental Sciences,Nanjing Agricultural University,Nanjing 210095,China)
(2 Hebei Province Instituteof Supervision and Inspection Product Quality,Shijiazhuang 050000,China)
【Objective】Interactions between aboveground and belowground herbivores sharing the same host plantare regarded as the basic linkage between aboveground and belowground ecosystems,though they are separated in space. Much work has been done demonstrating that soil nitrogen availability affects the herbivores via plant by regulating chemical composition,such as nitrogen content of the plant. However,little has been reported on effects of the interactions between the two groups of herbivores on soil nitrogen. In this paper,a microcosm experiment was designed to investigate effects of the interactions between the herbivores on nitrogen distribution in the plant and labile nitrogen(microbial biomass nitrogen,dissolved organic nitrogen,ammonium nitrogen and nitrate nitrogen)in the soil. 【Method】In this study,Nilaparvata lugens and Hirschmanniella oryzae were selected as representative of the two groups,aboveground and belowground herbivores,respectively,and a pot experiment was designed to have two rates of Nilaparvata lugens,(zero or eight individuals per plant),and two rates of Hirschmanniella oryzae(zero or 500 individuals per pot),totaling four treatments and four replicates for each treatment. The soil used in the pot was first sterilized under 121℃ to kill all the native nematodes,and then was inoculated with the soil bacterial suspension prepared out of the original soil that had been deprived of soil nematodes,and put into a dark incubator for 60 days of incubation under indoor temperature to restore and stabilize microbial communities in the soil. Rice seedlings were transplanted into the pots after 30 days of seedling nursing in nutrient solution. Hirschmanniella oryzae and Nilaparvata lugens was inoculated separately on the fifteenth and twenty fifth day after rice seedlings were transplanted. Ten days after the inoculation of Nilaparvata lugens,samples of the plants and soil were collected. Plant samples were separated into shoot and root for measurement of biomass and nitrogen content,separately. Soil samples were analyzed,separately,for microbial biomass nitrogen,dissolved organic nitrogen,ammonium nitrogen and nitrate nitrogen.【Result】Results showed that Nilaparvata lugens and Hirschmanniella oryzae negatively affected each other. Nilaparvata lugens tended to decrease shoot biomass,but did not affect much root biomass,while Hirschmanniella oryzae significantly reduced shoot and root biomass(p< 0.05). Nilaparvata lugens showed no significant effect on shoot and root nitrogen content,while Hirschmanniella oryzae significantly reduced root nitrogen content(p< 0.05),though they did not affect much shoot nitrogen content. Significantly interactive effects of aboveground and belowground herbivores on soil labile nitrogen were observed. In the absence of Hirschmanniella oryzae,Nilaparvata lugens significantly increased the content of microbial biomass nitrogen(p< 0.05)and decreased nitrate content significantly(p< 0.05),but did not have much effect on dissolved organic nitrogen and ammonium nitrogen,whereas in the presence of Hirschmanniella oryzae,they did not affect the content of soil labile nitrogen significantly. On the other hand,Hirschmanniella oryzae significantly and positively affected the content of microbial biomass nitrogen and total soil labile nitrogen(p<0.05). 【Conclusion】To sum up,the effects of the interactive suppressions between the aboveground and belowground herbivores on nitrogen content in shoot and root of the rice plants and on soil labile nitrogen are rather complicated. Moreover,compared with Nilaparvata lugens,Hirschmanniella oryzae tends to increase total soil labile nitrogen,which may in turn improve N-transformation-related ecological functions of the soil.
Nilaparvata lugens;Hirschmanniella oryzae;Nitrogen allocation;Microbial biomass nitrogen;Dissolved organic nitrogen;Inorganic nitrogen
S154.1
A
10.11766/trxb201605110159
(责任编辑:陈荣府)
* 国家自然科学基金项目(31170487)和江苏高校优势学科建设工程(PAPD)资助 Supported by the National Natural Science Foundation of China(No. 31170487)and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADA)
† 通讯作者 Corresponding author,E-mail:xychen@njau.edu.cn
郭瑞华(1986—),女,河南周口人,硕士研究生,主要研究地上与地下部生态系统之间的联系。E-mail:ruizhognrui@163.com
2016-05-11;
2016-07-04;优先数字出版日期(www.cnki.net):2016-07-22
