4种杀菌剂及其复配剂对番茄灰霉病菌的毒力
2017-03-29刘圣明车志平田月娥
刘圣明, 海 飞, 车志平, 田月娥, 刘 欣
(1. 河南科技大学林学院植物保护系,洛阳 471003;2. 河南农业职业学院农业工程系,郑州 451450)
4种杀菌剂及其复配剂对番茄灰霉病菌的毒力
刘圣明1*, 海 飞2, 车志平1, 田月娥1, 刘 欣1
(1. 河南科技大学林学院植物保护系,洛阳 471003;2. 河南农业职业学院农业工程系,郑州 451450)
采用菌丝生长速率法测定了咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑4 种杀菌剂及其两元复配剂对番茄灰霉病菌的毒力。结果显示,咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑对番茄灰霉病菌的有效抑制中浓度(EC50)分别为:0.018 0、0.018 1、1.896 8和2.087 4 μg/mL。复配剂啶酰菌胺∶苯醚甲环唑1∶5、1∶3和1∶1,咯菌腈∶苯醚甲环唑1∶5增效作用最明显;复配剂咯菌腈∶苯醚甲环唑1∶3,咯菌腈∶氟啶胺1∶3,啶酰菌胺∶咯菌腈5∶1,啶酰菌胺∶苯醚甲环唑3∶1具有增效作用,SR值范围为1.5~4.05,其中以复配剂啶酰菌胺∶苯醚甲环唑1∶5增效作用最好,其SR值为4.05;其余不同配比的各组合复配剂具有相加作用,其SR值范围为0.5~1.46。表明咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑4 种不同作用机制的杀菌剂可以交替或复配使用,以阻止或延缓灰霉病菌抗药性的进一步加剧,为灰霉病的综合防控和抗药性治理提供理论依据。
灰霉病; 灰葡萄孢; 杀菌剂; 复配剂
由灰葡萄孢BotrytiscinereaPers.exFr.,无性型Botryotiniafuckeliana(de Bary) Whetz引起的灰霉病是200多种作物,包括番茄、黄瓜和茄子等蔬菜以及草莓和葡萄等水果上的重要病害,能够造成花、叶、茎和果实的腐烂,影响作物的品质和产量,尤其在温室和大棚等设施蔬菜上危害尤为严重[1-5]。灰霉病在保护地番茄上严重发生时,能造成番茄花、叶和果实软腐,减产50%以上[6-8]。由于缺少高抗灰霉病的番茄品种,从20世纪70年代开始,生产上主要使用苯并咪唑类、二甲酰亚胺类、氨基甲酸酯类和苯胺基嘧啶类杀菌剂对灰霉病进行化学防治,但由于灰霉病菌具有较快的繁殖速率、较大的遗传变异性和较高的田间适合度等特点,极易对施用的杀菌剂产生抗药性,导致田间防治效果下降或丧失[6-13]。随着苯并咪唑类、二甲酰亚胺类、氨基甲酸酯类和苯胺基嘧啶类杀菌剂施药次数和施药量的不断增加,我国江苏、河南、山东、北京和辽宁等地相继出现了单抗、双抗、甚至多抗类型的灰霉病菌菌株[6-7, 10-12, 15-17]。2010年Sun等[12]研究发现江苏省南京市和淮阴市灰霉病菌对常用防治药剂苯并咪唑类杀菌剂多菌灵、二甲酰亚胺类杀菌剂腐霉利、氨基甲酸酯类杀菌剂乙霉威和苯胺基嘧啶类杀菌剂嘧霉胺的四抗菌株比例分别为51.6%和52.8%;山东省灰霉病菌对四类杀菌剂的四抗菌株比例为100%。2016年Liu等[10]研究发现河南省灰霉病菌对四类杀菌剂的四抗菌株比例为68.1%。先前的研究表明灰霉病菌对常用的苯并咪唑类、二甲酰亚胺类、氨基甲酸酯类和苯胺基嘧啶类防治药剂已经产生了较高比例的抗药性。为了阻止或延缓抗药性的进一步加剧,延长药剂的使用寿命,应科学选用合适的杀菌剂防治灰霉病。
采用不同作用机制的杀菌剂交替或复配使用,是阻止或延缓病原菌抗药性进一步加剧的主要策略。因此,本研究采用菌丝生长速率法,测定了咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑4种不同作用机制的杀菌剂单剂及其两元复配剂对番茄灰霉病菌的毒力,对生产上综合防控灰霉病和科学合理地用药具有重要的指导意义。
1 材料与方法
1.1 供试菌株
供试菌株为实验室保存的经单孢分离的番茄灰霉病菌。
1.2 供试药剂
97.9%咯菌腈原药,先正达(中国)有限公司提供;97%氟啶胺原药,江苏优士化学有限公司提供;95.4%苯醚甲环唑原药,先正达(中国)有限公司提供,上述3种药剂均预溶于甲醇,配制成104μg/mL母液。96.2%啶酰菌胺原药,巴斯夫(中国)有限公司提供,预溶于丙酮,配制成104μg/mL的母液。
1.3 供试培养基
PDA培养基:马铃薯200 g,葡萄糖20 g,琼脂15~20 g,加蒸馏水至1 000 mL。
1.4 4种杀菌剂毒力测定
采用菌丝生长速率法测定咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑对番茄灰霉病菌的离体毒力。将咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑母液稀释为系列浓度,按照一定的比例分别加入到灭菌热熔冷却至50℃左右的PDA培养基中,制成系列浓度(表1)的含药PDA平板,以不加药剂为对照。用直径为5 mm的打孔器,在25℃黑暗条件下培养3 d的灰霉病菌菌落边缘打孔制备菌饼,然后用接种针挑取菌饼分别接种于含咯菌腈、氟啶胺、啶酰菌胺或苯醚甲环唑系列浓度的PDA平板上。将各平板置于恒温培养箱内,25℃黑暗条件下培养3 d,测量并记录菌落直径。各处理3次重复,试验重复3次。根据灰霉病菌在不同浓度药剂平板上的线性生长速率,计算出各浓度药剂对病原菌的生长抑制率,用DPS统计软件进行处理,求出各药剂的EC50。
表1 4种杀菌剂毒力测定浓度
Table 1 Concentrations of four fungicides used for determination of their toxicity toBotrytiscinerea

杀菌剂Fungicide药剂浓度/μg·mL-1Fungicideconcentration咯菌腈Fludioxonil0,0.003125,0.00625,0.0125,0.025,0.1,0.4氟啶胺Fluazinam0,0.03125,0.0625,0.125,0.25,0.5,1啶酰菌胺Boscalid0,0.1,0.2,0.4,0.8,1.6,3.2苯醚甲环唑Difenoconazole0,0.3125,0.625,1.25,2.5,5,10
1.5 复配剂毒力测定
采用菌丝生长速率法测定复配剂对番茄灰霉病菌的离体毒力。将咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑4种药剂按照表2所示的比例分别进行两元复配后,分别加入到灭菌热熔冷却至50℃左右的PDA培养基中,制成含复配剂系列浓度(表2)的PDA平板。按照1.4测定和计算方法,求出各复配药剂的EC50。利用以下公式求出SR值[18],当SR值大于等于1.5时为增效作用,在0.5和1.5之间为相加作用,小于0.5为拮抗作用[19]。
A,B为复配的药剂;a,b为药剂在配方中所占的比例;EC50(Exp)为理论抑制中浓度,EC50(Obs)为实际测量抑制中浓度。
表2 复配剂不同比例浓度设计
Table 2 Concentrations of fungicides mixtures used for determination of their toxicity toBotrytiscinerea
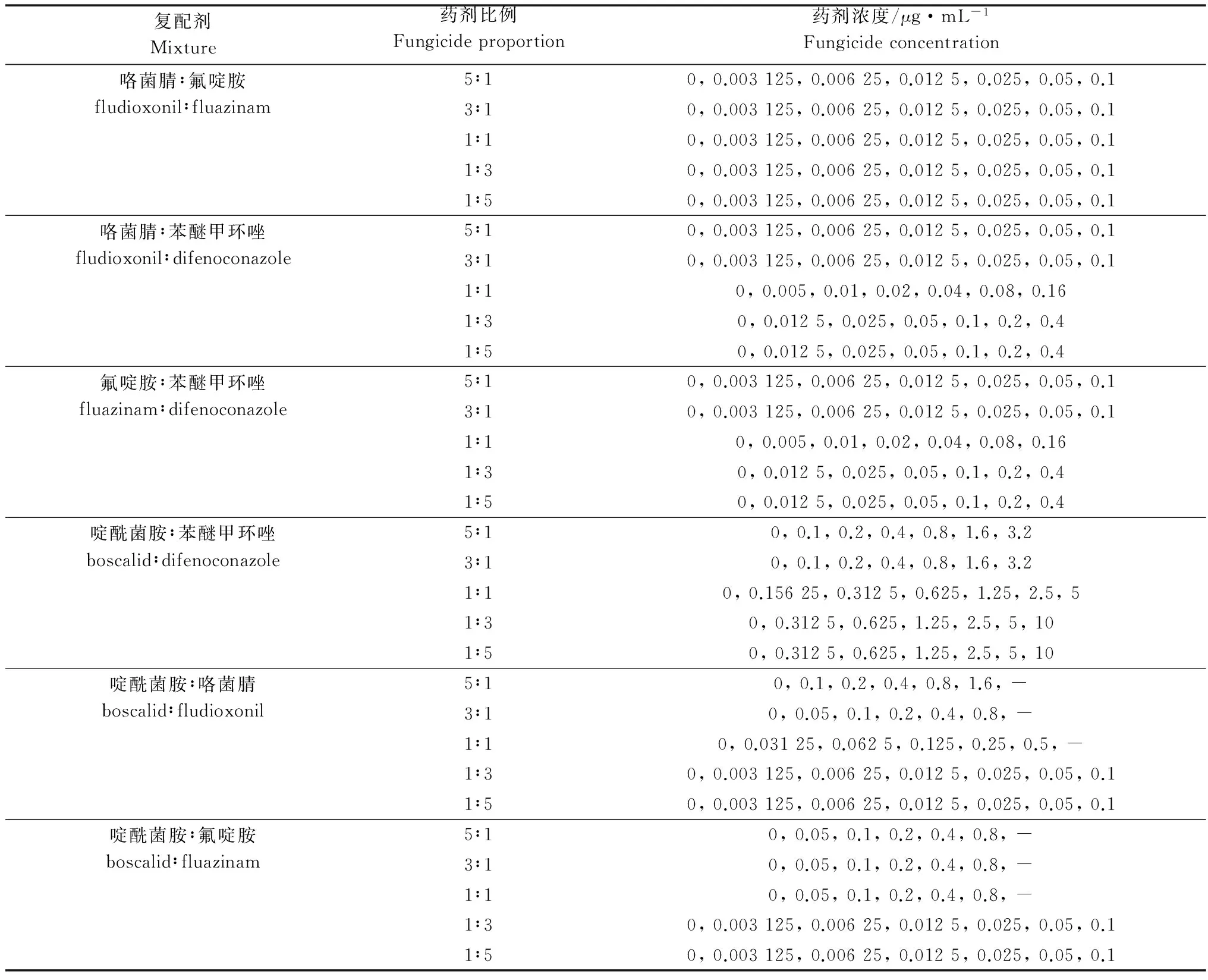
复配剂Mixture药剂比例Fungicideproportion药剂浓度/μg·mL-1Fungicideconcentration咯菌腈∶氟啶胺fludioxonil∶fluazinam5∶10,0.003125,0.00625,0.0125,0.025,0.05,0.13∶10,0.003125,0.00625,0.0125,0.025,0.05,0.11∶10,0.003125,0.00625,0.0125,0.025,0.05,0.11∶30,0.003125,0.00625,0.0125,0.025,0.05,0.11∶50,0.003125,0.00625,0.0125,0.025,0.05,0.1咯菌腈∶苯醚甲环唑fludioxonil∶difenoconazole5∶10,0.003125,0.00625,0.0125,0.025,0.05,0.13∶10,0.003125,0.00625,0.0125,0.025,0.05,0.11∶10,0.005,0.01,0.02,0.04,0.08,0.161∶30,0.0125,0.025,0.05,0.1,0.2,0.41∶50,0.0125,0.025,0.05,0.1,0.2,0.4氟啶胺∶苯醚甲环唑fluazinam∶difenoconazole5∶10,0.003125,0.00625,0.0125,0.025,0.05,0.13∶10,0.003125,0.00625,0.0125,0.025,0.05,0.11∶10,0.005,0.01,0.02,0.04,0.08,0.161∶30,0.0125,0.025,0.05,0.1,0.2,0.41∶50,0.0125,0.025,0.05,0.1,0.2,0.4啶酰菌胺∶苯醚甲环唑boscalid∶difenoconazole5∶10,0.1,0.2,0.4,0.8,1.6,3.23∶10,0.1,0.2,0.4,0.8,1.6,3.21∶10,0.15625,0.3125,0.625,1.25,2.5,51∶30,0.3125,0.625,1.25,2.5,5,101∶50,0.3125,0.625,1.25,2.5,5,10啶酰菌胺∶咯菌腈boscalid∶fludioxonil5∶10,0.1,0.2,0.4,0.8,1.6,-3∶10,0.05,0.1,0.2,0.4,0.8,-1∶10,0.03125,0.0625,0.125,0.25,0.5,-1∶30,0.003125,0.00625,0.0125,0.025,0.05,0.11∶50,0.003125,0.00625,0.0125,0.025,0.05,0.1啶酰菌胺∶氟啶胺boscalid∶fluazinam5∶10,0.05,0.1,0.2,0.4,0.8,-3∶10,0.05,0.1,0.2,0.4,0.8,-1∶10,0.05,0.1,0.2,0.4,0.8,-1∶30,0.003125,0.00625,0.0125,0.025,0.05,0.11∶50,0.003125,0.00625,0.0125,0.025,0.05,0.1
2 结果与分析
2.1 4种杀菌剂对番茄灰霉病菌的毒力
采用菌丝生长速率法测定了咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑对番茄灰霉病菌的毒力。结果(表3)显示,4种药剂对番茄灰霉病菌的有效抑制中浓度(EC50)分别为:0.018 0、0.018 1、1.896 8和2.087 4 μg/mL。表明4种药剂对番茄灰霉病菌的菌丝生长都有较好的抑制作用,4种单剂中咯菌腈对番茄灰霉病菌的毒力最高。
2.2 复配剂对番茄灰霉病菌的毒力
将咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑4 种药剂分别按照一定比例进行两元复配,采用菌丝生长速率法测定各复配剂对番茄灰霉病菌的毒力。结果(表 4)显示,不同组合、不同配比的复配剂对番茄灰霉病菌的毒力不同。复配剂啶酰菌胺:苯醚甲环唑1∶5、1∶3和1∶1、咯菌腈∶苯醚甲环唑1∶5增效作用最明显;复配剂咯菌腈∶氟啶胺1∶3、咯菌腈∶苯醚甲环唑1∶3、啶酰菌胺∶咯菌腈5∶1、啶酰菌胺∶苯醚甲环唑3∶1具有增效作用,SR范围为1.5~4.05,其中以复配剂啶酰菌胺∶苯醚甲环唑1∶5增效作用最好,其SR为4.05;其余不同配比的组合复配剂具有相加作用,其SR范围为0.5~1.46。
表3 4种药剂单剂对番茄灰霉病菌的毒力
Table 3 Toxicities of four fungicides againstBotrytiscinereabased on mycelial growthinvitro
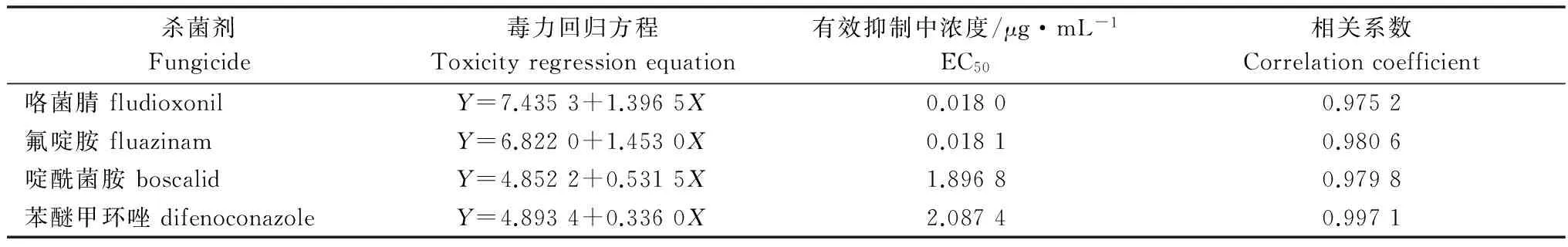
杀菌剂Fungicide毒力回归方程Toxicityregressionequation有效抑制中浓度/μg·mL-1EC50相关系数Correlationcoefficient咯菌腈fludioxonilY=7.4353+1.3965X0.01800.9752氟啶胺fluazinamY=6.8220+1.4530X0.01810.9806啶酰菌胺boscalidY=4.8522+0.5315X1.89680.9798苯醚甲环唑difenoconazoleY=4.8934+0.3360X2.08740.9971
表4 不同复配剂对番茄灰霉病菌的毒力
Table 4 Toxicities of mixtures of fungicides againstBotrytiscinereabased on mycelial growthinvitro
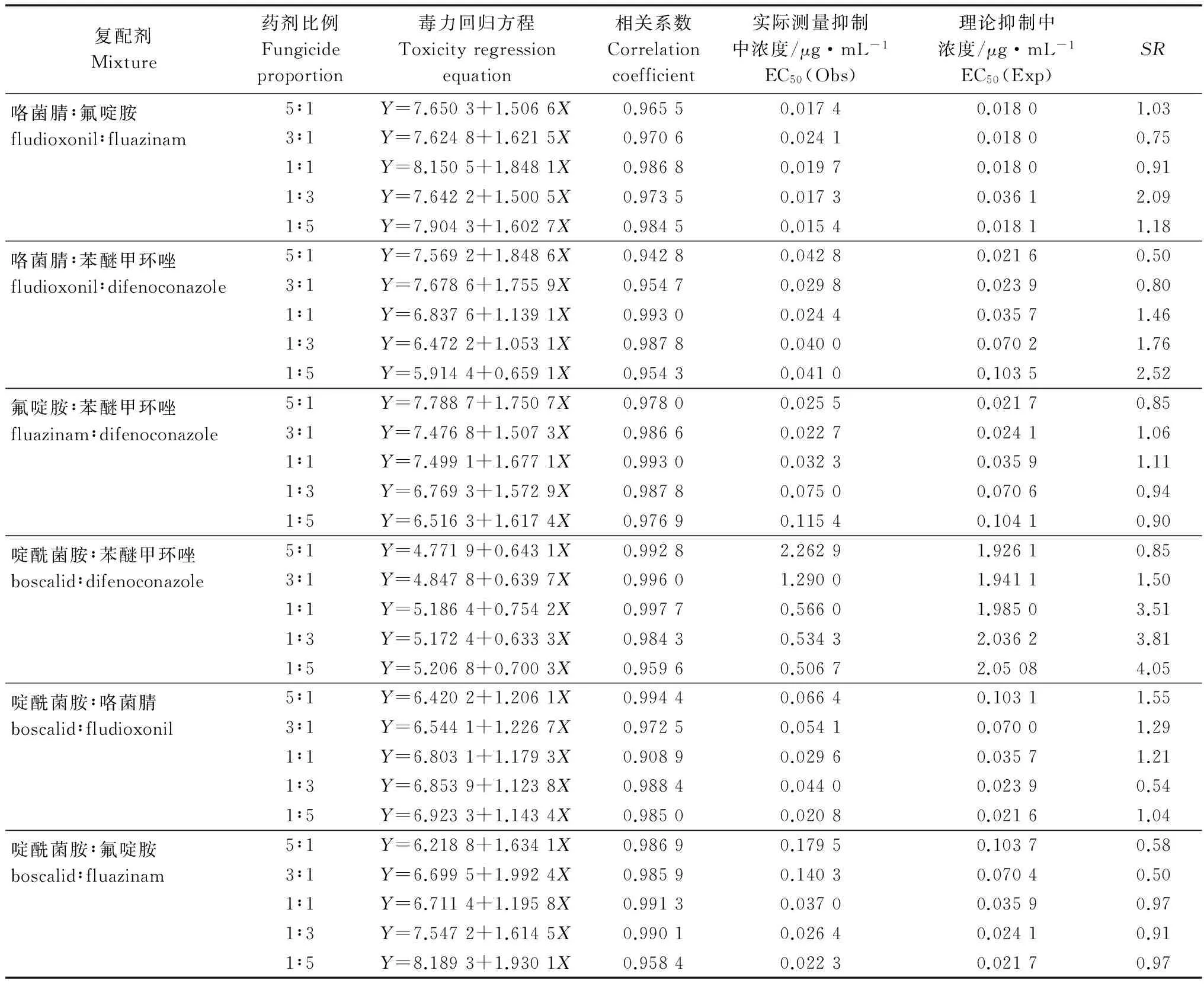
复配剂Mixture药剂比例Fungicideproportion毒力回归方程Toxicityregressionequation相关系数Correlationcoefficient实际测量抑制中浓度/μg·mL-1EC50(Obs)理论抑制中浓度/μg·mL-1EC50(Exp)SR咯菌腈∶氟啶胺fludioxonil∶fluazinam5∶1Y=7.6503+1.5066X0.96550.01740.01801.033∶1Y=7.6248+1.6215X0.97060.02410.01800.751∶1Y=8.1505+1.8481X0.98680.01970.01800.911∶3Y=7.6422+1.5005X0.97350.01730.03612.091∶5Y=7.9043+1.6027X0.98450.01540.01811.18咯菌腈∶苯醚甲环唑fludioxonil∶difenoconazole5∶1Y=7.5692+1.8486X0.94280.04280.02160.503∶1Y=7.6786+1.7559X0.95470.02980.02390.801∶1Y=6.8376+1.1391X0.99300.02440.03571.461∶3Y=6.4722+1.0531X0.98780.04000.07021.761∶5Y=5.9144+0.6591X0.95430.04100.10352.52氟啶胺∶苯醚甲环唑fluazinam∶difenoconazole5∶1Y=7.7887+1.7507X0.97800.02550.02170.853∶1Y=7.4768+1.5073X0.98660.02270.02411.061∶1Y=7.4991+1.6771X0.99300.03230.03591.111∶3Y=6.7693+1.5729X0.98780.07500.07060.941∶5Y=6.5163+1.6174X0.97690.11540.10410.90啶酰菌胺∶苯醚甲环唑boscalid∶difenoconazole5∶1Y=4.7719+0.6431X0.99282.26291.92610.853∶1Y=4.8478+0.6397X0.99601.29001.94111.501∶1Y=5.1864+0.7542X0.99770.56601.98503.511∶3Y=5.1724+0.6333X0.98430.53432.03623.811∶5Y=5.2068+0.7003X0.95960.50672.05084.05啶酰菌胺∶咯菌腈boscalid∶fludioxonil5∶1Y=6.4202+1.2061X0.99440.06640.10311.553∶1Y=6.5441+1.2267X0.97250.05410.07001.291∶1Y=6.8031+1.1793X0.90890.02960.03571.211∶3Y=6.8539+1.1238X0.98840.04400.02390.541∶5Y=6.9233+1.1434X0.98500.02080.02161.04啶酰菌胺∶氟啶胺boscalid∶fluazinam5∶1Y=6.2188+1.6341X0.98690.17950.10370.583∶1Y=6.6995+1.9924X0.98590.14030.07040.501∶1Y=6.7114+1.1958X0.99130.03700.03590.971∶3Y=7.5472+1.6145X0.99010.02640.02410.911∶5Y=8.1893+1.9301X0.95840.02230.02170.97
3 结论与讨论
灰霉病菌具有繁殖快、遗传变异性大和田间适合度高等特点,属于抗药性发生风险高的病原菌,极易对防治药剂产生抗药性[20]。采用不同作用机制的杀菌剂交替或复配使用,是阻止或延缓病原菌抗药性进一步加剧的主要策略。咯菌腈属于苯基吡咯类非内吸性杀菌剂,是渗透信号传导的分裂蛋白活化激酶/组氨酸激酶抑制剂[21];氟啶胺属于苯胺吡啶类杀菌剂,是解偶联剂,破坏氧化磷酸化[22];啶酰菌胺属于新型烟酰胺类内吸性杀菌剂,是呼吸作用抑制剂[23];苯醚甲环唑属于三唑类杀菌剂,是甾醇脱甲基化抑制剂[24]。本研究采用菌丝生长速率法测定了咯菌腈、氟啶胺、啶酰菌胺和苯醚甲环唑4种不同作用机制的杀菌剂单剂及其两元复配剂对番茄灰霉病菌的毒力。
单剂研究结果显示,4种药剂对番茄灰霉病菌的有效抑制中浓度由低到高依次为:咯菌腈(0.018 0 μg/mL)、氟啶胺(0.018 1 μg/mL)、啶酰菌胺(1.896 8 μg/mL)和苯醚甲环唑(2.087 4 μg/mL),表明4种药剂对番茄灰霉病菌菌丝生长均有较好的抑制作用。咯菌腈和氟啶胺单剂对番茄灰霉病菌具有较高的毒力,而且在田间尚没有发现抗咯菌腈和氟啶胺的番茄灰霉病菌菌株[25-26]。近年来,虽然陆续在田间发现了抗苯醚甲环唑和啶酰菌胺的番茄灰霉病菌菌株,但其抗药性水平和频率还处于较低水平[23, 27]。复配剂研究结果显示,不同配比的各组合复配剂具有增效或相加作用,没有拮抗作用,表明4种杀菌剂在两两复配使用时各药剂的作用机理互不影响。两元复配剂中以啶酰菌胺∶苯醚甲环唑1∶5增效作用最好,主要是由于啶酰菌胺是呼吸作用抑制剂,能够抑制病原菌能量的生成,苯醚甲环唑是甾醇脱甲基化抑制剂,能够抑制病原菌的生物合成,两者相辅相成。综上所述,4种不同作用机制的杀菌剂及其两元复配剂对番茄灰霉病菌均有较高的毒力,建议生产中采用这4种不同作用机制的杀菌剂进行交替或复配使用,以阻止或延缓灰霉菌抗药性的进一步发展,为灰霉病的综合防控和抗药性治理提供理论依据。
[1] Chapeland F, Fritz R, Lanen C, et al. Inheritance and mechanisms of resistance to anilinopyrimidine fungicides inBotrytiscinerea(Botryotiniafuckeliana)[J]. Pesticide Biochemistry and Physiology, 1999, 64: 85-100.
[2] Leroux P, Chapeland F, Desbrosses D, et al. Patterns of cross-resistance to fungicides inBotryotiniafuckeliana(Botrytiscinerea) isolates from French vineyards [J]. Crop Protection, 1999, 18(10): 687-697.
[3] Williamson B, Tudzynski B, Tudzynski P, et al.Botrytiscinerea: the cause of grey mould disease [J]. Molecular Plant Pathology, 2007, 8(5): 561-580.
[4] Widiastuti A, Yoshino M, Saito H, et al. Induction of disease resistance againstBotrytiscinereaby heat shock treatment in melon (CucumismeloL.)[J]. Physiological and Molecular Plant Pathology, 2011, 75(4): 157-162.
[5] Baptista F J, Bailey B J, Meneses J F. Effect of nocturnal ventilation on the occurrence ofBotrytiscinereain mediterranean unheated tomato greenhouses [J]. Crop Protection, 2012, 32: 144-149.
[6] 刘圣明, 高续恒, 张艳慧, 等. 河南省番茄灰霉病菌对3种杀菌剂的抗药性检测[J]. 植物保护, 2014, 40(4): 144-147.
[7] 刘圣明, 车志平, 陈根强. 河南省番茄灰霉病菌对嘧霉胺的抗药性检测[J]. 农药, 2014, 53(6): 442-444.
[8] 乔广行, 严红, 么奕清, 等. 北京地区番茄灰霉病菌的多重抗药性检测[J]. 植物保护, 2011, 37(5): 176-180.
[9] Rosslenbroich H J, Stuebler D.Botrytiscinerea-history of chemical control and novel fungicides for its management[J]. Crop Protection, 2000, 19: 557-561.
[10]Liu Shengming, Che Zhiping, Chen Genqiang. Multiple-fungicide resistance to carbendazim, diethofencarb, procymidone, and pyrimethanil in field isolates ofBotrytiscinereafrom tomato in Henan Province, China [J]. Crop Protection, 2016, 84: 56-61.
[11]周明国, 叶钟音, 刘经芬. 南京市郊灰霉菌对苯并咪唑类杀菌剂田间抗性的检测[J]. 南京农业大学学报, 1987, 10(2): 53-57.
[12]Sun Haiyan, Wang Hancheng, Chen Yu, et al. Multiple resistance ofBotrytiscinereafrom vegetable crops to carbendazim, diethofencarb, procymidone, and pyrimethanil in China[J]. Plant Disease, 2010, 94(5): 551-556.
[13]Shinpei B, Fumiyasu F, Akihiko I, et al. Genotyping of benzimidazole-resistant and dicarboximide-resistant mutations inBotrytiscinereausing real-time polymerase chain reaction assays [J]. Phytopathology, 2008, 98: 397-404.
[14]Bollen G J, Scholten G. Acquired resistance to benomyl and some other systemic fungicides in a strain ofBotrytiscinereain cyclamen[J]. Netherlands Journal Plant Pathology, 1971, 77: 83-90.
[15]宋晰, 肖露, 林东, 等. 番茄灰霉病菌对腐霉利的抗药性检测及生物学性状研究[J]. 农药学学报, 2013, 15(4): 398-404.
[16]潘以楼, 朱桂梅, 郭建. 江苏草莓灰霉病菌对5 种杀菌剂的抗药性[J]. 江苏农业学报, 2013, 29(2): 299-304.
[17]纪明山, 程根武, 张益先, 等. 灰霉病菌对多菌灵和乙霉威抗性研究[J]. 沈阳农业大学学报, 1998, 29(3): 213-216.
[18]Gisi U, Binder H, Rimbach E. Synergistic interactions of fungicides with different modes of action [J]. Transactions of the British Mycological Society, 1985, 85: 299-306.
[19]Gisi U. Synergistic interaction of fungicides in mixtures [J]. Phytopathology, 1996, 86: 1273-1279.
[20]Leroux P, Fritz R, Debieu D, et al. Mechanisms of resistance to fungicides in field strains ofBotrytiscinerea[J]. Pest Management Science, 2002, 58: 876-888.
[21]Leroux P. Recent developments in the mode of action of fungicides [J]. Pest Management Science, 1996, 47(2): 191-197.
[22]Cross R L, Müller V. The evolution of A-, F-, and V-type ATP synthases and ATPases: reversals in function and changes in the H+/ATP coupling ratio [J]. FEBS Letters, 2004, 576: 1-4.
[23]Zhang Chuanqing, Yuan Shankui, Sun Haiyan, et al. Sensitivity ofBotrytiscinereafrom vegetable greenhouses to boscalid[J]. Plant Pathology, 2007, 56: 646-653.
[24]范子耀, 王文桥, 孟润杰, 等. 吡唑醚菌酯与苯醚甲环唑混合物对茄链格孢的联合毒力及其对马铃薯产量的影响[J]. 农药学学报, 2011, 13(6): 591-596.
[25]赵建江, 张小风, 马志强, 等. 番茄灰霉病菌对咯菌腈的敏感基线及其与不同杀菌剂的交互抗性[J]. 农药, 2013, 52(9): 684-685.
[26]Shao Wenyong, Ren Weichao, Zhang Yu, et al. Baseline sensitivity of natural populations and characterization of resistant strains ofBotrytiscinereato fluazinam [J]. Australasian Plant Pathology, 2015, 44: 375-383.
[27]赵建江, 韩秀英, 张小风, 等. 灰葡萄孢(Botrytiscinerea)对苯醚甲环唑的敏感性及其对不同杀菌剂的交互抗药性[J]. 中国农学通报, 2010, 26(22): 282-286.
(责任编辑:杨明丽)
Toxicity of four fungicides and their mixtures toBotrytiscinereafrom tomato
Liu Shengming1, Hai Fei2, Che Zhiping1, Tian Yue’e1, Liu Xin1
(1.DepartmentofPlantProtection,CollegeofForestry,HenanUniversityofScienceandTechnology,Luoyang471003,China; 2.DepartmentofAgriculturalEngineering,HenanVocationalCollegeofAgriculture,Zhengzhou451450,China)
Toxicities of fludioxonil, fluazinam, boscalid and difenoconazole, and their mixtures toBotrytiscinereafrom tomato were detected by the method of mycelial growth assayinvitro. The results showed that the EC50values for fludioxonil, fluazinam, boscalid and difenoconazole were 0.018 0 μg/mL, 0.018 1 μg/mL, 1.896 8 μg/mL, and 2.087 4 μg/mL, respectively. The mixtures of the fungicides boscalid and difenoconazole with the ratio of 1∶5, 1∶3, 1∶1 and 3∶1, fludioxonil and difenoconazole with the ratio of 1∶5 and 1∶3, fludioxonil and fluazinam with the ratio of 1∶3, and boscalid and fludioxonil with the ratio of 5∶1 demonstrated synergistic inhibition effect, with the synergy ratio ranged from 1.5 to 4.05. Among them, the mixture of boscalid and difenoconazole (1∶5) had the strongest inhibition againstB.cinereawith the synergy ratio of 4.05, indicating synergistic inhibition, while the synergy ratio of other mixtures was 0.5-1.46, indicating additive inhibition. The above results indicated that fludioxonil, fluazinam, boscalid and difenoconazole, and their mixtures can be used alternately in controlling the gray mold disease caused byB.cinerea.
grey mould disease;Botrytiscinerea; fungicide; mixture
2016-04-12
2016-04-25
国家自然科学基金青年科学基金(31301688);公益性行业(农业)科研专项(201303023);河南省自然科学基金(162300410079);河南科技大学博士科研启动基金(09001589)
S 436.412
B
10.3969/j.issn.0529-1542.2017.02.042
* 通信作者 E-mail:liushengmingzb@163.com
