Stress injuries and autophagy in mouse hippocampus after chronic cold exposure
2017-04-07ZhanjunCuiXiaoqingWangLaiWang
Zhan-jun Cui Xiao-qing Wang Lai Wang
1 Institute of Neurobiology, College of Life Science, Henan University, Kaifeng, Henan Province, China
2 Nursing College, Henan Vocational College of Applied Technology, Zhengzhou, Henan Province, China
Stress injuries and autophagy in mouse hippocampus after chronic cold exposure
Ting-ting Qu1,2,#, Jie-xin Deng1,#, Rui-ling Li1, Zhan-jun Cui1, Xiao-qing Wang1, Lai Wang1,*, Jin-bo Deng1,*
1 Institute of Neurobiology, College of Life Science, Henan University, Kaifeng, Henan Province, China
2 Nursing College, Henan Vocational College of Applied Technology, Zhengzhou, Henan Province, China
How to cite this article:Qu TT, Deng JX, Li RL, Cui ZJ, Wang XQ, Wang L, Deng JB (2017) Stress injuries and autophagy in mouse hippocampus after chronic cold exposure. Neural Regen Res 12(3):440-446.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This study was supported by the Henan Province Foundation for Key University Teachers in China, No. 16A330001, 15A180031; the Henan Postdoctoral Foundation in China, No. 2015051; a grant from the Henan Province Research Program of Basic and Advanced Technology in China, No. 162300410102.
Graphical Abstract
Cold exposure is an external stress factor that causes skin frostbite as well as a variety of diseases. Estrogen might participate in neuroprotection after cold exposure, but its precise mechanism remains unclear. In this study, mice were exposed to 10°C for 7 days and 0-4°C for 30 days to induce a model of chronic cold exposure. Results showed that oxidative stress-related c-fos and cyclooxygenase 2 expressions, MAP1LC3-labeled autophagic cells, Iba1-labeled activated microglia, and interleukin-1β-positive pyramidal cells were increased in the hippocampal CA1 area. Chronic cold exposure markedly elevated the levels of estrogen in the blood and the estrogen receptor, G protein-coupled receptor 30. These results indicate that neuroimmunoreactivity is involved in chronic cold exposure-induced pathological alterations, including oxidative stress, neuronal autophagy, and neuroimmunoreactivity. Moreover, estrogen exerts a neuroprotective effect on cold exposure.
nerve regeneration; chronic cold exposure; oxidative stress; autophagy; microglial cells; neuroimmunoreactivity; hippocampal CA1 area; estrogen; neural regeneration
Introduction
Stress response refers to an organism’s reaction to external or internal environmental alterations. Physiological functions are optimal when performed under constant body temperature (Varela et al., 2015). Cold exposure is an external stress factor that causes skin frostbite and damages various physiological processes including movement, and the cardiovascular, immune, and nervous systems, resulting in various diseases (Mohr et al., 2009; Brazaitis et al., 2015). Terefore, it is of great significance to understand the risks of cold exposure to health. The mechanisms of an individual’s response to cold stress are very complex because many cells, organs, and systems are involved. At the system and organ level, cold stress activates the hypothalamic-pituitary-adrenal axis and excites sympathetic nerves. In addition, vasoconstriction reduces heat radiation. At the cellular level, a cold stimulus can induce oxidative stress, resulting in cell injury and even apoptosis (Venditti et al., 2007; Mihailidou et al., 2009; Ouellet et al., 2011).
Terrien et al. (2011) and Sugama et al. (2011) reported that acute cold induced microglial activation as early as 30minutes after exposure. In addition, cold exposure increased interleukin (IL)-1β immunoreactivity in the hippocampus and hypothalamus. Our previous study reported that estrogen might be involved in neuroprotection after chronic cold exposure (Cui et al., 2014).
The current study investigated changes in oxidative stress, neuronal autophagy, neuroimmunoreactivity, and estrogen levels after chronic cold exposure to elucidate the involved pathological alterations, cell stress responses, and estrogen-mediated neuroprotection mechanisms.
Materials and Methods
Establishment of a chronic cold exposure model
All experiments were carried out in accordance with the Institutional Guidelines of Henan University for Animal Welfare (MEWEAHUM2014-000). Adult male C57BL/6J mice (25-30 g) at postnatal day 40-50 were fed in standard laboratory animal housing with a 12-hour light/dark cycle at 20-25°C. The mice were randomly divided into a control group and a cold exposure group, with 20 mice in each group. To decrease the mortality from sudden exposure to 0-4°C, pre-exposure at 10°C was carried out for the cold exposure group. After pre-exposure for 7 days, the treatment mice were exposed to 0-4°C for 30 days. During cold exposure, each experimental mouse was housed in a separate cage to avoid mutual contact for warmth. During cold exposure, behavior and mortality were recorded. The control mice were housed under standard conditions at 20-25°C.
Sample collection
Both control and treatment mice were intraperitoneally anesthetized with sodium pentobarbital (20 mg/kg) after intervention. Mice were perfused transcardially with 4% paraformaldehyde. After brains were removed, immersion fixation was carried out at 4°C for 1-2 days. The hippocampus is an important organ for learning and memory, and the CA1 area of the hippocampus is very sensitive to stress, such as ischemia and hypoxia (Schmidt-Kastner, 2015). Terefore, the CA1 area (Schmidt-Kastner, 2015) was selected as a target for the measurement.
Immunofluorescence assay
Hippocampal coronal sections were cut and rinsed with 0.01 M phosphate buffer. Nonspecific antigens were blocked with 10% normal goat serum (with 0.3% Triton X-100, 1% bovine serum albumin in 0.1 M phosphate buffer) for 30 minutes. The slices were then incubated with primary antibodies at 4°C overnight. After rinsing three times, the slices were incubated with secondary antibodies for 3 hours at room temperature. The following primary antibodies were used: rabbit anti-IL-1β polyclonal antibody (1:500; Abcam, Cambridge, UK), rabbit anti-c-fos polyclonal antibody (1:500; Abcam, Cambridge, UK), rabbit anti-cyclooxygenase 2 (COX2) polyclonal antibody (1:500; Abcam), rabbit anti-MAP1LC3 polyclonal antibody (1:500; Abcam), rabbit anti-Iba1 polyclonal antibody (1:400; Abcam), rabbit anti-IL-6 polyclonal antibody (1:200; Abcam), and rabbit anti-G protein-coupled receptor 30 (GPR30) polyclonal antibody (1:300; Abcam). Secondary antibodies used were donkey anti-rabbit IgG Alexa Fluor 568 (1:600; Invitrogen, Carlsbad, CA, USA), and donkey anti-goat IgG Alexa Fluor 488 (1:500; Invitrogen). Sections were coverslipped with medium (65% glycerol in 0.01 M phosphate buffer + 1:10,000 DAPI for counterstaining). Ten, cells were imaged with an epifluorescence microscope (BX61; Olympus, Tokyo, Japan) under rhodamine or fluorescein isothiocyanate excitation. High-quality sections were photographed with an Olympus laser confocal microscope (FV1000; Olympus). The numbers of c-fos-, COX2-, MAP1LC3-, Iba1-, IL-1β-, IL-6- and GPR30-immunoreactive cells in the unit area were calculated. The number of immunoreactive cells was equal to the cell numbers/measured area (cells/mm2). For cell measurement, 20 mice in each group and 5 sections per mouse were used.
Western blot assay
To verify the results of the immunofluorescence assay, the expressions of c-fos, COX2, MAP1LC3, Iba1, nuclear factor-kappa B (NF-κB)-p65 and CD11b in hippocampus tissues were investigated using western blot assay. The proteins of hippocampal tissue were extracted with a cytoplasmic protein extraction kit (Beyotime Institute of Biotechnology, Haimen, China). The bicinchoninic acid assay was used to measure total protein concentrations according to a standard curve. The samples were subjected to electrophoresis and transferred to membranes. Primary antibodies, rabbit anti-cfos polyclonal antibody (1:1,000; Abcam), rabbit anti-COX2 polyclonal antibody (1:2,000; Abcam), rabbit anti-MAP1LC3 monoclonal antibody (1:2,000; Abcam), rabbit anti-Iba1 (1:2,000; Abcam), rabbit anti-NF-κB-p65 polyclonal antibody (1:500; Santa Cruz Biotechnology, Dallas, TX, USA) or mouse anti-CD11b (1:1,000; Abcam), were incubated at 4°C overnight. After washing with Tris Buffered Saline with Tween 20 three times (15 minutes each), corresponding horseradish peroxidase-labeled goat anti-rabbit IgG (1:2,000; Beyotime Institute of Biotechnology) and horseradish peroxidase-labeled rabbit anti-mouse IgG (1:1,000; Zhongshan Golden Bridge Biotechnology, Beijing, China), were incubated at room temperature for 2 hours. Finally, the membranes were incubated in enhanced chemiluminescence reagent for 3 minutes, and X-ray films were exposed and developed. β-Actin (Beyotime Institute of Biotechnology) was used as an internal reference. The grayscale ratio of target bands with an internal reference indicated the relative gray value of target bands. Objective cells in the CA1 area of the hippocampus were measured with ImageJ ProPlus 6.0 software (Media Cybernetics, Rockville, MD, USA). Protein expression levels were equal to the optical density value of the target band/ β-actin.
Blood estrogen test
After anesthetization with sodium pentobarbital (20 mg/kg, intraperitoneally), the eyeballs of mice were removed, and 2 mL of blood was collected from the orbit. Blood was self-clotted at room temperature for 1 hour, and centrifuged at 1,500 ×gfor 15 minutes. Afterwards, 400-500μL of serum was taken. Finally, chemiluminescence and microparticle immunoassays (Takeda et al., 2013) were used to measure the serum estrogen level using a specific kit and automated immunoassay analyzer (ARCHITECT, Abbott Park, IL, USA).
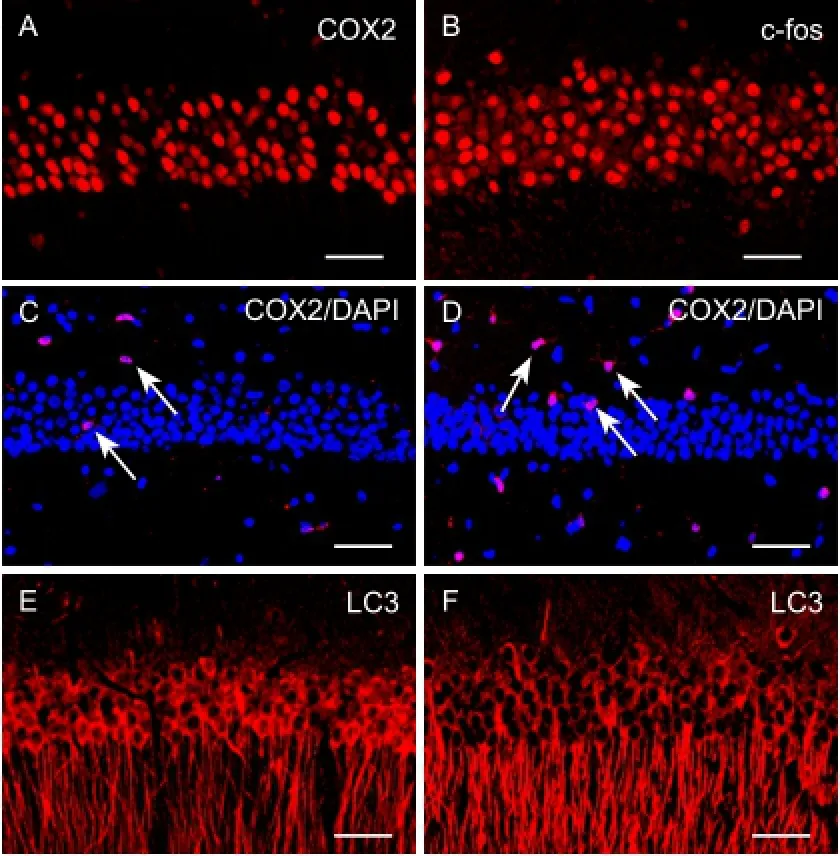
Figure 1 Cold-induced cellular oxidative stress, inflammatory injury, and autophagy in the hippocampal CA1 area (immunofluorescence assay).
Statistical analysis
The data were expressed as the mean ± SD. SPSS v11.5 statistical software (SPSS, Chicago, IL, USA) was used to compare measurements between control and cold exposure groups, and an independent-samplet-test analysis was carried out. A value ofP< 0.05 was accepted as statistically significant.
Results
Alterations of oxidative stress and autophagy after chronic cold exposure
To determine the effect of oxidative stress and inflammatory damage to neurons in the CA1 area after chronic cold exposure, an immunofluorescence assay was used to measure the relative levels of proteins including c-fos, COX2, and NF-κB. We found that c-fos-immunoreactive cells were mainly pyramidal cells and granule cells in the pyramidal and granular layers, but that COX2-immunoreactive cells were polygonal interneurons distributed in the polymorphic, pyramidal, and molecular layers. After cold exposure, numbers of c-fos-immunoreactive cells and COX2-immunoreactive cells in the CA1 region were increased significantly (Figure 1A-D). To confirm our observation, these immunoreactive cells were measured (Figure 2A,B). There was a statistically significant difference between the cold exposure and control groups (n= 20,P< 0.01). Western blot assay supported the conclusions from the immunofluorescence assay (n= 10,P< 0.01) (Figure 2C,D), suggesting that chronic cold exposure increased oxidative stress and inflammatory injury in hippocampal neurons.

Figure 4 Cold exposure increases neuroimmunoreactivity and estrogen receptor GPR30 expression (immunofluorescence assay).
We also used MAP1LC3 to label autophagic neurons with immunocytochemistry. MAP1LC3 was mainly expressed in the somas and dendrites of pyramidal cells. Compared with the control group, numbers of MAP1LC3-immunoreactive cells were significantly increased after cold exposure (Figure 1E,F). There was a significant difference between the cold exposure and control groups (n= 20,P< 0.01) (Figure 3A). Western blot assay showed that MAP1LC3 was over-expressed in the cold exposure group, supporting the immunofluorescence assay results (n= 10,P< 0.01) (Figure 3B,C).
Neuroimmunoreactivity after chronic cold exposure
Neuroimmunoreactivity may be involved in cold stress, as previously reported (Sugama et al., 2011). Immunofluorescence staining was used to identify microglial cells (Iba1+), stress cytokines (IL-6, IL-1β), and CD11b, which reflected the neuroimmunoreactivity present. The expressions ofIba1 and CD11b were also detected by western blot assay. Iba1-positive microglial cells were evenly distributed in the hippocampus with a star-like shape, and IL-1β and IL-6-immunoreactive cells were mainly located in the pyramidal and granule layers. The numbers of microglial cells and IL-1β-immunoreactive pyramidal cells in the CA1 region was increased significantly after cold exposure (Figure 4AF). Statistical analyses confirmed these observations (P<0.01) (Figure 5A-C). Western blot assay results were consistent with the immunofluorescence assay (P< 0.01) (Figure5D,E), indicating an overexpression of Iba1 and CD11b after cold exposure. These results suggest that chronic cold exposure induces microglia and stress cytokine secretion.
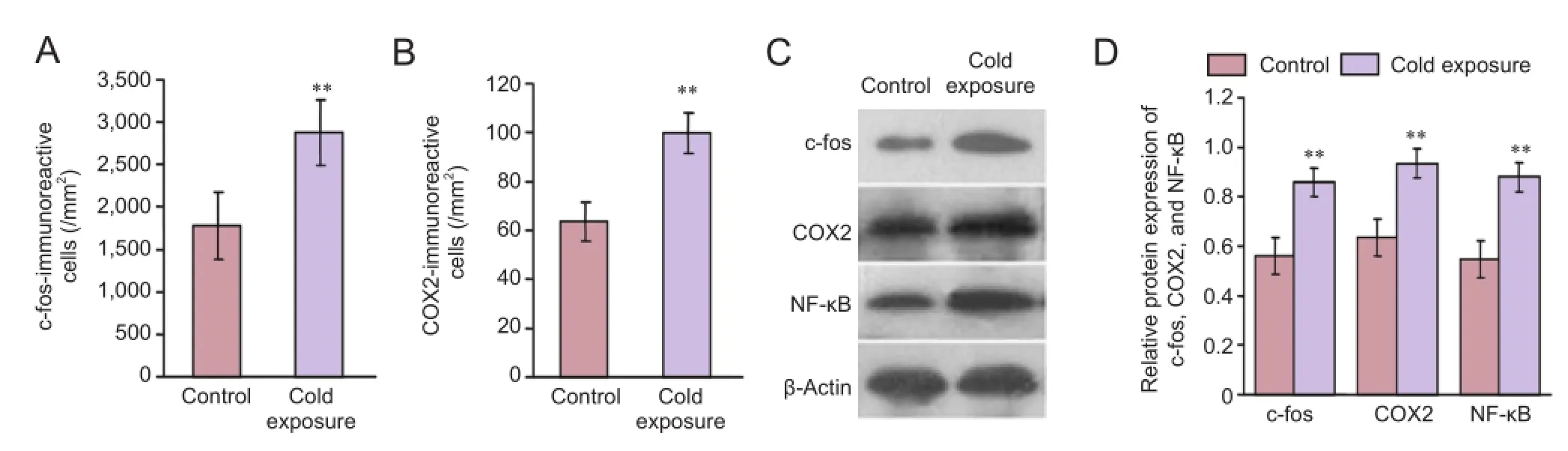
Figure 2 Expression of oxidative stress-related proteins after chronic cold exposure.
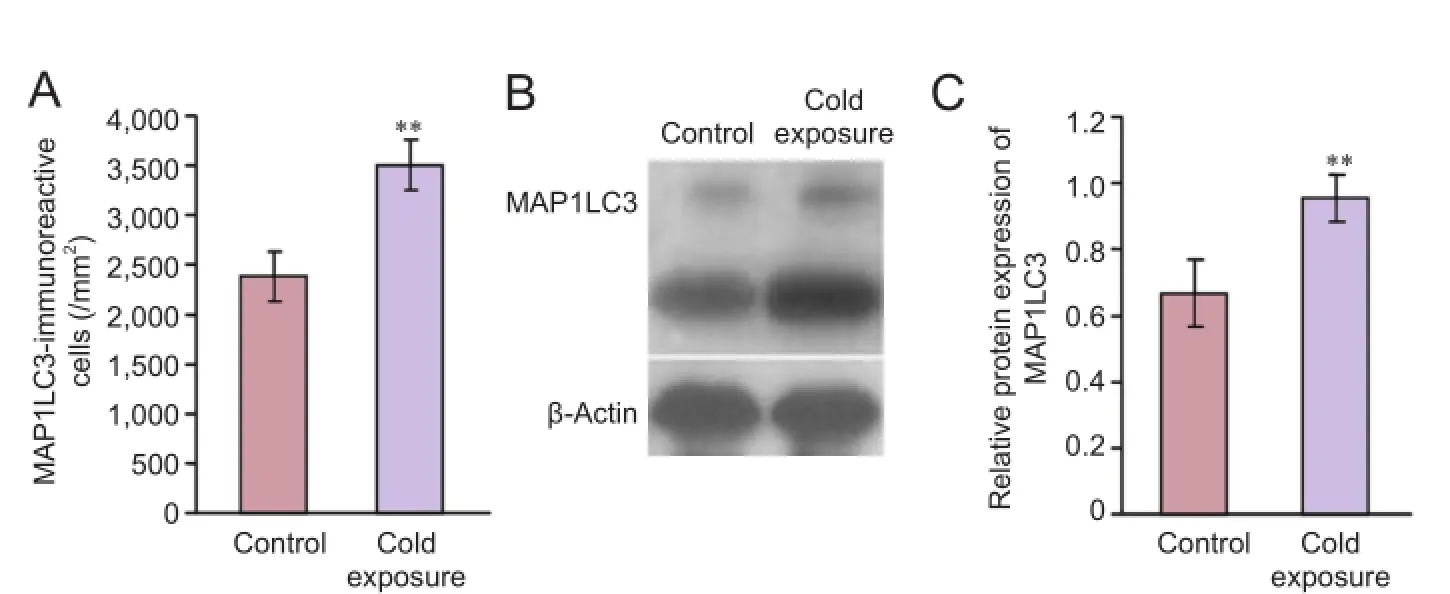
Figure 3 Expression of autophagy-related proteins after chronic cold exposure.

Figure 5 Expression of neuroimmunoreactivity-related proteins after chronic cold exposure.
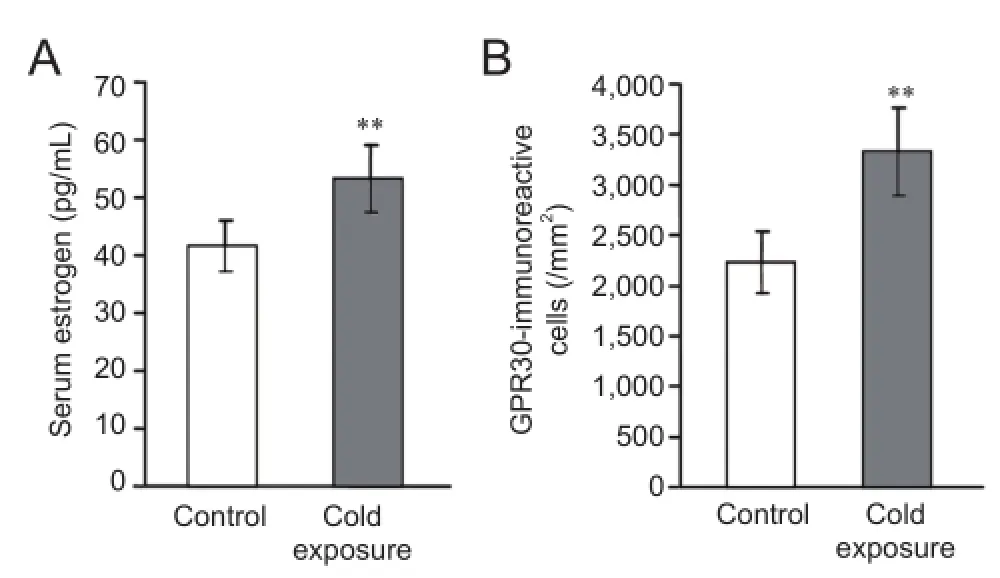
Figure 6 Estrogen levels and GPR30 expression after chronic cold exposure (immunofluorescence assay).
Changes of estrogen and estrogen receptor levels after chronic cold exposure
Estrogen is an important steroid hormone and might be involved in responses to cold stress (Uchida et al., 2010). To understand this further, estrogen levels in the blood and estrogen receptor, GPR30 expression in the CA1 area were measured. In this study, chronic cold exposure significantly enhanced blood estrogen levels (P< 0.01;Figure 6A). Because GPR30 is an active target of estrogen (Lee et al., 2012), the expression of GPR30 in the CA1 area was also measured. GPR30 was mainly located on pyramidal cells and their projections toward the molecular layer. After cold exposure, numbers of GPR30-immunoreactive cells were significantly increased (P< 0.01) (Figures 4G,Hand6B).
Discussion
Chronic cold exposure induced oxidative stress and neuronal autophagy
Oxidative stress is regarded as an early protective reaction to defend against injury caused by environmental changes. Subsequently, oxidative stress will develop into a cell inflammatory reactionviaparticipation of the immune system (Loane et al., 2014; Lee et al., 2015; Lu and Black, 2016). During oxidative and inflammatory reactions, cytokines are secreted. c-fos is an early/immediate stress gene involved in oxidative stress, which induces cell growth and differentiation (Gil-Mohapel et al., 2013). NF-κB and COX2 are also regarded as oxidative stress and inflammatory cytokines (Alvira, 2014; Zhou et al., 2014; Li et al., 2015). Once stress and inflammatory cytokine levels reach optimal levels, apoptosis and cellular and tissue damage occurs (Town, 2010; Vianna et al., 2011; Cho et al., 2015).
In our study, the expressions of c-fos, NF-κB, and COX2 were investigated using immunocytochemistry and western blot assay. Chronic cold exposure increased the expression of these proteins, suggesting that oxidative stress and inflammatory injuries occur in the hippocampus. Reactive oxygen species are critical for oxidative stress (Cano et al., 2014), and reactive oxygen species might be induced by cold exposure (Wang et al., 2015). Furthermore, intensive lipid peroxidation induced by cold stimuli might result in an increase in reactive oxygen species in cells. In this study, cold exposure induced neuronal autophagy. Cell autophagy often occurs during nutritional deficiencies. During autophagy, cells can survive by digesting their own organelles to obtain necessary nutrition and energy (Parzych and Klionsky, 2014). Excessive autophagy can trigger the release of hydrolase from lysosomes causing cell death (Levine and Kroemer, 2008). In a previous study, cold exposure induced autophagy (Kumar, 2007). A link was also reported between cell oxidative stress and autophagy (Lv and Zhou, 2012). Oxidative stress and inflammatory reactions can probably induce the autophagy pathway under a cold stimulus (Kathiria et al., 2012; Lu and Xu, 2013). We believe that cold-induced autophagy probably has an important neuroprotective function and provides sufficient nutrition and energy for body and cell survival.
Cold induced-oxidative stress caused neuroimmunoreactivity
Neural immunity is very important for an organism to maintain microenvironmental stability in the brain, and microglia are key for neural immunity in the central nervous system. Microglia are derived from macrophages and are widely distributed in the central nervous system (Lawson et al., 1992). Microglia are rapidly activated upon infection by bacteria and viruses (Glass et al., 2010). As calcium-binding proteins and markers of microglia, Iba1 and CD11b have specific functions in neural immunity. Activated microglia express Iba1 and CD11b (Blanchard et al., 2014). In addition, the inflammatory cytokines, IL-1β and IL-6, are also secreted by activated microglia (Awada et al., 2014), suggesting a close relationship between oxidative stress/inflammatory reaction and neuroimmunoreactivity.
In our study, Iba1-positive microglia were significantly increased in the CA1 area after chronic cold exposure, and this was consistent with the expressions of Iba1 and CD11b detected by western blot assay. This indicates causality between cold-induced oxidative stress and immune responses in the central nervous system. Indeed, during oxidative stress, transcription factor and inflammatory cytokines, such as NF-κB (Wang et al., 2010), IL-1β, IL-6, COX2, TNF-α (Jensen et al., 2011; Luheshi et al., 2011) and inducible nitric oxide synthase (Loane and Byrnes, 2010), are released by neurons in the central nervous system. These stress cytokines activate microglia in the central nervous system causing neurotoxicity (Xie et al., 2014), consistent with the results of our study. These cytokines also further activate glial cells and produce more inflammatory mediators (Morris et al., 2015; Yang et al., 2015a). Finally, these inflammatory mediators induce anti-inflammatory cytokines and neurotrophic factors that are neuroprotective (Correale, 2014). Excessive amounts of cytokines cause neurotoxicity and inflammatory injuries inthe brain (Xie et al., 2014). For example, the over-expression of NF-κB IL-1β, and IL-6 promotes the production of oxygen free radicals, which cause inflammatory damage to endothelial cells in the blood-brain barrier (Gil-Núñez and Villanueva, 2001; Yang et al., 2015b).
Regulation of estrogen to autophagy and neural immunity
Our previous study showed that after chronic cold exposure, the mortality of male mice was significantly higher than that of females, suggesting that estrogen was involved in cold stress responses (Deng et al., 2011). Previous studies also confirmed that stress and inflammatory responses are stronger in males than in females (Ide et al., 2002), and stronger post-menopause than pre-menopause (Bombelli et al., 2005), suggesting a close correlation between estrogen and oxidative stress. Therefore, we hypothesize that estrogen protects against cold-induced neural injury. Estradiol is a steroid hormone with an important role in the reproductive, circulatory, and nervous systems (Brann et al., 2007). GPR30 is an estrogen receptor mainly distributed in the brain, such as the cerebral cortex and hypothalamus. GPR30 is required for the neurophysiological functions of estrogen. Previous studies confirmed that estrogen participates in neuronal activity of the hypothalamic-pituitary-adrenal axis after signaling through GPR30 (Lebesgue et al., 2009; Tang et al., 2014), to maintain body temperature (Handa et al., 2011; Shivers et al., 2015).
Because the activity of estrogen requires GPR30 expression, we detected estrogen levels in the blood and quantified GPR30 in the CA1 area. Our study showed that estrogen levels in the blood and GPR30 expression in the CA1 region were increased significantly after chronic cold exposure. Considering neural oxidative stress responses and the appearance of neuronal autophagy after cold exposure, estrogen probably mediates oxidative stress responses and neuronal autophagy in the central nervous system during cold exposure. The regulative mechanism of estrogen for neural immune responses and autophagy is not fully understood. We hypothesize that the GPR30 pathway is activated once estrogen signals through its receptor (Lee et al., 2012; Pupo et al., 2016). Estrogen mediates neural immunity and has neuroprotective functionsviathe GPR30 pathway by the production of estrogen-induced cytoprotective factors, such as heat shock proteins (Gragasin et al., 2003) and neurotrophic factors (Hou et al., 2010). Estrogen increases the expression of Bcl-2, a negative regulator of cell apoptosis including autophagy (Bei et al., 2012; Brann et al., 2012; Naderi et al., 2015). Moreover, estrogen improves cerebral blood circulation in cortical infarct areas by increasing nitric oxide release (McNeill et al., 1999).
In summary, chronic cold exposure induces neural oxidative stress, inflammatory damage, and autophagy. Immune responses are induced by an increase in the numbers of microglia and IL-1β- and IL-6-positive cells. Furthermore, blood estrogen levels and GPR30 expression were increased after cold exposure. Our study suggests that oxidative stress, autophagy, and immune responses mediatedviaestrogen have neuroprotective functions during cold exposure responses. Our data further contributes to our knowledge of cryomedicine and new clinical treatment strategies. Furthermore, these data will be useful in research of military survival and athletic performance in extreme cold environments.
Author contributions:TTQ collected the main experimental data. JXD, RLL, ZJC and XQW provided technical assistances. LW and JBD wrote the paper and designed the assay. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Alvira CM (2014) Nuclear factor-kappa-B signaling in lung development and disease: one pathway, numerous functions. Birth Defects Res A Clin Mol Teratol 100:202-216.
Awada R, Saulnier-Blache JS, Grès S, Bourdon E, Rondeau P, Parimisetty A, Orihuela R, Harry GJ, d’Hellencourt CL (2014) Autotaxin downregulates LPS-induced microglia activation and pro-inflammatory cytokines production. J Cell Biochem 115:2123-2132.
Bei S, Yifan C, Kunlin J (2012) Estrogen, neuroprotection and neurogenesis after ischemic stroke. Curr Drug Targets 13:188-198.
Blanchard H, Taha AY, Cheon Y, Kim HW, Turk J, Rapoport SI (2014) iPLA2β knockout mouse, a genetic model for progressive human motor disorders, develops age-related neuropathology. Neurochem Res 39:1522-1532.
Bombelli M, Sega R, Facchetti R, Corrao G, Friz HP, Vertemati AM, Sanvito R, Banfi E, Carugo S, Primitz L, Mancia G (2005) Prevalence and clinical significance of a greater ambulatory versus office blood pressure (‘reversed white coat’ condition) in a general population. J Hypertens 23:513-520.
Brann D, Raz L, Wang R, Vadlamudi R, Zhang Q (2012) Oestrogen signalling and neuroprotection in cerebral ischaemia. J Neuroendocrinol 24:34-47.
Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM (2007) Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72:381-405.
Brazaitis M, Eimantas N, Daniuseviciute L, Vitkauskiene A, Paulauskas H, Skurvydas A (2015) Two strategies for the acute response to cold exposure but one strategy for the response to heat stress. Int J Hyperthermia 31:325-335.
Cano I, Selivanov V, Gomez-Cabrero D, Tegnér J, Roca J, Wagner PD, Cascante M (2014) Oxygen pathway modeling estimates high reactive oxygen species production above the highest permanent human habitation. PLoS One 9:e111068.
Cho YS, Shin MS, Ko IG, Kim SE, Kim CJ, Sung YH, Yoon HS, Lee BJ (2015) Ulinastatin inhibits cerebral ischemia-induced apoptosis in the hippocampus of gerbils. Mol Med Rep 12:1796-1802.
Correale J (2014) The role of microglial activation in disease progression. Mult Scler 20:1288-1295.
Cui ZJ, Deng JX, Zhao KB, Yu DM, Hu S, Shi SQ, Deng JB (2014) Effects of chronic cold exposure on murine central nervous system. J Neurosci Res 92:496-505.
Deng TX, Wang ZX, Deng JB, Ma Z, Yan ZY, Chang C, Gao XQ (2011) The study of sexual difference against coldness. Yixue Yanjiu Zazhi 40:44-46.
Gil-Mohapel J, Brocardo PS, Choquette W, Gothard R, Simpson JM, Christie BR (2013) Hippocampal neurogenesis levels predict WATERMAZE search strategies in the aging brain. PLoS One 8:e75125.
Gil-Núñez AC, Villanueva JA (2001) Advantages of lipid-lowering therapy in cerebral ischemia: role of HMG-CoA reductase inhibitors. Cerebrovasc Dis 11 Suppl 1:85-95.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918-934.
Gragasin FS, Xu Y, Arenas IA, Kainth N, Davidge ST (2003) Estrogen reduces angiotensin II-induced nitric oxide synthase and NAD(P) H oxidase expression in endothelial cells. Arterioscler Tromb Vasc Biol 23:38-44.
Handa RJ, Sharma D, Uht R (2011) A role for the androgen metabolite, 5alpha androstane 3beta, 17beta diol (3β-diol) in the regulation of the hypothalamo-pituitary-adrenal axis. Front Endocrinol (Lausanne) 2:65.
Hou Y, Wei H, Luo Y, Liu G (2010) Modulating expression of brain heat shock proteins by estrogen in ovariectomized mice model of aging. Exp Gerontol 45:323-330.
Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A (2002) Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Tromb Vasc Biol 22:438-442.
Jensen KD, Wang Y, Wojno ED, Shastri AJ, Hu K, Cornel L, Boedec E, Ong YC, Chien YH, Hunter CA, Boothroyd JC, Saeij JP (2011) Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9:472-483.
Kathiria AS, Butcher LD, Feagins LA, Souza RF, Boland CR, Teiss AL (2012) Prohibitin 1 modulates mitochondrial stress-related autophagy in human colonic epithelial cells. PLoS One 7:e31231.
Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14:32-43.
Lawson LJ, Perry VH, Gordon S (1992) Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48:405-415.
Lebesgue D, Reyna-Neyra A, Huang X, Etgen AM (2009) GPR30 differentially regulates short latency responses of luteinising hormone and prolactin secretion to oestradiol. J Neuroendocrinol 21:743-752.
Lee E, Sidoryk-Wêgrzynowicz M, Wang N, Webb A, Son DS, Lee K, Aschner M (2012) GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem 287:26817-26828.
Lee SG, Yoo DY, Jung HY, Nam SM, Kim JW, Choi JH, Yi SS, Won MH, Yoon YS, Hwang IK, Moon SM (2015) Neurons in the hippocampal CA1 region, but not the dentate gyrus, are susceptible to oxidative stress in rats with streptozotocin-induced type 1 diabetes. Neural Regen Res 10:451-456.
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27-42.
Li ZX, Lv JL, Chen P, Zhao RL (2015) Effects of ischemic postconditioning on related pathways and interleukin 8 expression in rat models of focal cerebral ischemia/reperfusion. Zhongguo Zuzhi Gongcheng Yanjiu 19:7938-7944.
Loane DJ, Byrnes KR (2010) Role of microglia in neurotrauma. Neurotherapeutics 7:366-377.
Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI (2014) Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol 73:14-29.
Lu Q, Black SM (2016) Iron metabolism, oxidative stress, and neonatal brain injury. Neural Regen Res 11:725-726.
Lu S, Xu D (2013) Cold stress accentuates pressure overload-induced cardiac hypertrophy and contractile dysfunction: role of TRPV1/ AMPK-mediated autophagy. Biochem Biophys Res Commun 442:8-15.
Luheshi NM, Kovács KJ, Lopez-Castejon G, Brough D, Denes A (2011) Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J Neuroinflammation 8:186-186.
Lv XC, Zhou HY (2012) Resveratrol protects H9c2 embryonic rat heart derived cells from oxidative stress by inducing autophagy: role of p38 mitogen-activated protein kinase. Can J Physiol Pharmacol 90:655-662.
McNeill AM, Kim N, Duckles SP, Krause DN (1999) Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke 30:2186-2190.
Mihailidou AS, Loan Le TY, Mardini M, Funder JW (2009) Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension 54:1306-1312.
Mohr WJ, Jenabzadeh K, Ahrenholz DH (2009) Cold injury. Hand Clin 25:481-496.
Morris G, Berk M, Walder K, Maes M (2015) Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med 13:28.
Naderi V, Khaksari M, Abbasi R, Maghool F (2015) Estrogen provides neuroprotection against brain edema and blood brain barrier disruption through both estrogen receptors α and β following traumatic brain injury. Iran J Basic Med Sci 18:138-144.
Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D (2011) Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 96:192-199.
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20:460-473.
Pupo M, Maggiolini M, Musti AM (2016) GPER Mediates Non-Genomic Effects of Estrogen. Methods Mol Biol 1366:471-488.
Schmidt-Kastner R (2015) Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience 309:259-279.
Shivers K-Y, Amador N, Abrams L, Hunter D, Jenab S, Quiñones-Jenab V (2015) Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine 72:121-129.
Sugama S, Takenouchi T, Fujita M, Kitani H, Hashimoto M (2011) Cold stress induced morphological microglial activation and increased IL-1β expression in astroglial cells in rat brain. J Neuroimmunol 233:29-36.
Takeda K, Maruki M, Yamagaito T, Muramatsu M, Sakai Y, Tobimatsu H, Kobayashi H, Mizuno Y, Hamaguchi Y (2013) Highly sensitive detection of hepatitis B virus surface antigen by use of a semiautomated immune complex transfer chemiluminescence enzyme immunoassay. J Clin Microbiol 51:2238-2244.
Tang H, Zhang Q, Yang L, Dong Y, Khan M, Yang F, Brann DW, Wang R (2014) Reprint of ”GPR30 mediates estrogen rapid signaling and neuroprotection”. Mol Cell Endocrinol 389:92-98.
Terrien J, Perret M, Aujard F (2011) Behavioral thermoregulation in mammals: a review. Front Biosci (Landmark Ed) 16:1428-1444.
Town T (2010) Inflammation, immunity, and Alzheimer’s disease. CNS Neurol Disord Drug Targets 9:129-131.
Uchida Y, Tokizawa K, Nakamura M, Mori H, Nagashima K (2010) Estrogen in the medial preoptic nucleus of the hypothalamus modulates cold responses in female rats. Brain Res 1339:49-59.
Varela S, Lima-Ribeiro MS, Diniz-Filho JA, Storch D (2015) Differential effects of temperature change and human impact on European Late Quaternary mammalian extinctions. Glob Chang Biol 21:1475-1481.
Venditti P, Bari A, Di Stefano L, Di Meo S (2007) Vitamin E attenuates cold-induced rat liver oxidative damage reducing H2O2mitochondrial release. Int J Biochem Cell Biol 39:1731-1742.
Vianna HR, Soares CM, Tavares MS, Teixeira MM, Silva AC (2011) Inflammation in chronic kidney disease: the role of cytokines. J Bras Nefrol 33:351-364.
Wang J, Du JR, Wang Y, Kuang X, Wang CY (2010) Z-ligustilide attenuates lipopolysaccharide-induced proinflammatory response via inhibiting NF-κB pathway in primary rat microglia. Acta Pharmacol Sin 31:791-797.
Wang X, Che H, Zhang W, Wang J, Ke T, Cao R, Meng S, Li D, Weiming O, Chen J, Luo W (2015) Effects of mild chronic intermittent cold exposure on rat organs. Int J Biol Sci 11:1171-1180.
Xie S, Liu B, Fu S, Wang W, Yin Y, Li N, Chen W, Liu J, Liu D (2014) GLP-2 suppresses LPS-induced inflammation in macrophages by inhibiting ERK phosphorylation and NF-κB activation. Cell Physiol Biochem 34:590-602.
Yang S, Gao L, Lu F, Wang B, Gao F, Zhu G, Cai Z, Lai J, Yang Q (2015a) Transcription factor myocyte enhancer factor 2D regulates interleukin-10 production in microglia to protect neuronal cells from inflammation-induced death. J Neuroinflammation 12:33.
Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y (2015b) Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation 12:26.
Zhou SF, Gao L, He WY, Cui ZJ, Deng JB (2014) Prenatal alcohol exposure inducing insulin resistance and stress injury in mouse hippocampi. Jiepou Xuebao 45:7-14.
Copyedited by Croxford L, Raye W, Yu J, Li CH, Qiu Y, Song LP, Zhao M
*Correspondence to: Lai Wang, M.D. or Jin-bo Deng, M.D., wanglai@henu.edu.cn or jinbo_deng@henu.edu.cn.
#These authors contributed equally to this study.
orcid: 0000-0002-3323-7210 (Lai Wang) 0000-0002-3166-2686 (Jin-bo Deng)
10.4103/1673-5374.202932
Accepted: 2017-01-19
杂志排行
中国神经再生研究(英文版)的其它文章
- Telomerase and mTOR in the brain: the mitochondria connection
- Impacts of the retinal environment and photoreceptor type on functional regeneration
- Tissue-type plasminogen activator is a homeostatic regulator of synaptic function in the central nervous system
- Novel insights into the role of NF-κB p50 in astrocytemediated fate specification of adult neural progenitor cells
- Anesthetic considerations for patients with acute cervical spinal cord injury
- Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator
