黄体酮阴道缓释凝胶在体外受精—胚胎移植黄体支持中的应用效果
2017-03-18王美仙张扬邵小光
王美仙+张扬+邵小光
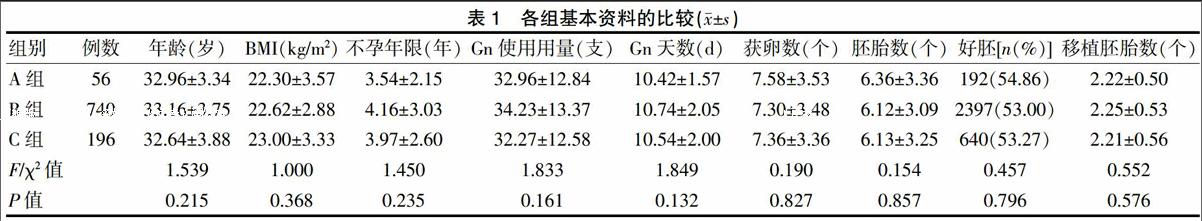
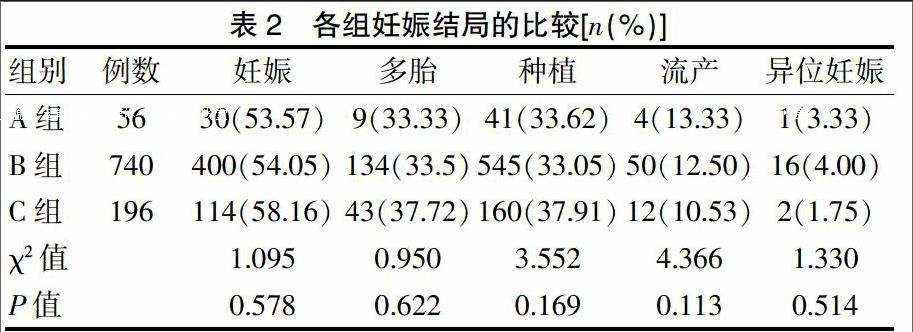
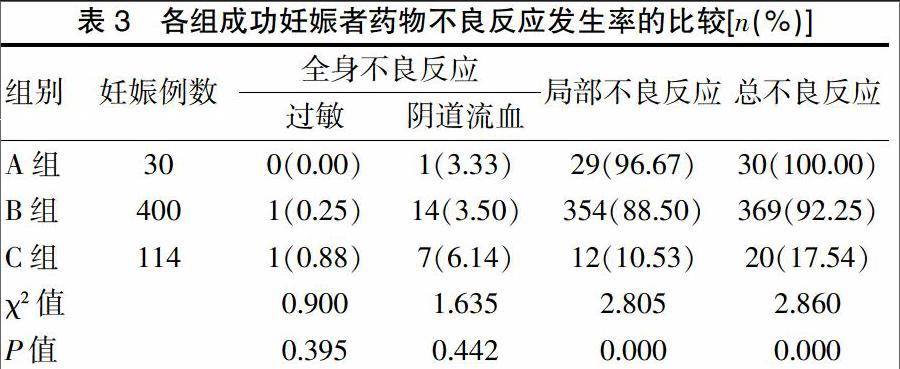
[摘要]目的 分析黄体酮阴道缓释凝胶在体外受精-胚胎移植(IVF-ET)黄体支持中的有效性和安全性。方法 选择2015年1~12月于我中心接受长方案IVF-ET助孕患者992例,按照不同的黄体支持方案进行分组:黄体酮60 mg肌内注射组(A组)56例,黄体酮40 mg肌内注射组(B组)740例,黄体酮阴道缓释凝胶外用组(C组)196例。比较三组患者的妊娠率、种植率、多胎率、流产率和异位妊娠率及药物不良反应发生率。结果 三组的妊娠率、种植率、多胎率、流产率、异位妊娠率比较,差异无统计学意义(P>0.05),但是C组妊娠率、多胎率、种植率有增高趋势,流产率有降低趋势。C组的药物总不良反应发生率明显低于A、B两组,差异有统计学意义(P<0.01)。结论 黄体酮阴道缓释凝胶是有效、安全的IVF-ET黄体支持方法之一。
[关键词]黄体酮阴道缓释凝胶;黄体酮针剂;体外受精-胚胎移植;黄体支持
[中图分类号] R714.8 [文献标识码] A [文章编号] 1674-4721(2017)01(c)-0071-03
[Abstract]Objective To analyze the efficacy and safety of progesterone sustained-released vaginal gel for luteal phase support in vitro fertilization-embryo transfer(IVF-ET).Methods From January to December 2015,992 patients who were agreed on long-term regimen of IVF-ET were selected.According to different progesterone-support regimens,they were divided into group A(muscular injection progesterone 60 mg,n=56),group B(muscular injection progesterone 40 mg,n=740),and group C(external application of progesterone sustained-released vaginal gel,n=196).The pregnancy rate,implantation rate,multiple-gestation pregnancy rate,miscarriage rate,ectopic pregnancy rate and incidence of adverse drug reactions were compared among the three groups.Results There was no significant difference in pregnancy rate,implantation rate,multiple gestation pregnancy rate,miscarriage rate,orectopic pregnancy rate among the three groups(P>0.05).However,the pregnancy rate,multiple gestation pregnancy rate and implantation rate had increasing tendency,and miscarriage rate was in a decrease tendency in group C.The total incidence of adverse drug reactions was significantly lower than that in the group A and B,the difference was statistically significant(P<0.01).Conclusion Progesterone sustained-released vaginal gel is one of the effective and safe methods for luteal phase support in IVF-ET.
[Key words]Progesterone sustained-released vaginal gel;Progesterone injection;In vitro fertilization-embryo transfer;Corpus luteum support
體外受精-胚胎移植(in vitro fertilization and embryo transfer,IVF-ET)中黄体支持非常重要。由于IVF调节后垂体功能被抑制,卵泡颗粒细胞被抽吸,所以需要大剂量孕酮做黄体支持[1]。常用的黄体支持方案有口服、肌内注射或阴道外用孕激素、肌内注射人绒毛膜促性腺激素(human chorionic gonadotropin,HCG)、皮下注射促性腺激素释放激素激动剂(gonadotropin releasing hormone agonist,GnRH-a)等,但目前尚无统一的黄体支持方案。黄体酮针剂是临床应用最久的药物,费用低、疗效好,但其不良反应亦明显。黄体酮阴道缓释凝胶在欧美国家被广泛应用于IVF-ET后黄体支持[2],我中心从2015年1月开始用黄体酮阴道缓释凝胶做黄体支持。本文就我中心长方案新鲜移植周期患者的黄体支持方法做比较分析,探讨黄体酮阴道缓释凝胶的应用效果。
1对象与方法
1.1对象
选择2015年1~12月于我中心IVF-ET患者992例,平均年龄(33.35±3.75)岁。纳入标准:①年龄<43岁;②长方案控制性卵巢刺激新鲜周期移植患者;③无IVF禁忌证。排除标准:①拮抗剂方案、微刺激方案、超长方案患者;②解冻移植患者;③染色体异常患者。按照不同的黄体支持方案分为三组:黄体酮60 mg肌内注射组(A组,56例),黄体酮40 mg肌内注射组(B组,740例),黄体酮阴道缓释凝胶外用组(C组,196例)。三组患者的基本资料比较差异无统计学意义(P>0.05)(表1),具有可比性。
