Anesthetic considerations for patients with acute cervical spinal cord injury
2017-04-07
Department of Anesthesiology, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, China
REVIEW
Anesthetic considerations for patients with acute cervical spinal cord injury
Fang-ping Bao, Hong-gang Zhang*, Sheng-mei Zhu
Department of Anesthesiology, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, China
How to cite this article:Bao FP, Zhang HG, Zhu SM (2017) Anesthetic considerations for patients with acute cervical spinal cord injury. Neural Regen Res 12(3):499-504.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Anesthesiologists work to prevent or minimize secondary injury of the nervous system and improve the outcome of medical procedures. To this end, anesthesiologists must have a thorough understanding of pathophysiology and optimize their skills and equipment to make an anesthesia plan. Anesthesiologists should conduct careful physical examinations of patients and consider neuroprotection at preoperative interviews, consider cervical spinal cord movement and compression during airway management, and suggest awake fiberoptic bronchoscope intubation for stable patients and direct laryngoscopy with manual in-line immobilization in emergency situations. During induction, anesthesiologists should avoid hypotension and depolarizing muscle relaxants. Mean artery pressure should be maintained within 85-90 mmHg (1 mmHg = 0.133 kPa; vasoactive drug selection and fluid management). Normal arterial carbon dioxide pressure and normal blood glucose levels should be maintained. Intraoperative neurophysiological monitoring is a useful option. Anesthesiologists should be attentive to postoperative respiratory insufficiency (carefully considering postoperative extubation), thrombus, and infection. In conclusion, anesthesiologists should carefully plan the treatment of patients with acute cervical spinal cord injuries to protect the nervous system and improve patient outcome.
nerve regeneration; cervical spine injury; cervical spinal cord injury; spinal cord injury; anesthesia; airway management; induction; intubation; neuroprotection; neurophysiological monitoring; neuroprotection; neural regeneration
Introduction
Cervical spine injury (CSI) occurs in 0.9% to 3% of all patients with blunt trauma, with a weighted average of 1.8% (Crosby et al., 1990; Casha et al., 2011). Acute cervical spinal cord injury (CSCI) can cause significant morbidity and mortality. Most of these patients will see an anesthesiologist, as patients with CSCI often require emergent airway management (intubation) and emergent surgery. Guidelines (Hadley et al., 2013) exist for the treatment of spinal cord injury (SCI), but these include few details for anesthesiologists, even though they always take part in patient management. Anesthesiologists may be able to prevent or minimize secondary injury to the nervous system and improve patient outcome by ensuring that they understand the pathophysiology of CSCI and make a patient-specific plan during the perioperative period. In this review, we highlight important factors for the optimal treatment of acute CSCI patients by anesthesiologists during preoperative interviews, airway management, induction administration, management during general anesthesia, and recovery phases. We hope that such information will provide anesthesiologists with useful information about CSCI, enabling them to provide optimal management (Figure 1).
Preoperative Assessment
During this period, the anesthesiologist has the opportunity to interrogate and examine patients to develop treatment strategies. As CSCI patients present a particular challenge, anesthesiologists should pay special attention to establishing an anesthesia plan for these patients.
History
First, it is necessary to determine the cause of injury, if possible. This will enable the anesthesiologist to anticipate likely injury patterns. For example, “rear end” motor vehicle collisions usually result in damage to central cord syndrome patterns. Second, the time span from injury to treatment is important as this dictates whether prophylactic anticoagulation and hypothermia can be used. Additionally, the time course of treatment may signal the potential for complications that require anesthesiologist management. Although the timing of decompressive surgery was not given in some CSCI studies (Casha et al., 2011; Hadley et al., 2013), early surgery has been found to be feasible and safe, and can improve clinical and neurological outcomes as well as reduce medical costs (Furlan et al., 2011).
Physical examination
An initial assessment of the airway, respiration, and circulation of the patient should always be conducted prior to evaluation of the spine. CSCI patients should be monitored in intensive care, and hemodynamic instability and respiratory insufficien-cy should be managed. Maintenance of mean arterial blood pressure (MAP) at 85 to 90 mmHg in the first week following injury can improve spinal perfusion (Hadley et al., 2013). Respiratory insufficiency is common after CSCI. CSCI disrupts the function of the diaphragm, muscles, accessory respiratory muscles, and abdominal muscles. This can lead to the loss of vital capacity and expiratory force, predisposing patients to atelectasis and the secretion retention, thus resulting in a high incidence of pneumonia. Patients with CSCI above C3-5often need respiratory care. Intubation and ventilator support should be used if the patient has the following conditions: (1) PaO2<60 mmHg (1 mmHg = 0.133 kPa), (2) PaCO2> 45 mmHg, or (3) respiratory rate > 35 breaths per minute despite medical management (Friedman et al., 1999). Intranasal oxygen is usually sufficient for patients with CSCI below C5.
Neurological examination is thus necessary to designate the injured segment of the spinal cord. Frankel classification (Table 1) concerns both motor and sensory functions, and is associated with long-term prognosis. To exclude “spinal shock”, it is not possible to diagnose patients until at least 24-48 hours after injury.
Radiographic assessment
Radiographic images are widely used to evaluate CSI and SCI. For symptomatic patients, the 2002 American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANSCNS) guidelines recommended a 3-views cervical spine series. At present, high-quality computer tomography (CT) has been recommended (Hadley et al., 2013). CT is ideal for visualization of most fractures. Magnetic resonance imaging (MRI) allows detailed visualization of the soft tissue structure, and is sufficiently sensitive to reveal abnormal cord signals. MRI is optimal for detecting edema, hemorrhage, and ligamentous injuries. MRI may be required in patients whose clinical presentation cannot be explained by plain film or CT. Radiographic imaging can help anesthesiologists evaluate the level of SCI and cervical spine stability, plan airway management, and determine whether a ventilator is necessary. Only 20% of traumatic injuries are limited to the cervical spine, and 25-50% of CSI patients have associated head injuries (Sekhon et al., 2001). Patients with additional organ damage are more likely to require emergency airway and surgical interventions, and are also susceptible to secondary nerve injury.
Neuroprotection
Immobilization
To reduce secondary injury and mortality, spinal immobilization is widely recommended for cervical spine or CSCI patients. Cervical collars, sandbags, tape, and axial-traction devices are common. Used alone, collars cannot effectively reduce spinal movement (Podolsky et al., 1983; Bednar et al., 2004), and they may limit mouth-opening movements and interfere with airway management (Goutcher et al., 2005).
Methylprednisolone
Although not included in the 2013 guidelines, the 2002 guidelines recommended a high dose of methylprednisolone for patients within eight hours of the injury (Hadley et al., 2013). Indeed, few has known the clinical benefits and various harmful side effects of methylprednisolone , such as hyperglycemia, depression of the immune system, gastrointestinal bleeding, and even death. If surgeons choose to use methylprednisolone, the anesthesiologist should consider the management of potential complications.
GM-1 ganglioside was not recommended for treatment of CSCI in the 2013 guidelines (Hadley et al., 2013). Other substances, such as thyrotropin-releasing hormone, have potential, but require further testing with large clinical trials (Tsai et al., 2005; LiVecchi et al., 2011).
Hypothermia
Moderate hypothermia has been used to target several neurological disorders, including traumatic brain injury and SCI, in a variety of experimental and clinical situations. Deep and sustained hypothermia may lead to complications, including coagulopathy, cardiovascular instability, and infection. Systemic introduction of moderate hypothermia (33°C) appears to be safe and provides some benefits in terms of long-term functional recovery (Dietrich et al., 2011). At present, mild hypothermia or low normothermia have been widely accepted in the management of CSCI patients. Some studies have focused on local hypothermia, but clinical application has been limited due to the small number of samples and the lack of randomization and control.
Airway Management
The primary objective when managing the airway in a CSCI patient is to minimize neck movement while rapidly and efficiently securing the airway.
All basic airway maneuvers have effects on the injured neck (Aprahamian et al., 1984). Mask ventilation is a commonly used method, and moves the cervical spine more than any other method (Hauswald et al., 1991). Although cricoid pressure is safe in CSI patients (Donaldson et al., 1997), other types of movement or force may enhance injury.
Direct laryngoscopy induces the greatest motion at the craniocervical junction, moderate motion at the C1to C2joints, and causes minimal displacement below C4in healthy patients (Sawin et al., 1996). Direct laryngoscopy can still cause spinal movement during manual in-line immobilization (MILI) involving a cervical collar and traction, although the movement magnitude is small and within physiological range in the injured spine model (Gerling et al., 2000; Lennarson et al., 2001). Different laryngoscope blades (Macintosh, McCoy, and Miller) are comparable in terms of their influence on spinal movement during direct laryngoscopy (Hastings et al., 1995; MacIntyre et al., 1999; Gerling et al., 2000).
MILI is most frequently used during airway management, and is applied to limited head and neck movement. Although MILI improves laryngoscopic grade in some patients, it increases the failure rate of successful intubations within the first 30 seconds (Nolan et al., 1993; Tiboutot et al., 2009).Compared with collar immobilization, MILI reduces total spinal movement (Majernick et al., 1986; Watts et al., 1997) and improves laryngeal visualization (Heath et al., 1994; Gerling et al., 2000) during oral tracheal intubation. When MILI is in place, the front portion of the collar can be removed to allow for larger openings, thereby facilitating airway interventions. MILI is recommended as an airway intervention standard in known or suspected cervical injury patients.
Video laryngoscopy is an alternative to conventional direct laryngoscopy. Several studies have compared video and direct laryngoscopy, with conflicting results. One study revealed that video laryngoscopy prolonged intubation time (Wetsch et al., 2012). The Airtraq intubation device reduced cervical motion (Hirabayashi et al., 2007; Maruyama et al., 2008) and facilitated intubation (Maharaj et al., 2007; McElwain et al., 2011), while the GlideScope prolonged intubation time. One study reported that the GlideScope reduced moment in a particular cervical segment (Turkstra et al., 2005), while another reported no difference in cervical segment movement (Robitaille et al., 2008). Other devices such as the fiberoptic laryngoscope (Bullard) (Watts et al., 1997; Gerling et al., 2000) and gum elastic bougie (Nolan et al., 1993; Nocera et al., 1996) are important adjuncts during laryngoscopy in patients with suspected CSCI, as these devices can minimize cervical spine movement.
Laryngeal mask airways can cause some posterior displacement at upper cervical levels (Keller et al., 1999; Kihara et al., 2000). Although the above-listed techniques may still produce movements in the cervical spine, this should not prevent them from being used to save lives (Brimacombe et al., 2000). In the “cannot intubate, cannot ventilate” scenario, surgical airway or cricothyroidotomy must be performed.
Although neurological deterioration has been reported in CSI patients with direct laryngoscopy (Hastings et al., 1991, 1993; Muckart et al., 1997), most studies find no deterioration caused by direct laryngoscopy or other airway maneuvers (Rhee et al., 1990; Scanell et al., 1993; Shatney et al., 1995). A wake-up cannula can be neurologically tested after intubation and positioning. However, it requires a co-operative patient. Intubations performed on patients who are awake are not better than those performed in sleeping patients (Crosby et al., 1990; Suderman et al., 1991).
When faced with CSI patients in stable situations (breathing spontaneously with stale vital signs), most anesthesiologists opt to perform awake fiberoptic bronchoscope intubation (Rosenblatt et al., 1998; Jenkins et al., 2002). However, this technique requires adequate training and time for intubation, and is difficult to perform in the presence of blood, vomit, secretions, or distorted anatomy. In emergency situations, most anesthesiologists rely on direct laryngoscopy with MILI for CSI patients. Hence, for each CSCI patient, an anesthesiologist may select an airway management method based on previous experience and the patient status.
Induction
There are no specific rules regarding the use of anesthetic for induction. Caution should be used during induction with propofol, benzodiazepines, or barbiturates, because these drugs may cause severe hypotension in patients with hypovolemia. Kematine may increase axial pressure, but this effect can be balanced by the parallel use of hypnotics such as propofol.
Between three days to nine months following CSCI, patients should avoid depolarizing muscle relaxants such as succinylcholine, as these can trigger fatal hyperkalemia (Cooperman, 1970). Non-depolarizing muscle relaxants are preferable because they do not increase axial pressure or induce fasciculation. Stimulation of airway tissue can lead to deep bradycardia, hypotension, and cardiac arrest (Yoo et al., 2003).
Intraoperative Neurophysiological Monitoring
Although intraoperative neurophysiological monitoring is not mentioned in the guidelines, several studies have focused on this method with respect to SCI patients. Intraoperative neurophysiological monitoring is useful for evaluating deterioration of spinal cord function; thus enabling correction of risk factors such as patient location (e.g., neck position and shoulder position), hypotension, hypothermia, and surgery-related factors.
Somatosensory evoked potential (SSEP) is the most widely used monitoring pattern in spinal surgery. SSEP directly monitors the dorsal column-medial lemniscus pathway, but not directly to the cortical spinal cord. Tus, SSEP may provide false negatives in patients with anterior spinal syndrome. The key limitation of SSEP monitoring is the time requirement for data summation. Thus, irreversible nerve damage can occur before the monitoring data can be analyzed. In addition, SSEP has low sensitivity to nerve root injury. When monitoring SSEPs, total intravenous anesthesia or balanced anesthesia with low concentrations of volatile anesthetics may be preferable (Loughnan et al., 1995).
Motor evoked potentials and muscle motor evoked potentials can be used to monitor motor pathways during spine surgery. Since stimulation induces movement, volatile anesthetics and nitrous oxide should be avoided; and when monitoring motor evoked potentials, a total intravenous technique without muscle relaxation is advised (Taniguchi et al., 1993).
Direct waves are generated by transcranial stimulation and are directly monitored at the level of the spinal cord. Direct waves are relatively resistant to anesthetic effects and permit the use of neuromuscular block paralysis.
During spinal cord surgery, spontaneous electromyography is widely used to monitor selective nerve root function. This monitoring prohibits the use of neuromuscular blockades, but allows the use of volatile anesthetics.
Triggered electromyography is used to assess the accuracy of pedicle screw placement. The main limitation of triggering electromyography is the high rate of false positive alarms. As with other forms of electromyography, neuromuscular blockade is prohibited.
Maintenance of Anesthesia
No convincing data have shown any advantages of one anes-thetic agent or technique over another. It is crucial that the regimen used enables the maintenance of adequate spinal cord perfusion. Ventilation should be adjusted to maintain normocapnia. With undisturbed CO2reactivity in the spinal cord perfusion, as exhibited in most patients, excessive hyperventilation may reduce blood flow to areas with inadequate perfusion.

Table 1 Frankel classification of spinal cord injury patients
Hyperglycemia must be avoided in patients with severe head trauma. Similarly, a blood glucose concentration above 200 mg/dl must be treated aggressively in acute SCI, because hyperglycemia is associated with poor neurologic outcomes (Drummond et al., 1989).
Cardiovascular System
In acute CSCI, the sympathetic nerve fibers in the superior ganglion can be physically or functionally disrupted, leading to reduced vascular tone and unbalanced vagal hyperactivity. Cardiovascular abnormalities, including bradycardia, hypotension, supraventricular arrhythmias, and cardiac arrest, are caused by autonomic nervous system imbalance.
Bradycardia: In the acute post-injury stage, 64-77% of patients experienced bradycardia with CSCI, especially those with high cervical (C1-5) lesions. Bradycardia peaks at four days after injury, and may persist in acute CSI patients for two weeks, during which period a positive sinus rhythm drug or pacemaker may be required (Lehmann et al., 1987; Bilello et al., 2003). Atropine is effective in increasing heart rate. Some procedures, such as oropharyngeal suction and endotracheal intubation, might increase vagal tone and cause cardiac arrest. Therefore, these should be carried out with atropine on hand in case of bradycardia.
Isoprenaline can increase sinus rhythm, but generally leads to arrhythmia. Tus, it is not widely used at present. Salbutamol is a β2-adrenergic receptor agonist used for treatment of asthma. It has a small but direct effect on β1-adrenoceptors, and can increase cardiac output and heat rate (Silke et al., 1999; Cekici et al., 2009).
Hypotension: Systemic hypotension frequently occurs after hemorrhage and neurogenic shock. Neurogenic shock resulting from the loss of central supraspinal sympathetic control and vasodilatation can lead to hypotension and inadequate tissue perfusion (Wuermser et al., 2007). Systemic hypotension reduces spinal cord perfusion pressure and contributes to secondary neurologic injury. It has been suggested that MAP be maintained within 85-90 mmHg, and systolic blood pressure kept above 90 mmHg for the first seven days.
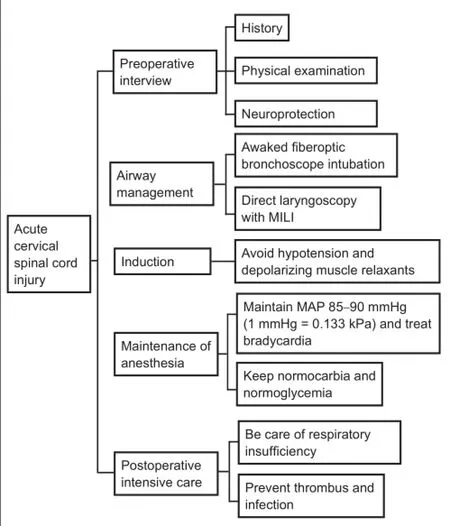
Figure 1 Guide for developing an appropriate anesthetic plan for acute cervical spinal cord injury.
Except for hypotension related to hypovolemia, excessive fluid administration is associated with cardiac failure, electrolyte abnormalities, coagulopathy, significant edema (including airway edema), and prolonged hospitalization in the postoperative intensive care (Rosenthal et al., 1999). Agents with inotropic, chronotropic, and vasoconstrictive properties should be used to maintain blood pressure. Hence, dopamine, norepinephrine, or epinephrine with their α1- and β1-agonist properties are acceptable options. Phenylephrine works as a α1-receptor agonist with minimal β1 effects, which may lead to reflex bradycardia. The use of dobutamine for treating SCI is limited due to its effect on vasodilation and the possible risk of reflex bradycardia (Nockels, 2001).
Acute blood loss leads to hypotension, which requires fluid management. Optimal fluid therapy for SCI patients is unknown. 5% dextrose in water and 0.45% normal saline made from hypotonic crystalloids should be avoided, as these may exacerbate spinal cord swelling. Cardiac output monitoring devices may improve intraoperative fluid delivery and may reduce morbidity resulting from excessive fluid administration. Major blood loss can lead to transfusions. In addition to the known risks of transfusing blood components, including potential transfusion reactions and alloimmunization, as well as the risk of contagion, the cost of blood replacement must be evaluated. Several techniques have been used to minimize intraoperative blood loss, including erythrocyteaugmentation, intraoperative antifibrinolytic administration, the use of topical hemostatic agents, intraoperative blood salvage, and postoperative blood salvage (Bess et al., 2006). Because it may exacerbate secondary SCI, hypotensive anesthesia should be avoided in SCI patients.
Coagulopathy can occur during spine surgery after a large number of blood transfusions. When performing multilevel instrumentation, significant blood loss should be anticipated. Homeostasis assays should be used to assess ongoing blood loss during surgery. Standard coagulation tests are too slow for use with actively bleeding patients. However, viscoelastic point-of-care coagulation assays have a rapid turn-around time (Plotkin et al., 2008).
Management in the Intensive Care Unit
All patients with acute SCI, especially those with severe CSCI, should be managed in the intensive care unit for adequate neurological, cardiac, hemodynamic, and respiratory monitoring. Life threatening cardiovascular instability and respiratory insufficiency may be transient and episodic, and may recur in the first 7-10 days after injury (Ryken et al., 2013). When deciding whether to immediately extubate patients postoperatively, anesthesiologists should consider the extent of the surgery, surgical complications, such as recurrent laryngeal nerve injury, the duration of surgery, prone position, degree of blood loss and subsequent fluid resuscitation, and the ease of intubation. For CSCI patients, respiratory insufficiency may occur not only immediately at postoperation, but may also be delayed.
These patients have a very high risk of thromboembolic complication. Thus, active prophylaxis is recommended for three months, using a combination of low-molecular-weight-heparins and a rotating bed, elastic or pneumatic compression stockings, or electrical stimulation (Dhall et al., 2013a).
Patients will have increased caloric requirements after SCI. Therefore, appropriate nutritional support should be provided. It is safe for SCI patients to meet caloric and nitrogen requirements, and such nutritional support can even reduce the deleterious effects of consumptive processes. Early enteral nutrition (within 72 hours) is safe, but does not affect neurological outcomes (Dhall et al., 2013b).
CSCI patients carry an increased risk of respiratory and/ or urinary tract infections, especially with the use of methylprednisolone. Other complications, such as occult peritonitis, may also occur and require attention.
Conclusions
Acute CSCI is a severe injury. Anesthesiologists managing patients with acute CSCI should make themselves aware of the relevant pathophysiology and provide specialized care to improve patient outcomes. Tey are advised to communicate effectively with surgeons, plan well in advance, and execute their plan at each step of the preoperative interview, airway management, induction, maintenance of anesthesia, and postoperative intensive care. In-depth CSCI research is underway to provide more efficient neuroprotective and neuroregenerative solutions for the future.
Author contributions:FPB wrote the paper. HGZ searched and analyzed data. SMZ designed the work. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Aprahamian C, Thompson BM, Finger WA, Darin JC (1984) Experimental cervical spine injury model: Evaluation of airway management and splinting techniques. Ann Emerg Med 13:584-587.
Bednar DA (2004) Efficacy of orthotic immobilization of the unstable subaxial cervical spine of the elderly patient: Investigation in a cadaver model. Can J Anaesth 47:251-256.
Bess RS, Lenke LG (2006) Blood loss minimization and blood salvage techniques for complex spinal surgery. Neurosurg Clin N Am 17:227-234.
Bilello JF, Davis JW, Cunningham MA, Groom TF, Lemaster D, Sue LP (2003) Cervical spinal cord injury and the need for cardiovascular intervention. Arch Surg 138:1127-1129.
Brimacombe J, Keller C, Künzel KH, Gaber O, Boehler M, Pühringer F (2000) Cervical spine motion during airway management: a cinefluoroscopic study of the posteriorly destabilized third cervical vertebrae in human cadavers. Anesth Analg 91:1724-1728.
Casha S, Christie S (2011) A systematic review of intensive cardiopulmonary management after spinal cord injuries. J Neurotrauma 28:1479-1495.
Cekici L, Valipour A, Kohansal R, Burghuber OC (2009) Short-term effects of inhaled salbutamol on autonomic cardiovascular control in healthy subjects: a placebo-controlled study. Br J Clin Pharmacol 67:394-402.
Cooperman LH (1970) Succinylcholine-induced hyperkalemia in neuromuscular disease. JAMA 213:1867-1871.
Crosby ET, Lui A (1990) The adult cervical spine: implications for airway management. Can J Anaesth 37:77-93.
Dhall SS, Hadley MN, Aarabi B, Gelb DE, Hurlbert RJ, Rozzelle CJ, Ryken TC, Teodore N, Walters BC (2013a) Deep venous thrombosis and thromboembolism in patients with cervical spinal cord injuries. Neurosugery 72 Suppl 2:244-254.
Dhall SS, Hadley MN, Aarabi B, Gelb DE, Hurlbert RJ, Rozzelle CJ, Ryken TC, Teodore N, Walters BC (2013b) Nutritional support after spinal cord injury. Neurosugery 72 Suppl 2:255-259.
Dietrich WD, Levi AD, Wang M, Green BA (2011) Hypothermic treatment for acute spinal cord injury. Neurotherapeutics 8:229-239.
Donaldson WFr, Heil BV, Donaldson VP, Silvaggio VJ (1997) The effect of airway maneuvers on the unstable C1-C2 segment. A cadaver study. Spine (Phila Pa 1976) 22:1215-1218.
Drummond JC, Moore SS (1989) The influence of dextrose administration on neurologic outcome after temporary spinal cord ischemia in the rabbit. Anesthesiology 70:64-70.
Friedman JH (1999) Vital signs stable. Med Health R I 82:346.
Furlan JC, Noonan V, Cadotte DW, Fehligs MG (2011) Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical sutuies. J Neurotrauma 88:1371-1399.
Gerling MC, Davis DP, Hamilton RS, Morris GF, Vilke GM, Garfin SR, Hayden SR (2000) Effects of cervical spine immobilization technique and laryngoscope blade selection on an unstable cervical spine in a cadaver model of intubation. annals of emergency medicine 36:293-300.
Goutcher CM, Lochhead V (2005) Reduction in mouth opening with semi-rigid cervical collars. Br J Anaesth 95:344-348.
Hadley MN, Walters BC (2013) Introduction of the guidelines for the management of acute cervical spine and spinal cord injuries. Neurosurgery 72:5-16.
Hadley MN, Aarabi B, Gelb DE, Hurlbert RJ, Rozzelle CJ, Ryken TC, Teodore N, Walters BC (2013) Nutritional support after spinal cord injury. Neurosurgery 72:255-259.
Hastings RH, Kelley SD (1993) Neurologic deterioration associated with airway management in a cervical spine-injured patient. Anesthesiology 78:580-583.
Hastings RH, Marks JD (1991) Airway management for trauma patients with potential cervical spine injuries. Anesth Analg 73:471-482.
Hastings RH, Vigil AC, Hanna R, Yang BY, Sartoris DJ (1995) Cervical spine movement during laryngoscopy with the Bullard, Macintosh and Miller laryngoscopes. Anesthesiology 82:859-869.
Hauswald M, Sklar DP, Tandberg D, Garcia JF (1991) Cervical spine movement during airway management: cinefluoroscopic appraisal in human cadavers. Am J Emerg Med 9:535-538.
Heath KJ (1994) The effect on laryngoscopy of different cervical spine immobilization techniques. Anaesthesia 49:843-845.
Hirabayashi Y, Fujita A, Seo N, Sugimoto H (2007) Cervical spine movement during laryngoscopy using the Airway Scope compared with the Macintosh laryngoscope. Anaesthesia 62:1050-1055.
Jenkins K, Wong DT, Correa R (2002) Management choices for the difficult airway by anesthesiologists in Canada. Can J Anaesth 49:850-856.
Keller C, Brimacombe J, Keller K (1999) Pressures exerted against the cervical vertebrae by the standard and intubating laryngeal mask airways: a randomized, controlled, cross-over study in fresh cadavers. Anesth Analg 89:1296-1300.
Kihara S, Watanabe S, Brimacombe J, Taguchi N, Yaguchi Y, Yamasaki Y (2000) Segmental cervical spine movement with the intubating laryngeal mask during manual in-line stabilization in patients with cervical pathology undergoing cervical spine surgery. Anesth Analg 91:195-200.
Lehmann KG, Lane JG, Piepmeier JM, Batsford WP (1987) Cardiovascular abnormalities accompanying acute spinal cord injury in humans: Incidence, time course and severity. J Am Coll Cardiol 10:46-52.
Lennarson PJ, Smith DW, Sawin PD, Todd MM, Sato Y, Traynelis VC (2001) Cervical spinal motion during intubation: efficacy of stabilization maneuvers in the setting of complete segmental instability. J Neurosurg 94:265-270.
LiVecchi MA (2011) spinal cord injury. Continuum (Minneap Minn) 17:568-583.
Loughnan BA, Fennelly ME (1995) spinal cord monitoring. Anaesthesia 50:101-102.
MacIntyre PA, McLeod AD, Hurley R, Peacock C (1999) Cervical spine movements during laryngoscopy: comparison of the Macintosh and McCoy laryngoscope blades. Anaesthesia 54:413-418.
Maharaj CH, Buckley E, Harte BH, Laffey JG (2007) Endotracheal intubation in patients with cervical spine immobilization: a comparison of macintosh and airtraq laryngoscopes. Anesthesiology 107:53-59.
Majernick TG, Bienek R, Houston JB, Hughes HG (1986) Cervical spine movement during orotracheal intubation. Ann Emerg Med 15:417-420.
Maruyama K, Yamada T, Kawakami R, Hara K (2008) Randomized cross-over comparison of cervical-spine motion with the AirWay Scope or Macintosh laryngoscope with in-line stabilization: a video-fluoroscopic study. Br J Anaesth 101:563-567.
McElwain J, Laffey JG (2011) Comparison of the C-MAC®, Airtraq®, and Macintosh laryngoscopes in patients undergoing tracheal intubation with cervical spine immobilization. Br J Anaesth 107:258-264.
Muckart DJ, Bhagwanjee S, van der Merwe R (1997) Spinal cord injury as a result of endotracheal intubation in patients with undiagnosed cervical spine fractures. Anesthesiology 87:418-420.
Nocera A (1996) A flexible solution for emergency intubation difficulties. Ann Emerg Med 27:665-667.
Nockels RP (2001) Nonoperative management of acute spinal cord injury. Spine (Phila Pa 1976) 26:31-37.
Nolan JP, Wilson ME (1993) Orotracheal intubation in patients with potential cervical spine injuries. Anaesthesia 48:630-633
Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, Perkins JG, Holcomb JB (2008) A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma 64:64-68.
Podolsky S, Baraff LJ, Simon RR, Hoffman JR, Larmon B, Ablon W (1983) Efficacy of cervical spine immobilization methods. J Trauma 23:461-465.
Rhee KJ, Green W, Holcroff JW, Mangili JA (1990) Oral intubation in the multiply injured patient: The risk of exacerbating spinal cord damage. Ann Emerg Med 19:511-514.
Robitaille A, Williams SR, Tremblay MH, Guilbert F, Tériault M, Drolet P (2008) Cervical spine motion during tracheal intubation with manual in-line stabilization: direct laryngoscopy versus GlideScope videolaryngoscopy. Anesth Analg 106:935-941.
Rosenblatt WH, Wagner PJ, Ovassapian A, Kain ZN (1998) Practice patterns in managing the difficult airway by anesthesiologists in the United States. Anesth Analg 87:153-157.
Rosenthal MH (1999) Intraoperative fluid management-what and how much? Chest 115:106-112.
Ryken TC, Hurlbert RJ, Hadley MN, Aarabi B, Dhall SS, Gelb DE, Rozzelle CJ, Teodore N, Walters BC (2013) The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery 72 Suppl 2:84-92.
Sawin PD, Todd MM, Traynelis VC, Farrell SB, Nader A, Sato Y, Clausen JD, Goel VK (1996) Cervical spine motion with direct laryngoscopy and orotracheal intubation. An in vivo cinefluoroscopic study of subjects without cervical abnormality. Anesthesiology 85:26-36.
Scanell G, Waxman K, Tommaga G, Barker S, Anna C (1993) Orotracheal intubations in trauma patients with cervical fractures. Arch Surg 128:903-905.
Sekhon LH, Fehlings MG (2001) Epidemiology, demographics and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 26:s2-12.
Shatney CH, Brunner RD, Nguyen TQ (1995) The safety of orotracheal intubation in patients with unstable cervical spine fracture or high spinal cord injury. Am J Surg 170:676-679.
Silke B, Hanratty CG, Riddell JG (1999) Heart-rate variability effects of beta-adrenoceptor agonists (xamoterol, prenalterol, and salbutamol) assessed nonlinearly with scatterplots and sequence methods. J Cardiovasc Pharmacol 33:859-867.
Suderman VS, Crosby ET, Lui A (1991) Elective oral tracheal intubation in cervical spine-injured adults. Can J Anaesth 38:785-789.
Taniguchi M, Cedzich C, Schramm J (1993) Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery 32:219-226.
Tiboutot F, Nicole PC, Trepanier CA, Turgeon AF, Lessard MR (2009) Effect of manual in-line stabilization of the cervical spine in adults on the rate of difficult orotracheal intubation by direct laryngoscopy: a randomized controlled trial. Can J Anaesth 56:412-418.
Tsai EC, Tator CH (2005) Neuroprotection and regeneration strategies for spinal cord repair. Curr Pharm Des 11:1211-1222.
Turkstra TP, Craen RA, Pelz DM, Gelb AW (2005) Cervical spine motion: a fluoroscopic comparison during intubation with lighted stylet, glidescope, and macintosh laryngoscope. Anesth Analg 101:910-915.
Watts AD, Gelb AW, Bach DB, Pelz DM (1997) Comparison of Bullard and Macintosh laryngoscopes for endotracheal intubation of patients with a potential cervical spine injury. Anesthesiology 87:1335-1342.
Wetsch WA, Spelten O, Hellmich M, Carlitscheck M, Padosch SA, Lier H, Böttiger BW, Hinkelbein J (2012) Comparison of different video laryngoscopes for emergency intubation in a standardized airway manikin with immobilized cervical spine by experienced anaesthetists. A randomized, controlled crossover trial. Resuscitation 2012:6.
Wuermser LA, Ho CH, Chiodo AE, Priebe MM, Kirshblum SC, Scelza WM (2007) Spinal cord injury medicine. 2. Acute care management of traumatic and nontraumatic injury. Arch Phys Med Rehabil 88:55-61.
Yoo KY, Jeong SW, Kim SJ, Ha IH, Lee J (2003) Cardiovascular responses to endotracheal intubation in patients with acute and chronic spinal cord injuries. Anesth Analg 97:1162-1167.
Copyedited by Koke S, Pack M, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*Correspondence to: Hong-gang Zhang, M.D., zhg1979@ hotmail.com.
orcid: 0000-0002-0161-3736 (Hong-gang Zhang)
10.4103/1673-5374.202916
Accepted: 2016-12-25
杂志排行
中国神经再生研究(英文版)的其它文章
- Transplantation of autologous peripheral blood mononuclear cells in the subarachnoid space for amyotrophic lateral sclerosis: a safety analysis of 14 patients
- Anatomical distributional defects in mutant genes associated with dominant intermediate Charcot-Marie-Tooth disease type C in an adenovirusmediated mouse model
- Mechanisms responsible for the inhibitory effects of epothilone B on scar formation after spinal cord injury
- The mechanism of Naringin-enhanced remyelination after spinal cord injury
- Estrogen affects neuropathic pain through upregulating N-methyl-D-aspartate acid receptor 1 expression in the dorsal root ganglion of rats
- Combination therapy using evening primrose oil and electrical stimulation to improve nerve function following a crush injury of sciatic nerve in male rats
