突破衍射极限的成像方法综述
2017-04-10乌拉郑玉祥
乌拉 郑玉祥
摘要: “衍射極限”实际上不是一个真正的障碍,除非处理远场和定位精度。这种衍射障碍并不是坚不可摧的,可以利用一些智能技术来突破光学衍射极限。讨论了四种技术,近场扫描光学显微镜(NSOM)法,受激发射损耗(STED)显微镜法,光激活定位显微镜(PALM)法或随机光学重建显微镜(STORM)法和结构照明显微镜(SIM)法,并且介绍了各自的基本原则与优劣。NSOM利用纳米级探测器检测通过光纤的极小汇聚光斑,从而获得单个像素的分辨率;PALM和STORM利用荧光探针,实现暗场和荧光的转换,从而观察到极小的荧光团;SIM则是利用栅格图案与样品叠加成像来实现。其中,STORM具有相对较高的潜力,能够更为有效地突破衍射极限。
关键词:
衍射极限; 近场显微镜; 三维显微
中图分类号: O 43文献标志码: Adoi: 10.3969/j.issn.10055630.2017.01.014
A review on imaging methods to break the diffraction limit
Ramzan Ullah1,2, ZHENG Yuxiang1
(1.Shanghai UltraPrecision Optical Manufacturing Engineering Center, Department of Optical Science and Engineering,
Fudan University, Shanghai 200433, China;
2.Department of Physics, COMSATS Institute of Information Technology, Islamabad 45550, Pakistan)
Abstract:
Notorious term 'diffraction limit' is not actually a true barrier unless we are dealing with far field and localization precision.This diffraction barrier is not impenetrable and can be broken with some intelligent techniques.We discuss here four powerful techniques,nearfield scanning optical microscopy(NSOM),stimulated emission depletion(STED) microscopy,photoactivated localization microscopy(PALM) & stochastic optical reconstruction microscopy(STORM) and structured illumination microscopy(SIM),along with their underlying principles together with pros and cons.NSOM uses a nanometer scale detector or source which compels the light to pass through the tiny tip of a fiber while keeping the distance between the tip and sample less than λ.At any given moment in a STED microscope,laser light is focused into a small spot by the objective and as a result,all fluorophores within this focused spot radiate fluorescence,which is then gathered by the objective and headed to the detector where it forms a single pixel.Fluorescent probes are employed by STORM/PALM,which are able to toggle between dark states and fluorescent so that with every snapshot taken,only a tiny,optically resolvable portion of the fluorophores is observed.Structured illumination is a wide field technique in which a grid pattern is produced by the interference of diffraction orders which are superimposed on the sample while taking images.STORM has the relatively high potential to effectively break the conventional diffraction barrier with fewer hurdles.
Keywords:
diffraction limit; nearfield microscopy; threedimensional microscopy
Introduction
A microscope is a device used to see objects in intricate detail usually up to the order ofnanoscale.The main factor in determining the quality of a microscope is its resolution which is fundamentally bounded by diffraction limit.Normally,determining the diffraction limit of an imaging system is based on Abbe and Rayleigh criterions[1] which in turn depend on numerical aperture of the lens and wavelength of light being used.With the advent of new technologies different types of microscopes working beyond the limit of diffraction,have been developed which include electron microscopes[23] using electrons as well as optical microscopes using smart optical techniques.Each type has its own pros and cons.We present a short review of some of these optical microscopes.
1Nearfield scanning optical microscopy(NSOM)
NSOM sometimes abbreviated as SNOM for "Scanning Nearfield Scanning Optical Microscopy" was firstly suggested in 1928[4].NSOM uses a very innovative concept to penetrate the diffraction barrier which is to use a detector or source whose size is in nanometer scale.NSOM compels the light to pass through the tiny tip(whose aperture size is on the order of tens of nanometers) of a fiber.Now if this tip is brought very close to the object,the resolution is no more limited by the diffraction,but by the size of the tip aperture as elucidated in Fig 1.So it means the distance between the tip and object must be much smaller than λ.So it breaks the far field resolution limit.Probe resolution is mainly quantified by the diameter of the aperture[5].With the passage of time,more and more advanced techniques have been developed and some are even specific to the type of sample[6].This technique has revolutionized the field of material characterization especially for nano materials[7].So basically NSOM/SNOM utilizes the near field component of the electromagnetic wave whose propagation is limited to very short distance as opposed to far field light which smears out infinitely until absorbed,refracted or scattered whatever is the case.The propagation distance of a near field photon is proportional to the physical dimensions of its source;hence in order to be observable by the nearfield,the objects have to be in very close proximity of the field.The distance between the tip and object must be less than the dimensions of the aperture of the tip.The amplitude of the nearfield light decays exponentially as the negative of the 1st or higher power of the distance from its source.A detailed analysis of the NSOM can be found here[8].Similarly another technique called apertureless near field microscopy reaches beyond the range of simple NSOM[9].
1.1Advantages
(1) NSOM offers direct relationship between surfacenano features and optical or electronic characteristics along with concurrent mensuration of the topography as well as optical properties(fluorescence).
Fig.1Schematic diagram of a NSOM
(2) NSOM is substantially effective in characterizing the inhomogeneous materials or surfaces,like nano particles,polymer blends,porous silicon,and biological systems[10].
1.2Disadvantages
(1) The chief drawback to NSOM is the restricted number of photons coming out of the tiny tip and the miniscule collection efficiency.
(2) Long scan time for high resolution images or large areas to be scanned.
(3) Only surface features can be studied.
2Stimulated emission depletion(STED) microscopy
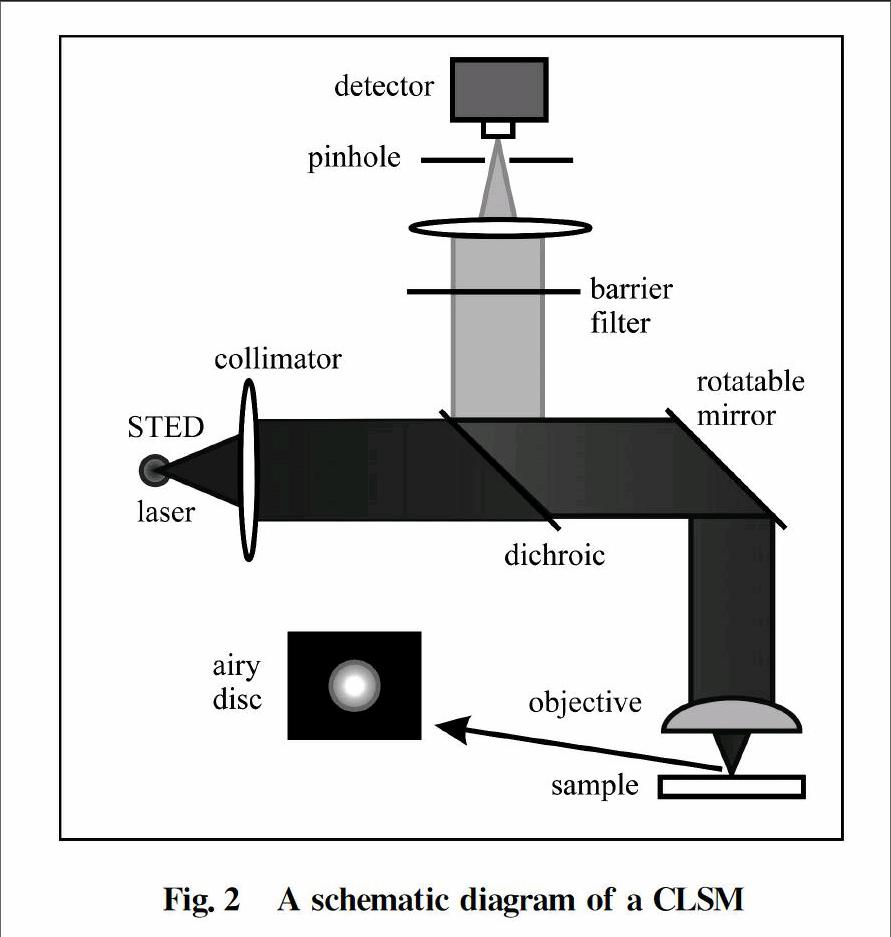
A STED microscope is built on the basis of aconfocal laser scanning microscope(CLSM).A layout of a CLSM is shown in Fig.2.At any given moment,laser light is focused into a small spot by the objective and as a result,all fluorophores within this focused spot radiate fluorescence,which is then gathered by the objective and headed to the detector.The detected signal forms a single pixel.Then the scanning mirror moves in XY plane to take the next pixels and this goes on until the whole sample is scanned and as a result whole image of the sample is formed.Sometimes,the sample stage is moveable so sample is moved in the XY plane and whole image is formed.A single pixel is obtained for each location.So in order to get a high resolution image,it would take considerable time.
Fig.2A schematic diagram of a CLSM
The intensity of the light at the focused spot spreads out in accordance with the point spread function(PSF).For a circular aperture,the PSF exhibits a pattern called “Airy disk”,whose size is proportional to λ/NA where λ is the wavelength of light & NA is numerical aperture.
The resolution of CLSM is decided by the size of the PSF:If the focal spot is smaller,so does the each pixel acquired and the resultant image will be crisp and sharp.But if not,resultant image will be blurred.So the main challenge is to achieve smaller and smaller PSF to get better and better resolution.However,there is a natural diffraction limit in doing this like in any other system and this situation was first described in 1870s by a German physicist Ernst Abbe(1840—1905) who indicated that the PSF size has a lower limit which is proportional to λ/NA(circular aperture) due to diffraction.This is called the Abbes diffraction limit.The basic idea behind STED microscopy is the utilization of nonlinear optics to design a smaller PSF below Abbes diffraction limit.
It was Albert Einstein,who in 1917 theoretically anticipated the occurrence of stimulated emission.Stimulated emission is the basic building block of lasers and it also functions as the foundation of STED that cracks the diffraction limit.
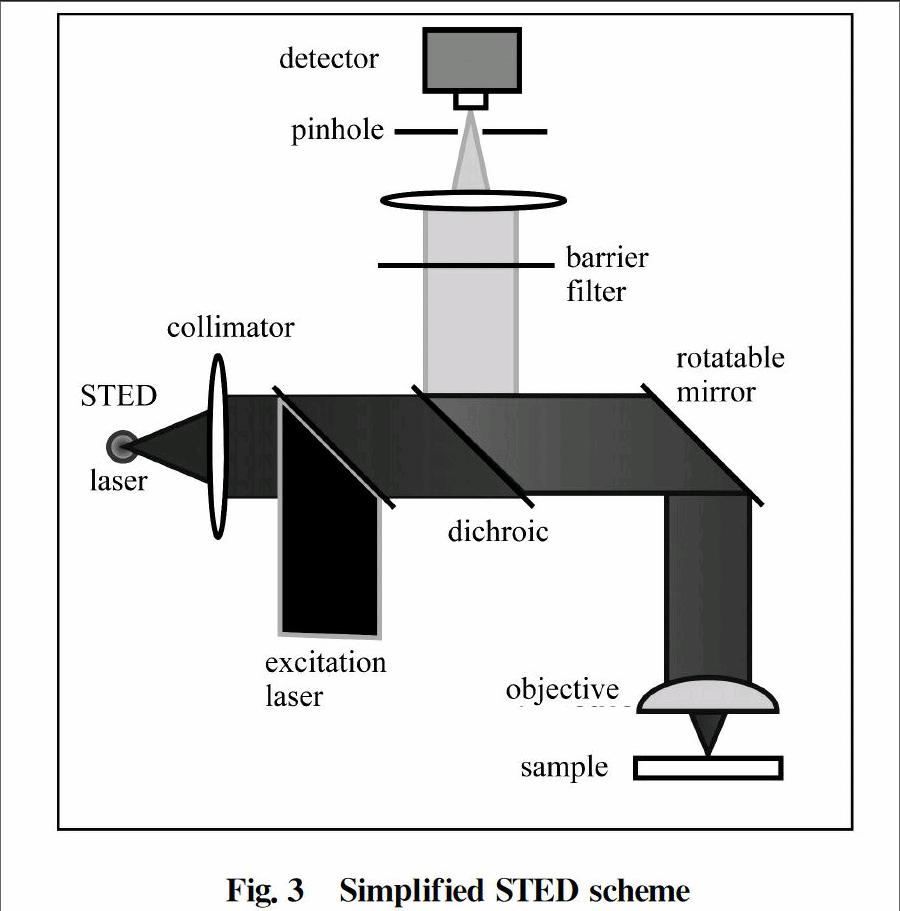
The STEDmicroscope is largely dependent on two laser pulses which are synchronized.These two synchronized laser pulses are named as 'STED laser' and 'excitation laser' in Fig.3.As can be seen,excitation is carried out by a subpicosecond laser pulse which is tuned to the absorption spectrum of the dye.The excitation pulse is irradiated and focused onto the sample,generating a typical diffraction limited spot of excited molecules.The excitation pulse is instantly chased by a depletion pulse named as STED pulse.The STED pulse is redshifted(increased in wavelength) in frequency to the emission spectrum of the dye,in such a way that its lower energy photons operate only on the excited molecules of the dye under ideal condition,hence,extinguish them to the ground state by stimulated emission.The overall result of the STED pulse is that the influenced excited molecules cannot radiate in the fluorescence regime because their energy is disposed of in the STED pulse.By arranging the STED pulse in doughnut mode spatially,only the molecules in the proximity of the spot are quenched under ideal condition.Fluorescence ideally remains intact at the center of the doughnut,where the STED pulse is evanescing.
Fig.3Simplified STED scheme
By increasing the intensity of the STED pulse,the depletion becomes increasingly more functional towards the middle and sufficiently complete at the proximity of the spot.However,the fluorescence is ideally not affected at all at the doughnut hole.Therefore,by increasing the intensity of the doughnutshaped STEDpulse,the fluorescent spot can be gradually shrunk down,theoretically,even up to the size of a single molecule.This intriguing concept is manifesting the fundamental smashing of the diffraction barrier.The crucial element is the saturated diminishing of the fluorescence at any coordinate except the focal point.
This microscopy technique is unique in a way that presently the well known super resolution methods like multiphoton fluorescence,the confocal or related microscopes,which can never transcend Abbes barrier by more than a factor of 2.In a way,confocal fluorescence and twophoton microscopes just cross the border of the diffraction limitation,without breaking it[11].The resolution of these systems is still restricted by diffraction,as opposed to the STEDmicroscope[12].
The actual physical reason behind the breakage of the diffraction barrier is not that fluorescence is hindered,but the saturation of the fluorescence diminishing.Fluorescence diminishing alone is not conducive to the breakage of the diffraction barrier since the focused STEDpulse is also limited by diffraction.However,in this context,saturation means that when the fluorescence at the middle of the doughnut is intact,it is completely stopped at the closest proximity of the doughnut.Thus the fluorescent region is gradually shrunk down without any limit[13].
3Photoactivated localization microscopy(PALM) & stochastic optical reconstruction microscopy(STORM)
Superresolution optical microscopy technique which is founded on stochastic switching of single molecule fluorescence signal is named as PALM or sometimes also called STORM[14].As in the case of conventional fluorescence microscopy where all fluorophores in the sample are fluorescent and their corresponding images,being diffraction limited,overlap and thus form a smooth but somewhat obscure image.Fluorescent probes are employed by STORM/PALM,which are able to toggle between dark states and fluorescent so that with every snapshot taken,only a tiny,optically resolvable portion of the fluorophores is observed[15].In this way,deduction of their locations with ultra high accuracy from the central locations of the fluorescent spots is possible.With many snapshots of the sample,a final superresolution image can be reenacted from the assembled positions,each catching a random subset of the fluorophores[16].
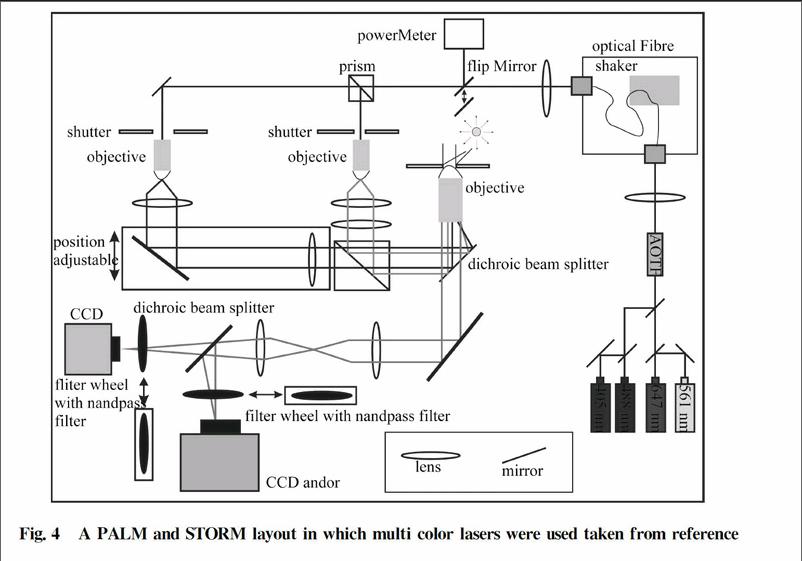
Since its inception in 2006,STORM has gathered many more functionalities[17].With either different emission wavelengths or different activation wavelengths,multicolor imaging can be attained with photoswitchable fluorophores.3D imaging has been actualized with the help of several 3D singleparticle localization methods,inclusive of PSF engineering,biplane imaging,astigmatic imaging and interference.A typical STORM /PALM setup is shown in Fig.4 in which multi color lasers were used.The detail can be found[18].Similarly,Nikon made a new STORM microscope which they name NSTORM with superresolution capable to reconstruct 2D and 3D high resolution images with crystal clear clarity.The detail and specifications can be found here[19].
4Structured illumination microscopy(SIM)
Structured illumination is awide field technique in which a grid pattern is produced by the interference of diffraction orders which are superimposed on the sample while taking images.The grid pattern is relocated or revolved in steps between recordings of each snapshot set.The snapshot set consists of individual subsets,where each subset is recorded after rotating the grid.Succeeded by the processing with a specially designed algorithm[20],highfrequency information can be extricated from the raw data to develop a reconstructed image with a lateral resolution roughly twice to that of diffractionlimited microscopes[21] and an axial resolution between 150 and 300 nm.
Fig.4A PALM and STORM layout in which multi color lasers were used taken from reference
Structuredillumination(SI) leans on both exclusive microscopy procedures and extensive software analysis after exposure.But,because SI is a widefield technique,it is normally capable to capture images at a higher rate than confocalbased schemes like STED[22].The leading concept of SI is to illuminate a sample with patterned light and increase the resolution by measuring the fringes in the Moiré pattern[23] and sample information(which is otherwise unobservable) is extracted from these fringes and computationally reinstated[24].
There are some limitations associated with SI.Firstly,the saturating excitation powers induce more photo damage and decline fluorophore photo stability.Secondly,sample drift must be retained well below the resolving distance which is also very challenging.The first limitation can be resolved by combining with other microscopy techniques which use some other nonlinearity like reversible photo activation and stimulated emission depletion.The second limitation delimits livecell imaging and may necessitate faster frame rates.In spite of that,SI is undoubtedly,a strong rival in the competition of applications in the field of superresolution microscopy[25].
A comparison table of pros and cons of all of these microscopy techniques given above together with many others can be found at reference[26].
5Conclusion
After discussing these four very powerful techniques,nearfield scanning optical microscopy(NSOM),stimulated emission depletion(STED) microscopy,photoactivated localization microscopy(PALM) & stochastic optical reconstruction microscopy(STORM) and structured illumination microscopy(SIM),of breaking the diffraction limit,we conclude STORM has the high potential to effectively break the conventional diffraction barrier with less hurdles.However,this conclusion is relative as it depends upon the application for which a microscope is required.
參考文献:
[1]PAWLE Y J.Handbook of biological confocal microscopy[M].New York:Plenum Press,1990.
[2]CLARKED R.Review:transmission scanning electron microscopy[J].Journal of Materials Science,1973,8(2):279285.
[3]VERNONPARRY K D.Scanning electron microscopy:an introduction[J].IIIVs Review,2000,13(4):4044.
[4]NOVOTNY L.From nearfield optics to optical antennas[J].Physics Today,2011(7):4752.
[5]LEWENG D,NAHATA A,LEZEC H J,et al.Surface Plasmonenhanced transmission for high throughput NSOM probes[J/OL].[20150810].http:∥www.foresight.org/Conference/MNT9/Papers/Lewen/index.html.
[6]MICHAELIS J,HETTICH C,MLYNEK J,et al.Optical microscopy using a singlemolecule light source[J].Nature,2000,405:325328.
[7]TISLER J,OECKINGHAUS T,STHR R J,et al.Single defect center scanning nearfield optical microscopy on graphene[J].Nano Letters,2013,13(7):31523156.
[8]DUNN R C.Nearfield scanning optical microscopy[J].Chemical Reviews,1999,99(10):28912928.
[9]YANG T J,LESSARD G A,QUAKE S R.An apertureless nearfield microscope for fluorescence imaging[J].Applied Physics Letters,2000,76(3):378380.
[10]HERMAN M A.Scanning nearfield optical microscopy[J].OptoElectronics Review,1997,5(4):295298.
[11]HUANG B,BATES M,ZHUANG X W.Super resolution fluorescence microscopy[J].Annual Review of Biochemistry,2009,78:9931016.
[12]HELL S W,WICHMANN J.Breaking the diffraction resolution limit by stimulated emission:stimulatedemissiondepletion fluorescence microscopy[J].Optics Letters,1994,19(11):780782.
[13]HELL S W.Increasing the resolution of farfield fluorescence light microscopy by pointspreadfunction engineering[M]∥LAKOWICZ J.Topics in fluorescence spectroscopy:volume 5:nonlinear and twophotoninduced fluorescence.New York:Plenum Press,1997:361426.
[14]HUANG B,BABCOCK H,ZHUANG X W.Breaking the diffraction barrier:superresolution imaging of cells[J].Cell,2010,143(7):10471058.
[15]HELL S W.Microscopy and its focal switch[J].Nature Methods,2009,6(1):2432.
[16]HELL S W.Farfield optical nanoscopy[J].Science,2007,316(5828):11531158.
[17]KAMIYAMA D,HUANG B.Development in the STORM[J].Developmental Cell,2012,23(6):11031110.
[18]CHEMIE P.Establishment and optimization of superresolution fluorescence microscopy for multicolour studies of biological systems[D].München,2010.
[19]Nikon instrumants Inc.SupperResolution microscope system offering ten times the resolution of convention optical microscopes[EB/OL].[20160402].http:∥www.nikoninstruments.com/Products/Superresolution/NSTORMSuperResolution.
[20]BARLOW A L,GUERIN C J.Quantization of widefield fluorescence images using structured illumination and image analysis software[J].Microscopy Research and Technique,2007,70(1):7684.
[21]NEIL M A A,WILSON T,JUKAITIS R.A light efficient optically sectioning microscope[J].Journal of Microscopy,1998,189(2):114117.
[22]WILSON T,JUKAITIS R,NEIL M A A,et al.Confocal microscopy by aperture correlation[J].Optics Letters,1996,21(23):18791881.
[23]CHASLES F,DUBERTRET B,BOCCARA A C.Optimization and characterization of a structured illumination microscope[J].Optics Express,2007,15(24):1613016140.
[24]JUKAITIS R,WILSON T,NEIL M A A,et al.Efficient realtime confocal microscopy with white light sources[J].Nature,1996,383(6603):804806.
[25]KARADAGLI D,WILSON T.Image formation in structured illumination widefield fluorescence microscopy[J].Micron,2008,39(7):808818.
[26]WILSON S M,BACIC A.Preparation of plant cells for transmission electron microscopy to optimize immunogold labeling of carbohydrate and protein epitopes[J].Nature Protocols,2012,7(9):17161727.
(編辑:张磊)
