Neurorestoration from medicinal plants: an opportunity to treat painful neuropathies
2017-04-07LorenzoDiCesareMannelli,CarlaGhelardini
Neurorestoration from medicinal plants: an opportunity to treat painful neuropathies
Neuropathic pain originates from damages to the somatosensory nervous system (IASP - International Association for the Study of Pain - taxonomy). Lesions (traumas, compression, iatrogenic and pharmacological causes) or diseases (infections, diabetes, ischemia, cancer) lead to the establishment of hypersensitivity. Afterwards, the maladaptive plasticity of the nervous structures facilitates pain chronicization (von Hehn et al., 2012; Di Cesare Mannelli et al., 2013). Currently, clinicians have very limited therapeutic resources available often associated to a burden of side effects (Hurley et al., 2013). Recently, we showed (Di Cesare Mannelli et al., 2016, summarized inFigure 1) the efficacy of a selected rosemary (Rosmarinus officinalisL.) extract, obtained by an innovative extraction process. In rats, it is able to control neuropathic pain (induced by the chronic constriction injury of the sciatic nerve; CCI) and prevents the alterations of the nervous system induced by nerve injury. Data offer a preclinical evaluation for a prospective industrial scale up and therapeutic application of a natural, safe, disease-modifying product for the treatment of neuropathies.
The use of medicinal plants for treating complex, chronic pathologies related to severe alterations of the nervous system, like neuropathies, is an intriguing perspective. The scientific and clinical relevance of this approach is often compromised by evidences of activity obtained with not standardized procedures and referred to vegetal products scarcely characterized as extraction methods, chemical composition and pharmacodynamic profile. Starting by this awareness, selected extracts from rosemary leaves were obtained by the cooperation between the University of Florence and the University of Turin. The extracts were characterized and compared in terms of phenolic composition to define the more active constituents. The progressive increase of terpenoid contents paralleled with higher pain relieving efficacy till to suggest the fundamental role of carnosic acid. The strong anti-hyperalgesic and anti-allodynic effect of the pure compound let us to produce a new terpenoid-enriched rosemary extract (URE). A technological improvement was reached using the innovative ultrasounds assisted extraction procedure. The method is clean, the low bulk temperature and the rapid execution avoid degradative processes offering several advantages in terms of higher yields and higher selectivity, improving the processing time, reducing chemical and physical hazards.
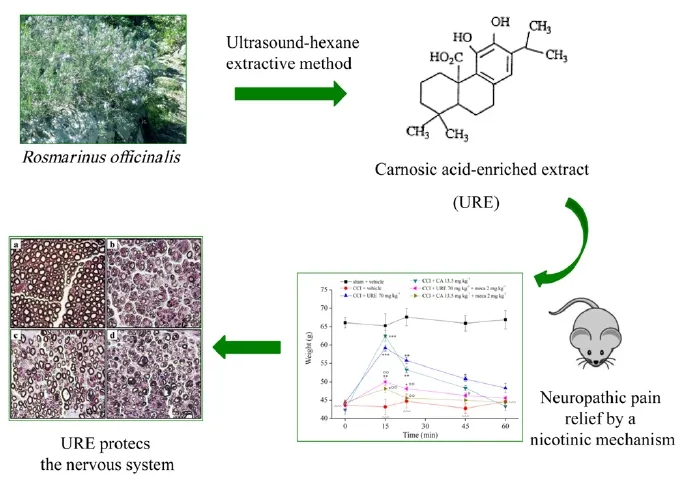
Figure 1 Schematic representation of the study.
The final extraction product URE was able to reduce the development of CCI-dependent painful response to suprathreshold stimulation and to increase pain threshold to non-noxious stimuli. URE reduced the postural unbalance related to spontaneous, non-evoked pain, a feature of neuropathy progression mainly related to the somatosensory component. The pain-relieving effect of URE progressively increased during treatment suggesting a rescue mechanism that protects the nervous tissuefrom damages that result in chronic pain. Consistently with previous reports (Pacini et al., 2010; Di Cesare Mannelli et al., 2014), CCI induces morphometric alterations of the sciatic nerve. We analyzed the sciatic nerve at different distances from the injury individuating 900 μm as optimal to evaluate damage characteristics and protective effects promoted by treatments. Repeated administrations of URE preserved the number and diameter of nerve fibers and significantly prevented the reduction in myelin sheet thickness and axonal diameter, both signs of Wallerian degeneration. Moreover, URE attenuated the degree of peripheral nerve inflammation, reducing edema and infiltrate which generally complicate the neuropathic condition after a trauma.
This interesting profile has been related to the stimulation of the nicotinic acetylcholine receptors (nAChRs) offering, uncommon with vegetal extracts, a pharmacodynamic explanation. The cholinergic system intervenes on pain perception by different signal pathways in acute and chronic pain. The nicotinic component is prevalent in chronic pain modulation (Di Cesare Mannelli et al., 2009), indirect cholinomimetic drugs as well as direct modulators effective against neuropathic pain acts by nAChRs. In particular, the homopentameric α7 and the α9α10 nAChR subtypes are involved in neuropathic conditions (Di Cesare Mannelli et al., 2014a; Pacini et al, 2016). Characteristically, the α7 and the α9α10 nAChR modulators are able to reduce pain and improve the restoration of the damaged nervous system. The double role of pain relieving and neuroprotective compounds has been shown in different models of neuropathic pain like trauma- or chemotherapy-induced neuropathies (Pacini et al., 2010, 2016; Di Cesare Mannelli et al., 2014a, b). Finally, a special consideration deserves the properties of nAChRs regarding glial cells. The plasticity of glia in spinal cord and brain areas participates in the central sensitization mechanisms: activated microglia and astrocytes have been recognized as powerful modulators of pain (Scholz and Woolf, 2007). It is usually accepted that glial inhibitors may reduce pain (Scholz and Woolf, 2007), on the other hand the downregulation of glial functionality strongly limits the physiological mechanisms of neurorestoration since neuroconservation is one of the housekeeping functions of glia (Milligan and Watkins, 2009). nAChRs possess the properties to reduce neuropathic pain and induce neuroprotection concomitantly to glial cell activation (Di Cesare Mannelli et al., 2014a, 2016; Pacini et al., 2016). The nicotinic signal may “modulate” glia functionality influencing the maladaptive plasticity of glial cells reducing pain and favouring neurorestoration. Accordingly, the nicotinic face of rosemary could offer a functional switch of glial cell signalling able to re-establish the nervous homeostasis.
As regards the safety profile, oleoresins of rosemary, characterized by a high concentration of these terpenoids, are frequently used as natural food preservative. The EFSA panel has accepted a NOAEL (No Observed Adverse Effect Level) value in the range of 20-60 mg/kg of carnosic acid plus carnosol in the rat (EFSA, 2008). The ADI (Acceptable Food Intake) has not been defined but the EFSA opinion is that the margin of safety is high enough to conclude that dietary exposure is not of safety concern (EFSA, 2008).
Medicinal plants are a generous source of biologically active compounds. Chemical composition and doses of plant extracts make the difference between a therapeutic product and a venom. Natural derivatives are not safe for sure, the traditional/ popular curative use of plants cannot substitute a scientific validation. Thecultivar, the region where the plant grew, the method of extraction are dramatically relevant differences among products derived from the same plant species. Moreover, the usefulness of the total extract, the so called phytocomplex, in comparison to a single molecule must be carefully analyzed. Phytocomplex may offer pharmacodynamic or pharmacokinetic advantages but it could reveal dangerous interactions or exacerbate toxicity. The scientific and rigorous approach to the study of pharmacognosy is the conditionsine qua nonto learn by nature for individuating active products for curative purposes or adjuvant therapies against pain.
Lorenzo Di Cesare Mannelli*, Carla Ghelardini
Department of Neuroscience, Psychology, Drug Research and Child Health - NEUROFARBA - Pharmacology and Toxicology Section, University of Florence, Florence, Italy
*Correspondence to:Lorenzo Di Cesare Mannelli, Ph.D., lorenzo.mannelli@unifi.it.
Accepted:2017-03-14
orcid:0000-0001-8374-4432 (Lorenzo Di Cesare Mannelli)
Di Cesare Mannelli L, Ghelardini C, Calvani M, Nicolai R, Mosconi L, Toscano A, Pacini A, Bartolini A (2009) Neuroprotective effects of acetyl-L-carnitine on neuropathic pain and apoptosis: a role for the nicotinic receptor. J Neurosci Res 87:200-207.
Di Cesare Mannelli L, Pacini A, Bonaccini L, Zanardelli M, Mello T, Ghelardini C (2013) Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain 14:1585-1600.
Di Cesare Mannelli L, Pacini A, Matera C, Zanardelli M, Mello T, De Amici M, Dallanoce C, Ghelardini C (2014a) Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: effects of selective activation. Neuropharmacology 79:37-48.
Di Cesare Mannelli L, Cinci L, Micheli L, Zanardelli M, Pacini A, McIntosh JM, Ghelardini C (2014b) α-conotoxin RgIA protects against the development of nerve injury-induced chronic pain and prevents both neuronal and glial derangement. Pain 155:1986-1995.
Di Cesare Mannelli L, Micheli L, Maresca M, Cravotto G, Bellumori M, Innocenti M, Mulinacci N, Ghelardini C (2016) Anti-neuropathic effects of Rosmarinus officinalis L. terpenoid fraction: relevance of nicotinic receptors. Sci Rep 6:34832.
EFSA-Q-2003-140 (2008) Food additives, flavourings, processing aids and materials in contact with food (AFC), Panel Members, Opinion of the Scientific Committee/Scientific Panel Published 12 June 2008.
Hurley RW, Adams MC, Benzon HT (2013) Neuropathic pain: treatment guidelines and updates. Curr Opin Anaesthesiol 26:580-587.
Milligan ED, Watkins LR (2009) Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10:23-36.
Pacini A, Di Cesare Mannelli L, Bonaccini L, Ronzoni S, Bartolini A, Ghelardini C (2010) Protective effect of alpha7 nAChR: behavioural and morphological features on neuropathy. Pain 150:542-549.
Pacini A, Micheli L, Maresca M, Branca JJ, McIntosh JM, Ghelardini C, Di Cesare Mannelli L (2016) The α9α10 nicotinic receptor antagonist α-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment. Exp Neurol 282:37-48.
Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10:1361-1368.
von Hehn CA, Baron R, Woolf CJ (2012) Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73:638-652.
10.4103/1673-5374.202941
How to cite this article:Di Cesare Mannelli L, Ghelardini C (2017) Neurorestoration from medicinal plants: an opportunity to treat painful neuropathies. Neural Regen Res 12(3):403-404.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Telomerase and mTOR in the brain: the mitochondria connection
- Impacts of the retinal environment and photoreceptor type on functional regeneration
- Tissue-type plasminogen activator is a homeostatic regulator of synaptic function in the central nervous system
- Novel insights into the role of NF-κB p50 in astrocytemediated fate specification of adult neural progenitor cells
- Anesthetic considerations for patients with acute cervical spinal cord injury
- Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator
