核-壳结构氧还原反应电催化剂
2017-03-13常乔婉邵敏华
常乔婉 肖 菲 徐 源 邵敏华
(香港科技大学化学工程与生物分子工程学系,香港)
核-壳结构氧还原反应电催化剂
常乔婉 肖 菲 徐 源 邵敏华*
(香港科技大学化学工程与生物分子工程学系,香港)
金属铂是最高效的氧还原反应催化剂而被广泛应用于质子交换膜燃料电池,但其高成本阻碍了燃料电池的商业化进程。此专论主要总结了近年来核-壳结构纳米催化剂的设计、合成及在燃料电池中的应用,讨论了多种提高核-壳结构纳米催化剂的策略包括去合金化、形貌控制、表面改性等,以及在放大制备及燃料电池测试中遇到的问题。
核-壳结构;质子交换膜燃料电池;电催化;铂合金;钯;形貌控制
1 Introduction
Proton exchange membrane fuel cells(PEMFCs)involve two reactions:oneisthehydrogenoxidationreaction(HOR,H2→2H++ 2e-)at the anode,the other is the oxygen reduction reaction(ORR, 1/2O2+2H++2e-→H2O)at the cathode.The commercialization of PEMFCs has been hindered by costly Pt-based electrocatalysts at both electrodes,especially at the cathode since the slow reaction kinetics of ORR requires a higher Pt loading to achieve a desirable fuel cell performance.For the past five decades,great efforts have been spent on developing cost-competitive and highly active electrocatalysts for ORR1-3,including but not limited to advanced Pt alloys4-9,other noble-metal based materials10-13,core-shell structures14-18,and carbon-based non-noble metal composites19-23. This Account mainly focuses on the recent work of core-shell electrocatalysts for ORR in acidic media.Compared to conventional electrocatalysts,the core-shell nanostructures consisting of a thin Pt-based shell can significantly improve the utilization of Pt as only the surface atoms participate in electrocatalytic reactions.In addition,the use of less expensive cores,for example,Pd and Ru nanoparticles(NPs),reduces the overall cost of theelectrocatalysts.Furthermore,the possible strain and ligand effects from the core materials on the Pt shell may further improve the catalytic activity of Pt atoms in the surface24-29.Core-shell nanostructures can be synthesized by various methods1.In this Account,only the Cu-mediated-Pt deposition and chemical reduction methods are discussed.For other methods,the readers are referred to recent literature1,3,6,30.
2 Core-shell synthesis
2.1 Cu-mediated-Pt displacement method
The Cu-mediated-Pt displacement method involves the deposition of a Cu monolayer(ML)at potentials higher than its bulk deposition(underpotential deposition,UPD)and subsequent displacement of the Cu ML by Pt atoms with a one-to-one ratio (surface limited redox replacement,SLRR)17.Ideally,a Pt ML is formed on a foreign metal core(typically another noble metal). Fig.1 illustrates the overall procedure of the Pt shell deposition using a Pd nanoparticle as the core1.The Adzic group17,24,31,32at Brookhaven National Laboratory pioneered this process and extensively investigated this kind of core-shell structure with various core and shell compositions.

Fig.1 Model for Pt monolayer deposition on a foreign metal core (Pd as an example)involving the Cu UPD(underpotential deposition)and subsequent Pt displacement1

CHANG Qiao-Wan,received a BS in Chemical Engineering from Tianjin University and BS(Double Degree)in Finance from Nankai University in 2014.She is now undertaking an MPhil degree at the Hong Kong University of Science and Technology under the supervision of Professor SHAO Min-Hua. Her main research interests are advanced materials for fuel cells.

XIAO Fei,received a BS in Materials Science and Engineering from University of Science and Technology Beijing.She is now undertaking an MPhil degree at the Hong Kong University of Science and Technology under the supervision of Professor SHAO Min-Hua.Her main research interests are electrocatalysts for oxygen reduction reaction.

XU Yuan,received a BS in Chemistry from South China Normal University in 2014,and a MSc in Chemical and Biomolecular Engineering from the Hong Kong University of Science and Technology in 2015.She is now a Research Assistant at the Hong Kong University of Science and Technology under the supervision of Professor SHAO Min-Hua. Her main research interests are advanced materials for fuel cells.

SHAO Min-Hua,earned his BS(1999)and MS(2002)degrees in Chemistry from Xiamen University,and a PhD degree in Materials Science and Engineering from State University of New York at Stony Brook (2006).He joined UTC Power in 2007 to lead the development of advanced catalysts and supports for PEMFC and PAFC.In 2013,he joined Ford Motor Company to conduct research on lithium-ion batteries for electrified vehicles.He then joined the Hong Kong University of Science and Technology in the Department of Chemical and Biomolecular Engineering as anAssociate Professor in 2014.His research mainly focuses on electrocatalysis and advanced batteries.
According to the Sabatier principle,a good catalyst for ORR should form a moderate bond with the reaction intermediates in order to balance the reaction kinetics of O―O bond breaking/ electron transfer and promote the reduction of adsorbed oxygencontaining species33.However,it was found that the oxygen binding energy on pure Pt is somewhat too strong.The Adzic group24demonstrated that the oxygen binding energy of a Pt ML deposited on a Pd(111)surface was slightly weaker mainly due to the electronic effect from the substrate.Ahigher ORR activity than Pt(111)was observed when a Pt ML supported on Pd(111)34. Besides,a similar improvement was achieved by using Pd NPs as the core.In addition,it was showed that the ORR activity of the Pt ML can be further improved by tuning the composition of the core,for instance,alloying Pd with other transition metals by introducing additional strain and ligand effects25-27,32.Other materials such asAu-,Ir-,and Ru-based NPs have been also explored as cores to support Pt shells35-37.We also found that small Au NPs (~3 nm)were also suitable cores.The specific activity of Pt ML-modified Au NPs(Au@Pt)was 60%higher than that of Pd@Pt with the similar particle size(Fig.2)38and the higher activity of Au@Pt was also confirmed by other groups39.These results were somewhat surprising since it was known that the Pt ML on a bulk Au(111)single crystal showed a lower ORR activity than Pt(111) or Pt ML on Pd(111)due to a large tensile strain in Pt ML causedby Au24.Surprisingly,our density functional theory(DFT)calculation results demonstrated the existence of a significant compressive strain in the surface of small Au NPs38.The existence of the compressive strain led to a weaken oxygen binding energy by down-shifting of the d-band center of the Pt shell,which played an important role in improving the ORR activity.

Fig.2 Comparisons of normalized specific activities of Pt and Pt monolayer catalysts38
ORR activities of core-shell electrocatalysts are not only dependent on the composition of the core,but also its structure, including the shape,particle size,and porosity16,40-42.We did a systematic study on the structural effect of Pd core on the ORR activity.Without surface modification,the ORR activity of Pd cubes enriched with{100}facets was 10 times higher than that of octahedra enriched with{111}facets in a 0.1 mol·L-1HClO4solution43.Whereas the specific activity of Pd octahedra(~5 nm) was improved by 30 times with a Pt ML on it,while the activity of Pd cubes with a similar size was almost no change44.As a result, the ORR activity of the Pt shell supported on Pd octahedra was 3.5 times higher than that on cubes(Fig.3).According to our DFT calculation,one of the possible reasons for this discrepancy was the difference in oxygen binding energy change upon Pt ML modification.When a Pt ML was deposited on the Pd(111)surface,a significant decrease(>10%)of the oxygen binding energy was found,while there was no change on the(100)surface.
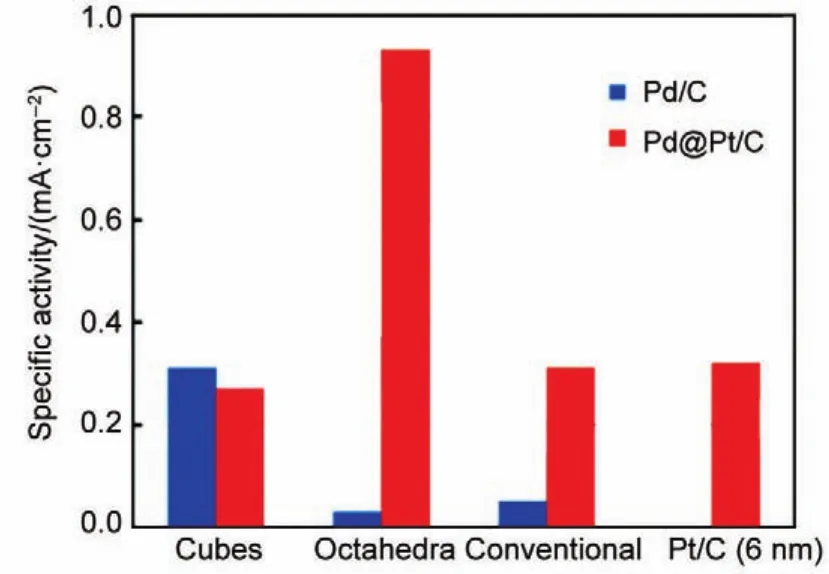
Fig.3 Comparisons of activities of the Pd and Pd@Pt catalysts44
The Adzic group24,45,46proved that a Pd interlayer between Pt shells and other metal cores(Au,Ru,etc.)was beneficial for ORR activity.We,on the other hand,found that an Au interlayer was also effective to further enhance the activity of Pt shell on Pd cores.Depending on the structure of the Pd core,the enhancement extent was different.For example,with an Au interlayer,the Pt mass activity was improved by 2 times on conventional Pd NPs (from 0.75 to 1.4A·mg-1),while on shape controlled Pd cubes and octahedra,the Pt mass activities increased from 0.63 and 2.2 to 2.0 and 2.6A·mg-1,respectively.The effect of Au interlayer on ORR activity was much smaller on(111)surface than that on(100)as the respective enhancement factors for Pd cubes and octahedra were 3 and 1.2 times.The difference in activity enhancement may mainly be due to the different degree of oxygen binding energy changes in Pt MLs.We found that the oxygen binding energies were weakened by 0.275 and 0.075 eV upon Pt modification for Pd(111)and(100),respectively.Since adding an Au interlayer does not change the strain in the Pt monolayer,only the electronic effect from theAu interlayer might be responsible for the observed oxygen binding energy changes47.

Fig.4 An HAADFTEM image of PdCu6/C after dealloying48
Reduction of other noble metals,such as Pd in the core is also of considerable interest to further reduce the loading of precious metals in the catalyst.We explored the idea of using porous instead of solid Pd NPs as the core.The porous Pd-based NPs were synthesized by dealloying of Pd-Cu(Fig.4)and Pd-Ni alloy NPs via a potential cycling or acid washing protocol41,48.Atiny amount of transition metal(less than 1%(w,mass fraction))left in the core after dealloying changed the electronic properties of Pd,and in turn the Pt shell49.The nanopores generated during dealloying mayalso generate additional compressive strain in the core.As a result, Pt MLs on porous Pd NPs were 3.5 times more active than that deposited on conventional ones48.Pt mass activities on porous Pd prepared from Pd-Cu and Pd-Ni alloys achieved 2.8 and 1.9 A· mg-1,respectively.Even though Pt ML on dealloyed Pd-Ni was slightly less active than that on Pd-Cu,the former was more suitable for fuel cell application.During fuel cell operation,the Cu or Ni in the dealloyed core dissolves forming cations that poison the ionomer and membrane.Cu2+can be reduced at the anode and cover the active sites for HOR,while Ni2+cannot be redeposited in the potential range of the anode.
2.1.1 Gram batch synthesis
Ideally,a uniform Pt monolayer is deposited on the core after all the Cu atoms are replaced in the Cu-mediated method.However,our study demonstrated that Pt clusters rather than a uniform Pt shell were formed on Pd nanoparticles50.The mechanism underlying this observation is that the SLRR process involves the electron transfer from the substrate(Pd for example)toions,rather than direct electron exchange from Cu.That means electrons generated anywhere on the surface can move freely through Pd substrate,reducing PtCl42-ions wherever their activity and surface energy are greatest51.In other words,the Pt atom may not deposit on the same site left by the Cu dissolution,but rather on Pt that was already deposited on the core leading to the formation of Pt clusters.Due to the incomplete Pt coverage and low Pt usage,it is expected to have lower activity and stability than a perfect core-shell structure.Thus,to synthesize core-shell materials with good quality on a large scale,the key is to force Pt atoms to deposit on the surface of the core rather than on Pt atoms already deposited by manipulating the Cu-Pt displacement reaction kinetics.We demonstrated that adding proper additives in the Cumediated method could help Pt atoms to form a thin and uniform shell by avoiding the tendency to form clusters52.For example,the Pt mass activity of Pd@Pt/C synthesized by adding citric acid in the Cu-Pt displacement reaction solution was(0.95±0.10)A· mg-1,which was 4.8 times higher than that of Pt/C((0.20±0.02) A·mg-1)at 0.9 V.The electrochemical surface area of the coreshell catalysts was(160±10)m2·g-1,while it was(85±2)m2·g-1for Pt/C(~2.5 nm).For comparison,the Pt mass activity of coreshell catalysts synthesized without adding citric acid was only ~0.4 A·mg-1.The same synthesis protocol was applied in the deposition of Pt shell on porous Pd-Ni core.The Pt mass activity could achieve 1.4A·mg-1,which was higher than that of Pd@Pt/ C41.
The role of citric acid in controlling the uniformity of Pt monolayers on Pd was studied by electrochemical techniques and theoretical approaches53.It was found that citric acid strongly adsorbed on Pd,Pt,Cu@Pd(Cu UPD layer)and Pd@Pt surfaces, especially in the double layer region in acid solutions.The strong adsorption of citric acid slowed down the Cu-Pt displacement reaction as indicated by the decrease of the rising rate of the open circuit potential during the reaction(Fig.5).This phenomenon might be caused by the fact that Pt atoms deposited on the Pd surface in the early stage of the Cu-Pt displacement reaction were covered by a dense layer of citric acid in the potential range where the reaction occurred.As a result,further deposition of Pt on the already existing Pt atoms covered by a citric acid layer to form 3D clusters was prohibited.

Fig.5 (a)Open circuit potential(OCP)changes for Pd/C covered with a Cu monolayer;(b)the OCPchanges for the graphite electrode in the gram scale synthesis53
The durability of core-shell electrocatalysts were evaluated on RDE using a square-wave signal between 0.65 and 1.00 V for 5 s at each potential.After 10000 cycles,the Pt mass activity of Pd@Pt/C dropped by 16%53.This result suggested that Pd@Pt/C was even more stable than Pt/C,which degraded 42%under the same testing condition.For Pt shell on dealloyed Pd-Ni,the stability was somewhat worse.After 5000 cycles,its Pt mass activity decreased by 35%,which was comparable to that of Pt/C41.
2.2 Chemical reduction method
2.2.1 Pt shell on Pd core
There have been significant efforts to deposit Pt-based shell on foreign metals without using Cu UPD layer as the sacrificing template54-65.A special focus has been given to Pd@Pt core-shell materials18,60,61,66-70.We explored a simple method to synthesize Pd@Pt NPs with an ultrathin(0.4 nm)Pt shell in a gram batch with the assistance of citric acid15.In this synthesis protocol,the cleaned Pd/C particles were mixed with a K2PtCl4solution containing citric acid.The Pt shell deposition might involve threedifferent pathways:galvanic displacement reaction between Pd atoms and Pt cations,chemical reduction by citric acid,and reduction by negative charges on Pd surfaces.The Pd@Pt NPs were characterized by scanning transmission electron microscopy (STEM)and evaluated by RDE(Fig.6).Results showed that the Pt mass activity was 4 times higher than that of Pt/C and comparable to the one synthesized by a complicated Cu UPDmethod50.
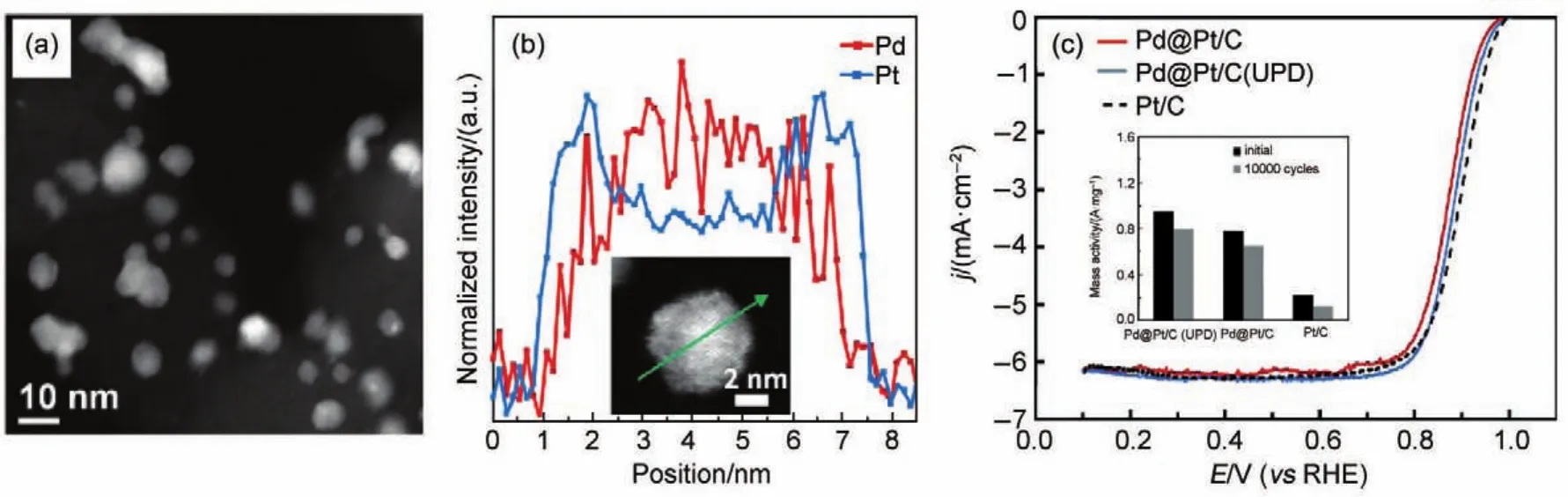
Fig.6 (a)HAADF-STEM image of Pd@Pt/C core-shell catalysts after 60 min of reaction.(b)EELS line scan profiles of Pt(blue)and Pd(red)of a single Pd@Pt core-shell particle(inset).(c)Comparisons of oxygen reduction polarization curves(positive-going)of Pd@Pt/C,Pd@Pt/C(UPD)and Pt/C.The inset of(c)compares the Pt mass activities of Pd@Pt/C(UPD),Pd@Pt/C and Pt/C before and after 10000 potential cycles between 0.65 and 1.0 V15
2.2.2 Pt alloy shell on Pd core
Pt alloys are more active toward ORR than pure Pt due to the strain and ligand effects from the transition metals in the alloys71,72. Thus,when Pt alloys instead of pure Pt are deposited on foreign metal cores,the ORR activity is expected to be further improved. The epitaxial overgrowth of thin Pt-Fe shells(0.3-1.3 nm)on Pd and Au NPs were achieved by Sun group73,74.For instance, Au@Pt3Fe showed 5-fold higher mass activity than that of Pt/C.
It has been recognized that the ORR activities of Pt-Ni(111) plane has a much higher activity than(100)and(110)8.In order to take advantage of this structural effect,{111}facets of nanocatalysts exposed are more desirable.Some groups have demonstrated that Pt-Ni octahedral NPs enriched with{111}facets showed much higher activity than conventional Pt alloys7,75-80.We synthesized Pt-Ni octahedra with an edge length of 9 nm by reducing Pt(acac)2and Ni(acac)2in the mixture of oleylamine,oleic acid and benzyl ether with the presence of W(CO)65.The mass activity at 0.9 Vcould reach as high as 3.3A·mg-1,which was 17-fold higher than that of Pt/C.As for a 9 nm particle,most of Pt atoms are buried inside and do not participate in the catalytic reaction.To further reduce the Pt loading,a thin Pt-Ni shell can be deposited on a cheaper material core,for instance Pd,and maintain the octahedral shape.This approach indeed combines three strategies including high Pt utilization and low Pt loading from the core-shell structure,alloying effect from the transition metal,and structural effect from the{111}facets.The key to depositing a Pt-Ni shell on Pd cores is to find a proper solvent, which can not only keep good compatibility between the solvents used for Pt-Ni growth(non-aqueous)and Pd seed synthesis (aqueous),but also help reduce the coverage of surfactants on the final product.The protocol of transferring Pd seeds from a hydrophilic solvent to an amphiphilic reaction medium was illustrated in Fig.7.The 1 nm Pt2.5Ni shell was deposited on a 5 nm Pd octahedral seed to form an octahedral Pd@Pt-Ni core-shell structure14(Fig.8).The preliminary results showed that the Pt mass and specific activities were 2.5 A·mg-1and 2.7 mA·cm-2,respectively.

Fig.7 Schematic illustration of a procedure about the transformation of the Pd nanocrystals from a hydrophilic solvent to am amphiphilic reaction medium and the preparation of Pd@Pt-Ni octahedra14
2.3 Fuel cell testing

Fig.8 (a)HAADF-STEM image of a single Pd@Pt-Ni octahedron; (b)energy-dispersive X-ray spectroscopy(EDS)mapping of elemental distributions for Pd,Pt,and Ni; (c)comparison of Pt mass and specific ORR activities for the Pd@Pt-Ni/C and state-of-the-art Pt/C(TKK)catalysts at 0.9 V
A limited number of core-shell materials have been tested in fuel cells due to the difficulty to obtain high quality samples52,81,82. We found that the fuel cell performance of Pd@Pt/C was approximately 20 and 60 mV in the low and high current density regions in H2-air environment,respectively52(Fig.9(a)),which were both higher than that of Pt/C.The cell voltage could achieve 0.775 V at a current density of 400 mA·cm-2with a Pt loading of 0.1 mg·cm-2(80°C,100%relative humidity and 140 kPa backpressure).However,it did not show the improvement at the low current density as observed in RDE(44 mV improvement for a 4.8-fold higher mass activity).The lower than predicted performance may be due to the ink fabrication and spraying steps.We also tested the fuel cell performance of Pt ML on dealloyed Pd-Ni core with the same Pt loading41.The performance was better than Pd@Pt/C,as expected from its higher activity in RDE.The dealloyed Pd-Ni@Pt/C outperformed at least 40 mV through the whole potential range in both pure oxygen and air compared to Pt/ C(Fig.9(b)).The cell voltage achieved 0.791 V at a current density of 400 mA·cm-2(80°C,80%relative humidity and 100 kPa backpressure).Again,the core-shell catalyst did not show theimprovement as observed in RDE(65 mV improvement for a 7-fold higher mass activity).

Fig.9 (a)Fuel cell performance of Pd@Pt/C and(b)d-PdNi@Pt/C comparing with Pt/C

Table 1 Summary of ORR activities of recent work of core-shell catalysts(measured at 0.9 V)
3 Conclusions and perspectives
This Account mainly discusses the recent progress of core-shell electrocatalysts for oxygen reduction reaction in our group.The rational design,synthesis,characterization,and evaluation of Ptbased shells supported on Pd-based cores were summarized.The structure and composition of the core play a significant role in controlling the electronic properties of Pt shells.For instance,Pd nanocrystals enriched with{111}facets were better cores than those enriched with{100}facets;dealloyed Pd alloys were better than conventional Pd cores.The Pt mass and specific activities of various core-shell catalysts published by our group are summarized in Table 1.The highest Pt mass activity(~2.8 A·mg-1)was observed on Pt ML on octahedral Pd and dealloyed Pd-Cu cores synthesized on RDE.For the samples synthesized in a gram scale batch,Pt ML on dealloyed Pd-Ni showed the highest activity(1.4 A·mg-1).Based on our and others′results,the general strategies of design core-shell electrocatalysts for ORR are summarized below:(1)choosing proper materials as cores to modify the dband center of the Pt shell and in turn alter the binding energy of oxygen-containing species;(2)controlling morphologies of the core and core-shell structures with only the highest active facets (for example{111})exposed to electrolytes;(3)reducing noble metal loadings in the core by using porous/hollow structures and non-noble metal-based cores;(4)developing more feasible protocols for mass production of core-shell materials.We realize that it is still a big challenge to make high performance ORR electrocatalysts suggested by RDE to exhibit the same improvement factor in a real fuel cell.More studies on demonstrating their feasibility in fuel cell applications with desired performance and durability are required.
(1)Shao,M.H.;Chang,Q.W.;Dodelet,J.P.;Chenitz,R.Chem. Rev.2016,116(6),3594.doi:10.1021/acs.chemrev.5b00462
(2)Nie,Y.;Li,L.;Wei,Z.Chem.Soc.Rev.2015,44(8),2168. doi:10.1039/C4CS00484A
(3)Shao,M.H.Electrocatalysis in Fuel Cells:a Non-and Lowplatinum Approach;Springer Science&Business Media: Heidelberg,2013;pp 1-745.
(4)Wanjala,B.N.;Fang,B.;Shan,S.;Petkov,V.;Zhu,P.; Loukrakpam,R.;Chen,Y.;Luo,J.;Yin,J.;Yang,L.Chem. Mater.2012,24(22),4283.doi:10.1021/cm301613j
(5)Choi,S.I.;Xie,S.;Shao,M.H.;Odell,J.H.;Lu,N.;Peng,H. C.;Protsailo,L.;Guerrero,S.;Park,J.;Xia,X.Nano Lett.2013, 13(7),3420.doi:10.1021/nl401881z
(6)Strasser,P.;Koh,S.;Anniyev,T.;Greeley,J.;More,K.;Yu,C.; Liu,Z.;Kaya,S.;Nordlund,D.;Ogasawara,H.Nat.Mater. 2010,2(6),454.doi:10.1021/nl3032795
(7)Cui,C.;Gan,L.;Li,H.H.;Yu,S.H.;Heggen,M.;Strasser,P. Nano Lett.2012,12(11),5885.doi:10.1021/nl3032795
(8)Stamenkovic,V.R.;Fowler,B.;Mun,B.S.;Wang,G.;Ross,P. N.;Lucas,C.A.;Marković,N.M.Science 2007,315(5811), 493.doi:10.1126/science.1135941
(9)Stamenkovic,V.R.;Mun,B.S.;Arenz,M.;Mayrhofer,K.J.; Lucas,C.A.;Wang,G.;Ross,P.N.;Markovic,N.M.Nat. Mater.2007,6(3),241.doi:10.1038/nmat1840
(10)Shao,M.H.J.Power Sources 2011,196(5),2433.doi:10.1016/ j.jpowsour.2010.10.093
(11)Shao,M.H.;Odell,J.;Humbert,M.;Yu,T.;Xia,Y.J.Phys. Chem.C 2013,117(8),4172.doi:10.1021/jp312859x
(12)Shao,M.H.;Huang,T.;Liu,P.;Zhang,J.;Sasaki,K.; Vukmirovic,M.;Adzic,R.Langmuir 2006,22(25),10409. doi:10.1021/la0610553
(13)Shao,M.H.;Sasaki,K.;Adzic,R.R.J.Am.Chem.Soc.2006, 128(11),3526.doi:10.1021/ja060167d
(14)Choi,S.I.;Shao,M.H.;Lu,N.;Ruditskiy,A.;Peng,H.C.; Park,J.;Guerrero,S.;Wang,J.;Kim,M.J.;Xia,Y.ACS Nano 2014,8(10),10363.doi:10.1021/nn5036894
(15)Zhang,L.L.;Zhu,S.Q.;Chang,Q.W.;Su,D.;Yue,J.;Du,Z.; Shao,M.H.ACS Catal.2016,6(6),3428.doi:10.1021/ acscatal.6b00517
(16)Adzic,R.R.Electrocatalysis 2012,3(3-4),163.doi:10.1007/ s12678-012-0112-3
(17)Adzic,R.R.;Zhang,J.;Sasaki,K.;Vukmirovic,M.B.;Shao, M.H.;Wang,J.;Nilekar,A.U.;Mavrikakis,M.;Valerio,J.; Uribe,F.Top.Catal.2007,46(3-4),249.doi:10.1021/ ja9067645
(18)Zhang,G.;Shao,Z.G.;Lu,W.;Xie,F.;Xiao,H.;Qin,X.;Yi,B. Appl.Catal.B 2013,132,183.doi:10.1016/j.apcatb.2012.11.029
(19)Akinpelu,A.;Merzougui,B.;Bukola,S.;Azad,A.M.;Basheer, R.A.;Swain,G.M.;Chang,Q.W.;Shao,M.H.Electrochim. Acta 2015,161,305.doi:10.1016/j.electacta.2015.02.072
(20)Jaouen,F.;Proietti,E.;Lefèvre,M.;Chenitz,R.;Dodelet,J.P.; Wu,G.;Chung,H.T.;Johnston,C.M.;Zelenay,P.Energy Environ.Sci.2011,4(1),114.doi:10.1039/C0EE00011F
(21)Wu,G.;More,K.L.;Johnston,C.M.;Zelenay,P.Science 2011, 332(6028),443.doi:10.1126/science.1200832
(22)Lefèvre,M.;Proietti,E.;Jaouen,F.;Dodelet,J.P.Science 2009, 324(5923),71.doi:10.1126/science.1170051
(23)Proietti,E.;Jaouen,F.;Lefèvre,M.;Larouche,N.;Tian,J.; Herranz,J.;Dodelet,J.P.Nat.Commun.2011,2,416. doi:10.1038/ncomms1427
(24)Zhang,J.;Vukmirovic,M.B.;Xu,Y.;Mavrikakis,M.;Adzic,R. R.Angew.Chem.Int.Ed.2005,44(14),2132.doi:10.1002/ anie.200462335
(25)Rodriguez,J.Surf.Sci.Rep.1996,24(7),223.doi:10.1016/ 0167-5729(96)00004-0
(26)Brankovic,S.;Wang,J.;Adzic,R.Surf.Sci.2001,474(1), L173.doi:10.1016/S0039-6028(00)01103-1
(27)Hammer,B.;Nørskov,J.K.Adv.Catal.2000,45,71.doi:10.1016/S0360-0564(02)45013-4
(28)Shao,M.H.;Odell,J.H.;Peles,A.;Su,D.Chem.Commun. 2014,50(17),2173.doi:10.1002/cssc.201400051
(29)Calle-Vallejo,F.;Koper,M.T.;Bandarenka,A.S.Chem.Soc. Rev.2013,42(12),5210.doi:10.1039/C3CS60026B
(30)Oezaslan,M.;Hasché,F.D.R.;Strasser,P.J.Phys.Chem.Lett. 2013,4(19),3273.doi:10.1021/jz4014135
(31)Zhang,J.;Sasaki,K.;Sutter,E.;Adzic,R.Science 2007,315 (5809),220.doi:10.1126/science.1134569
(32)Zhou,W.P.;Sasaki,K.;Su,D.;Zhu,Y.;Wang,J.X.;Adzic,R. R.J.Phys.Chem.C 2010,114(19),8950.doi:10.1021/ jp100283p
(33)Xu,Y.;Ruban,A.V.;Mavrikakis,M.J.Am.Chem.Soc.2004, 126(14),4717.doi:10.1021/ja031701+
(34)Zhang,J.;Mo,Y.;Vukmirovic,M.;Klie,R.;Sasaki,K.;Adzic, R.J.Phys.Chem.B 2004,108(30),10955.doi:10.1021/ jp0379953
(35)Zhang,L.;Iyyamperumal,R.;Yancey,D.F.;Crooks,R.M.; Henkelman,G.ACS Nano 2013,7(10),9168.doi:10.1021/ nn403788a
(36)Karan,H.I.;Sasaki,K.;Kuttiyiel,K.;Farberow,C.A.; Mavrikakis,M.;Adzic,R.R.ACS Catal.2012,2(5),817. doi:10.1021/cs200592x
(37)Brankovic,S.;McBreen,J.;Adzic,R.J.Electroanal.Chem. 2001,503(1),99.doi:10.1016/S0022-0728(01)00349-7
(38)Shao,M.H.;Peles,A.;Shoemaker,K.;Gummalla,M.;Njoki,P. N.;Luo,J.;Zhong,C.J.J.Phys.Chem.Lett.2010,2(2),67. doi:10.1021/jz1015789
(39)Iijima,Y.;Kondo,T.;Takahashi,Y.;Bando,Y.;Todoroki,N.; Wadayama,T.J.Electrochem.Soc.2013,160(8),F898. doi:10.1149/2.011309jes
(40)Cai,Y.;Adzic,R.R.Adv.Phys.Chem.2011,530397. doi:10.1155/2011/530397
(41)Shao,M.H.;Smith,B.H.;Guerrero,S.;Protsailo,L.;Su,D.; Kaneko,K.;Odell,J.H.;Humbert,M.P.;Sasaki,K.;Marzullo, J.Phys.Chem.Chem.Phys.2013,15(36),15078.doi:10.1039/ C3CP52252K
(42)Gong,K.;Choi,Y.;Vukmirovic,M.B.;Liu,P.;Ma,C.;Su,D.; Adzic,R.R.Z.Phys.Chem.2012,226(9-10),1025. doi:10.1524/zpch.2012.0239
(43)Xiao,L.;Zhuang,L.;Liu,Y.;Lu,J.J.Am.Chem.Soc.2008, 131(2),602.doi:10.1021/ja8063765
(44)Shao,M.H.;He,G.;Peles,A.;Odell,J.H.;Zeng,J.;Su,D.; Tao,J.;Yu,T.;Zhu,Y.;Xia,Y.Chem.Commun.2013,49(79), 9030.doi:10.1039/C3CC43276A
(45)Xing,Y.;Cai,Y.;Vukmirovic,M.B.;Zhou,W.P.;Karan,H.; Wang,J.X.;Adzic,R.R.J.Phys.Chem.Lett.2010,1(21), 3238.doi:10.1021/jz101297r
(46)Yang,L.;Vukmirovic,M.B.;Su,D.;Sasaki,K.;Herron,J.A.; Mavrikakis,M.;Liao,S.;Adzic,R.R.J.Phys.Chem.C 2013, 117(4),1748.doi:10.1021/jp309990e
(47)Shao,M.H.;Peles,A.;Odell,J.J.Phys.Chem.C 2014,118 (32),18505.doi:10.1021/jp503296s
(48)Shao,M.H.;Shoemaker,K.;Peles,A.;Protsail,L.J.Am. Chem.Soc.2010,132,9253.doi:10.1021/ja101966a
(49)Peles,A.;Shao,M.H.;Protsailo,L.Catalysts 2015,5(3),1193. doi:10.3390/catal5031193
(50)Humbert,M.P.;Smith,B.H.;Wang,Q.;Ehrlich,S.N.;Shao, M.H.Electrocatalysis 2012,3(3-4),298.doi:10.1007/s12678-012-0103-4
(51)Thambidurai,C.;Gebregziabiher,D.K.;Liang,X.;Zhang,Q.; Ivanova,V.;Haumesser,P.H.;Stickney,J.L.J.Electrochem. Soc.2010,157(8),D466.doi:10.1149/1.3454213
(52)Khateeb,S.;Guerreo,S.;Su,D.;Darling,R.M.;Protsailo,L. V.;Shao,M.H.J.Electrochem.Soc.2016,163(7),F708. doi:10.1149/2.1301607jes
(53)Zhu,S.Q;J.Y.;Qin X.P.;Wei,Z.;Liang,Z.D.;Adzic,R.R.; Brankovic,S.;Du,Z.;Shao,M.H.J.Electrochem.Soc.2016, 163(12),D3040.doi:10.1149/2.0061612jes
(54)Long,N.V.;Ohtaki,M.;Hien,T.D.;Randy,J.;Nogami,M. Electrochim.Acta 2011,56(25),9133.doi:10.1016/j. electacta.2011.07.090
(55)Chen,Y.;Liang,Z.;Yang,F.;Liu,Y.;Chen,S.J.Phys.Chem.C 2011,115(49),24073.doi:10.1021/jp207828n
(56)Du,B.;Zaluzhna,O.;Tong,Y.J.Phys.Chem.Chem.Phys. 2011,13(24),11568.doi:10.1039/C1CP20761J
(57)Atienza,D.O.;Allison,T.C.;Tong,Y.J.J.Phys.Chem.C 2012,116(50),26480.doi:10.1021/jp310313k
(58)Jung,N.;Chung,Y.H.;Chung,D.Y.;Choi,K.H.;Park,H.Y.; Ryu,J.;Lee,S.Y.;Kim,M.;Sung,Y.E.;Yoo,S.J.Phys.Chem. Chem.Phys.2013,15(40),17079.doi:10.1039/C3CP52807C
(59)Chen,Y.;Shi,J.J.Fuel Cell Sci.Technol.2015,12(2),021005. doi:10.1115/1.4028149
(60)Xie,S.;Choi,S.I.;Lu,N.;Roling,L.T.;Herron,J.A.;Zhang, L.;Park,J.;Wang,J.;Kim,M.J.;Xie,Z.Nano Lett.2014,14 (6),3570.doi:10.1021/nl501205j
(61)Park,J.;Zhang,L.;Choi,S.I.;Roling,L.T.;Lu,N.;Herron,J. A.;Xie,S.;Wang,J.;Kim,M.J.;Mavrikakis,M.ACS Nano 2015,9(3),2635.doi:10.1021/nn506387w
(62)Peng,Z.;Yang,H.J.Am.Chem.Soc.2009,131(22),7542. doi:10.1021/ja902256a
(63)Alia,S.M.;Jensen,K.O.;Pivovar,B.S.;Yan,Y.ACS Catal. 2012,2(5),858.doi:10.1021/cs200682c
(64)Liu,X.;Eileen,H.Y.;Scott,K.Appl.Catal.B 2015,162,593. doi:10.1016/j.apcatb.2014.07.038
(65)Zhang,L.;Roling,L.T.;Wang,X.;Vara,M.;Chi,M.;Liu,J.; Choi,S.I.;Park,J.;Herron,J.A.;Xie,Z.Science 2015,349 (6246),412.doi:10.1126/science.aab0801
(66)Choi,R.;Choi,S.I.;Choi,C.H.;Nam,K.M.;Woo,S.I.;Park, J.T.;Han,S.W.Chem.Eur.J.2013,19(25),8190.doi:10.1002/ chem.201203834
(67)Zhang,Y.;Hsieh,Y.C.;Volkov,V.;Su,D.;An,W.;Si,R.;Zhu,Y.;Liu,P.;Wang,J.X.;Adzic,R.R.ACS Catal.2014,4(3), 738.doi:10.1021/cs401091u
(68)Zhang,G.;Shao,Z.G.;Lu,W.;Xiao,H.;Xie,F.;Qin,X.;Li,J.; Liu,F.;Yi,B.J.Phys.Chem.C 2013,117(26),13413. doi:10.1021/jp401375b
(69)Liu,L.;Samjeske,G.;Nagamatsu,S.I.;Sekizawa,O.; Nagasawa,K.;Takao,S.;Imaizumi,Y.;Yamamoto,T.;Uruga, T.;Iwasawa,Y.J.Phys.Chem.C 2012,116(44),23453. doi:10.1021/jp308021a
(70)Anderson,R.M.;Zhang,L.;Loussaert,J.A.;Frenkel,A.I.; Henkelman,G.;Crooks,R.M.ACS Nano 2013,7(10),9345. doi:10.1021/nn4040348
(71)Wang,Y.J.;Zhao,N.;Fang,B.;Li,H.;Bi,X.T.;Wang,H. Chem.Rev.2015,115(9),3433.doi:10.1021/cr500519c
(72)Hwang,S.J.;Kim,S.K.;Lee,J.G.;Lee,S.C.;Jang,J.H.; Kim,P.;Lim,T.H.;Sung,Y.E.;Yoo,S.J.J.Am.Chem.Soc. 2012,134(48),19508.doi:10.1021/ja307951y
(73)Guo,S.;Zhang,S.;Su,D.;Sun,S.J.Am.Chem.Soc.2013,135 (37),13879.doi:10.1021/ja406091p
(74)Wang,C.;Van der Vliet,D.;More,K.L.;Zaluzec,N.J.;Peng, S.;Sun,S.;Daimon,H.;Wang,G.;Greeley,J.;Pearson,J.Nano Lett.2010,11(3),919.doi:10.1021/nl102369k
(75)Wu,J.;Gross,A.;Yang,H.Nano Lett.2011,11(2),798. doi:10.1021/nl104094p
(76)Carpenter,M.K.;Moylan,T.E.;Kukreja,R.S.;Atwan,M.H.; Tessema,M.M.J.Am.Chem.Soc.2012,134(20),8535. doi:10.1021/ja300756y
(77)Cui,C.;Ahmadi,M.;Behafarid,F.;Gan,L.;Neumann,M.; Heggen,M.;Cuenya,B.R.;Strasser,P.Farad.Discuss.2013, 162,91.doi:10.1039/C3FD20159G
(78)Zhang,C.;Hwang,S.Y.;Trout,A.;Peng,Z.J.Am.Chem.Soc. 2014,136(22),7805.doi:10.1021/ja501293x
(79)Huang,X.;Zhao,Z.;Cao,L.;Chen,Y.;Zhu,E.;Lin,Z.;Li,M.; Yan,A.;Zettl,A.;Wang,Y.M.Science 2015,348(6240),1230. doi:10.1126/science.aaa8765
(80)Wu,Y.;Cai,S.;Wang,D.;He,W.;Li,Y.J.Am.Chem.Soc. 2012,134(21),8975.doi:10.1021/ja302606d
(81)Sasaki,K.;Naohara,H.;Cai,Y.;Choi,Y.M.;Liu,P.; Vukmirovic,M.B.;Wang,J.X.;Adzic,R.R.Angew.Chem.Int. Ed.2010,49(46),8602.doi:10.1002/anie.201004287
(82)Kongkanand,A.;Subramanian,N.P.;Yu,Y.;Liu,Z.;Igarashi, H.;Muller,D.A.ACS Catal.2016,6(3),1578.doi:10.1021/ acscatal.5b02819
Core-Shell Electrocatalysts for Oxygen Reduction Reaction
CHANG Qiao-Wan XIAO Fei XU Yuan SHAO Min-Hua*
(Department of Chemical and Biomolecular Engineering,The Hong Kong University of Science and Technology, Hong Kong,P.R.China)
The high cost of platinum in catalyst layers hinders the commercialization of proton exchange membrane fuel cells.This Account reviews recent progress on core-shell nanostructures for oxygen reduction reaction(ORR)in acidic media,which is the cathodic reaction in fuel cells.The synthesis,characterization and evaluation of different types of core-shell electrocatalysts are summarized.Various strategies to improve the performance of core-shell electrocatalysts,including dealloying,morphology control,and surface modification are presented.The issues of mass production and fuel cell performance of core-shell electrocatalysts are also discussed.
Core-shell structure;Proton exchange membrane fuel cell;Electrocatalysis;Platinum alloy; Palladium;Shape control
O646
tureArticle]
10.3866/PKU.WHXB201609202www.whxb.pku.edu.cn
Received:August 2,2016;Revised:September 20,2016;Published online:September 20,2016.
*Corresponding author.Email:kemshao@ust.hk;Tel:+852-34692269.
The project was supported by the Research Grant Council of the Hong Kong SpecialAdministrative Region,China(26206115).
香港特别行政区研究资助局(26206115)资助项目
©Editorial office ofActa Physico-Chimica Sinica
