野生矮桃生物量分布及其对硒与矿质元素的利用特性
2017-02-18倪奎
倪 奎
(安徽新闻出版职业技术学院 新闻传播系, 合肥 230061)
野生矮桃生物量分布及其对硒与矿质元素的利用特性
倪 奎
(安徽新闻出版职业技术学院 新闻传播系, 合肥 230061)
分析野生矮桃生物量分布规律,以及对硒与矿质元素的吸收、迁移及其相关性的探究。采取野生矮桃样本,测出其生物量;分别采用原子发光色谱法和原子荧光法,对不同居群的矮桃植株及土壤中矿质元素和硒元素进行测定,并进行相关性分析。结果显示:矮桃叶的生物量分配率最高,矮桃根和果实中的硒与钾含量成显著负相关,矮桃叶中的硒含量最高。为探究矮桃作为富硒植物开发提供了基本科学依据。
矮桃;生物量;矿质元素;硒;相关性
矮桃(LysimachiaclethroidesDuby)隶属报春花科(Primulaceae)珍珠菜属(LysimachiaL.)[1]。我国珍珠菜属植物资源十分丰富,是一类具有丰富利用价值的植物类群,常生于山坡林缘和草丛中[2],是一种具有较高食用价值的绿色食品[3],种子脂肪油含量达32%,可用于制作肥皂等[2]。其植物体含14 种以上黄酮类化合物,在抗肿瘤方面具有重要应用前景与价值[4-6]。
硒是人体和动物的必需营养元素[7],缺硒可导致人、畜产生多种疾病[8-10],我国大约有72 %的县处于缺硒或严重缺硒状态[11]。研究显示,安徽池州产野生珍珠菜中的锌硒含量高[3]。研究从矮桃的个体生物量分布规律、矮桃对硒及矿质元素的吸收、转移与利用等特性角度探究矮桃作为富硒植物开发具有一定价值。
我国珍珠菜属植物资源十分丰富,有138种[1]。《安徽植物志》记载安徽广布有该属植物23种1变型[2]。近年还发现右旋过路黄(L.dextralflora)与祁门过路黄(L.qimenensis)两新种及距萼过路黄(L.crista-galli)一个地理分布新记录种[12-13]。研究表明,该属植物在民间作为药用植物资源非常普遍[14],多数种类具有清热解毒、利尿排石、活血散瘀等功效,可用于治疗跌打损伤、骨折、风湿疼痛及咳喘等病症;现代医学研究也证明该属植物在治疗肾炎、肝炎、尿路结石、心肌缺血、妇科疾病、抗肿瘤等方面具有明显疗效[15-17]。该属植物除了具有上述重要的药用价值外,还具有较好的观赏价值、工业价值及食用价值[18]。有研究表明,安徽池州产野生珍珠菜中的锌硒含量高。然而,关于矮桃的个体生物学基础研究较为缺乏,如生物量分布规律,矮桃对硒及矿质营养元素的吸收、转移、分布与利用等特性。本研究通过对安徽产不同地区的矮桃为研究材料,初步掌握其相关规律,为矮桃的合理高效持续的开发利用提供依据。
1 材料和方法
1.1 材料来源
2012年8月,分别在安徽金寨县青山镇汤店村、岳西县天堂镇叶畈村和芜湖市南陵县籍山镇千峰村采集野生矮桃结果期样品。在3个采集地分别选取3个代表性野生矮桃居群,生境条件皆为林缘,分根、茎、叶、果实4大植物功能器官以及土壤(0~30 cm)同时收集,每个居群重复采集3次,并分装于牛皮纸袋中带回实验室。
1.2 研究方法
1.2.1 土壤样品采集与处理
在每个调查居群内用内径 25 mm 的土钻钻取0~30 cm土壤样品,带回实验室,晾置于通风处风干,用研钵将土壤碾碎,过100目筛,装入自封袋,待测。
1.2.2 植物样品采集与处理
将来自不同地区,分不同器官的样品首先用自来水冲洗掉泥沙及污染物,然后用去离子水清洗3次,再将其置于75℃烘箱中烘干24 h至恒重,待测。
1.2.3 矮桃生物量测定
将烘干至恒重的不同样品用万分之一电子天平分别称量质量。
1.2.4 矿质元素测定
将烘干的植物器官样品用粉碎机粉碎,以及土壤样品各称取0.5 g于开氏瓶中,加浓HNO3∶HClO4混合酸(12∶3)15 mL,放置数小时后,在电炉上慢慢升温加热,使黄棕色烟慢慢挥发,再适当提高温度继续消化,至冒出大量白烟,消化液呈白色透明状,约2 mL时为止,取下,待冷却后定容至50 mL。然后采用原子发色光谱法测定矿质元素含量。共检测K、Na、Mg、Ca、Al、Cu、Fe、Zn、Mn等9种矿质元素含量。
1.2.5 硒元素测定
将烘干的植物器官样品用粉碎机粉碎,再用研钵研磨后过100目筛,以及土壤样品各称取0.2 g于开氏瓶中,加浓HNO3∶HClO4混合酸(8∶2)10 mL,放置数小时后,在电炉上慢慢升温加热,使黄棕色烟慢慢挥发,再适当提高温度继续消化,至冒出大量白烟,消化液呈白色透明状,约2 mL时为止,取下,待冷却加5 mL HCl(优级纯)过夜,定容至25 mL。然后采用原子荧光法测定硒元素含量。
1.3 数据处理与分析
生物吸收系数(Ax) =Ep/Es。式中,Ep和Es分别代表化学元素在植物干物质中的含量与其生长地土壤中的含量。
元素累积量=器官生物量×元素含量
本实验以生物吸收系数衡量矮桃对9种矿质元素及硒的富集能力,以元素含量及累积量衡量9种矿质元素及硒从土壤到生物体及生物体形态学下端到形态学上端的吸收与迁移特性。
以不同器官生物量差异性反应矮桃植株的生物量分布特征。
采用SPSS 13.0 for Windows软件进行差异显著性检验与多重比较,差异显著性检验前对数据进行转换,以确保符合方差分析的基本条件。数据结果以“平均值±标准误”表示,采用Microsoft Office Excel 2007软件作图。
2 结果与分析
2.1 矮桃生物量分布特征
2.1.1 不同地区单株矮桃总生物量及根冠比
不同地区矮桃单株平均总生物量如图1所示,其中岳西县产矮桃单株平均总生物量最高,为(8.796±1.239)g。方差分析与多重比较结果显示,3个不同地区产矮桃单株总生物量无显著差异(F=2.194,P>0.05)。从实验结果分析,在相似生境条件下,矮桃表现出对资源竞争利用能力的一致性特点。
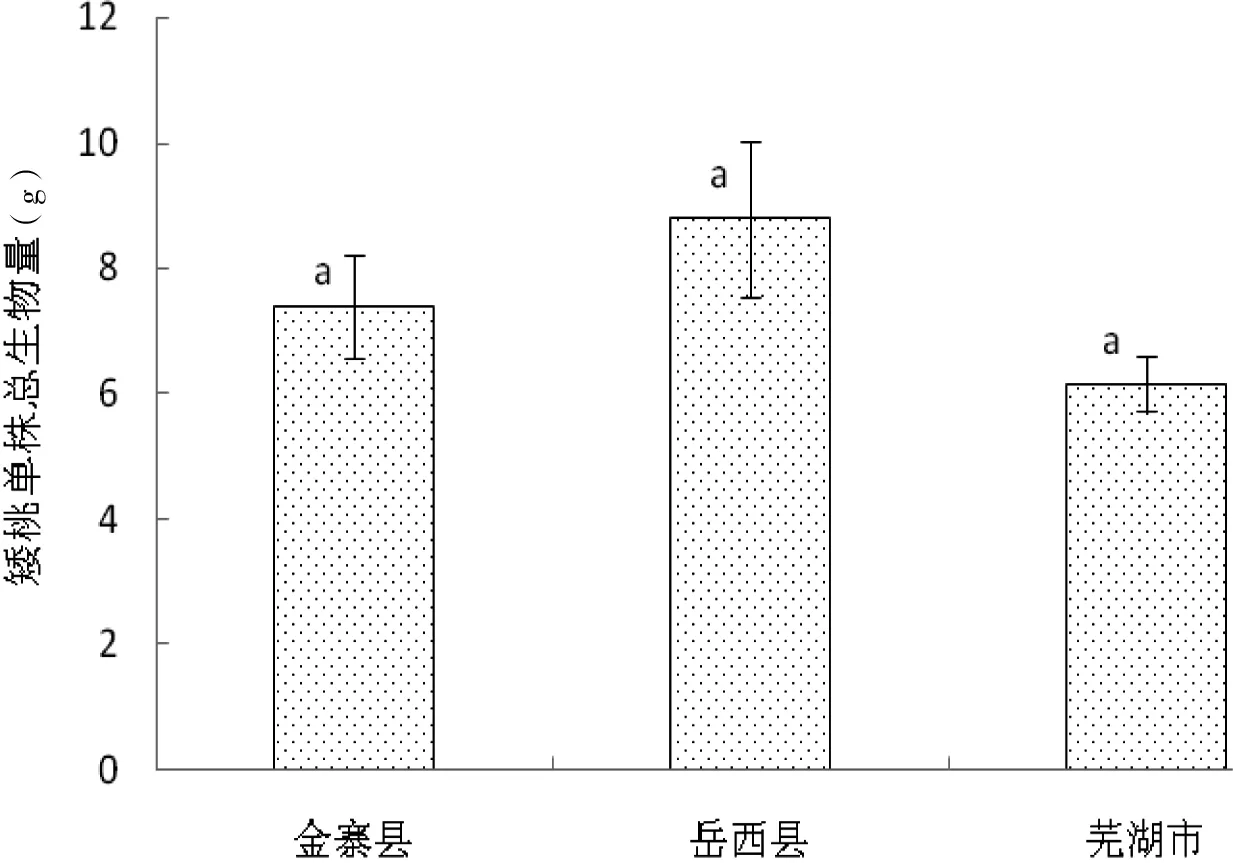
图1 不同地区矮桃单株平均总生物量Fig 1 The average general biomass of L. clethroides in different areas
如表1所示,不同地区产矮桃植株根冠比表现出显著差异(F=13.415,P﹤0.05),从大到小为金寨县>岳西县>芜湖市,总平均根冠比为(0.396±0.070)。这表明矮桃在资源分配上具有一定可塑性,以增加其在不同生境条件下的生态适应性。

表1 不同地区矮桃植株根冠比(平均值±标准误)Table1 The root-shoot ratio of L. clethroidesin different areas )
注:根冠比值后不同字母表示在0.05水平上有显著差异
从图2可以看出,不同地区矮桃植株的根、茎、叶及果实生物量分布有显著差异(金寨县样本,F=13.219,P﹤0.05;岳西县样本,F=14.989,P﹤0.05;芜湖市样本,F=59.914,P﹤0.05)。从营养器官生物量与繁殖器官生物量比较看,3个地区的矮桃都表现出两者间均具有显著差异,即矮桃对营养器官的资源投入明显大于对繁殖器官的资源投入。从对根、茎及叶3大营养器官的资源投入分析来看,不同地区表现出不同差异性,其中岳西县样本差异性不明显,而金寨县样本与芜湖市样本存在显著差异;从对繁殖器官的资源投入分析来看,3个不同地区样本间却未表现出差异性(F=0.992,P=0.424)。以上分析也表明,矮桃在对营养器官的资源投入上具有一定可塑性,但对繁殖器官的资源投入上却表现出一定程度的稳定性,这是矮桃生活史对策中的一种生殖资源保障对策。

图2 矮桃植株生物量分布Fig 2 The biomass distribution of clethroides
2.1.2 矮桃生物量分配特征
从表2可以看出,矮桃根、茎、叶与果实的生物量分配率在不同地区表现出一定的变异幅度。从整体而言,矮桃叶的生物量分配率最高,平均达37.5%,其次是茎,平均为28.4%,两者生物量分配率之和达到65.9%,占较大比例。从表2可有看出,作为依靠叶和茎类资源植物开发对象,叶和茎其在生物量指标上具有一定优势。
2.2 土壤中总硒及矿质元素含量特征
植物所需的矿质营养元素来自于土壤,因此土壤的矿质元素组成、含量及其供应养料的能力对于植物生长发育十分重要。表3为土壤中总硒及9种矿质营养元素的测定结果。从元素相对含量水平看,不同地区土壤硒及9种矿质元素含量存在一些差异。其中,金寨县土壤总硒及9种矿质营养元素顺序为:Al> Fe> Mg> Ca> K> Na> Mn> Zn> Cu> Se;岳西县土壤总硒及9种矿质营养元素顺序为:Al> Fe> Mg> K> Ca> Na> Mn> Zn> Cu> Se;芜湖市土壤总硒及9种矿质营养元素顺序为:Al> Fe> K> Ca> Mg> Na> Mn> Cu> Zn> Se。这些元素按相对含量可分为3级:A级,含量大于10 000 μg/g,有Al和Fe;B级,含量介于500~10 000 μg/g,有Na、Ca、Mg、K和Mn(金寨县);C级,含量小于500 μg/g,有Zn、Cu、Mn(除金寨县)和Se。分类中,除Mn元素在不同地区存在分类差异外,其他元素按含量分类均一致。但从3个取样品点来看,芜湖的硒含量明显高于金寨县和岳西县。
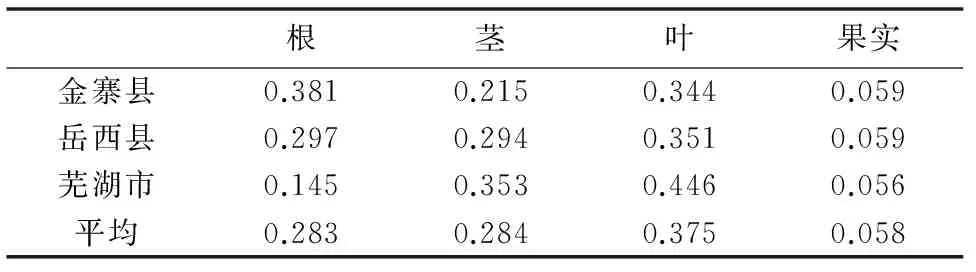
表2 不同地方矮桃不同器官生物量分配率Table 2 The biomass distribution rates different organs ofL. clethroides in from different areas
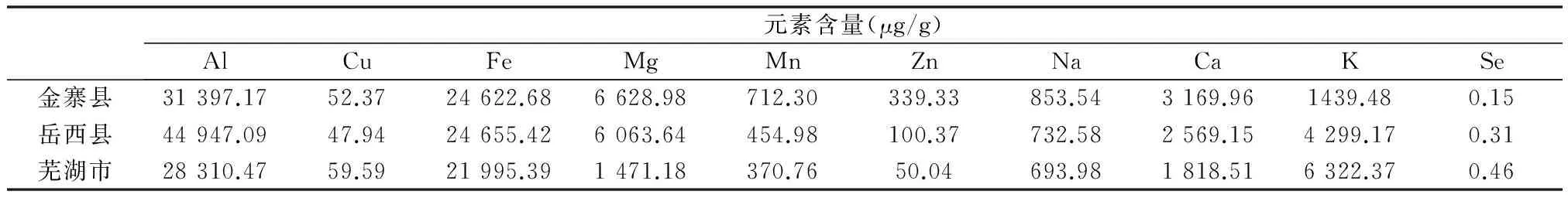
表3 土壤中硒及矿质元素含量Table 3 The contents of mineral elements and selenium in soil
2.3 矮桃植物体内总硒及矿质元素含量及分布特征
植物对矿质营养元素的吸收、迁移、分配和利用具有重要生态学意义。植物根冠比、对矿质营养元素的生物吸收系数及累积量常被用于衡量矿质营养元素从土壤库到生物库迁移特征的实验统计指标。以分析和评价土壤养分与植物生长之间的生态适应性。
2.3.1 矮桃植物体内硒元素总含量及分布特征

图3 矮桃植株内硒元素分布Fig 3 The content distribution of selenium in L. Clethroides
由图3可知,不同地区矮桃植株体内不同器官硒元素含量存在明显差别。但从形态学下端向形态学上端迁移中,一般都表现出先增加后减少的趋势。其中,叶的硒元素总含量较高,尤其是金寨县样本叶硒总含量最高,达到955.53 μg/kg,比来自岳西县样本叶硒总含量104.52 μg/kg高9倍多。这可能与该地区土壤中硒存在形态及其他环境因子有关。
2.3.2 矮桃植物体内矿质元素含量及分布特征

图4 矿质元素在矮桃植株内分布Fig 4 The confent distribution of the mineral elements in L. Clethroides
由图4可以看出,不同地方矮桃中的各矿质元素总体含量大小顺序为:K>Ca>Mg>Na>Al>Fe>Mn,Zn和Cu含量最少,两者含量差异不明显;各地矮桃中的K的总体含量大小顺序为:岳西县>金寨县>芜湖市;Ca和Mg在各器官中的含量大小顺序为:叶>果实>茎>根;3个地区矮桃中的Na含量基本相同,在各器官中的含量也大致相同,说明Na含量比较稳定;Al和Fe的含量在各器官中以根含量最高,茎含量最少;Mn的含量在各器官中以叶中最高;Zn和Cu的含量在各器官中偏低,分布差异不明显。
2.4 矮桃各营养器官对硒及矿质元素的富集特征
由表4可以看出,矮桃各器官中K的吸收系数最大(叶除外,叶中Ca的吸收系数最大),Ca、Na、Zn、Cu、Mn、Mg、Se的吸收系数较大,Fe的吸收系数较小,Al的吸收系数最小,在矮桃各器官对硒的吸收系数中,叶的吸收系数最大。Al、Fe、Zn、Na元素在根中的吸收系数大于在茎、叶、果实中的吸收系数,Mg、Mn、Ca、Se元素在叶中的吸收系数大于在根、茎、果实中的吸收系数,Cu、K在果实中的吸收系数大于在根、茎、叶中的吸收系数。
由表5可以看出,矮桃各器官中K的累积量最高(叶除外,叶中Ca的累积量最高,其次是K),其次是Ca,Al、Mg、Na、Fe的累积量较高,Mn、Zn、Cu的含量较低,在矮桃各器官中,叶中的Se累积量最高。Al、Fe元素在根中的累积量大于在茎、叶、果实的累积量,在根中富集,Cu、Mg、Mn、Zn、Na、Ca、K、Se元素在叶中的累积量大于在根、茎、果实的累积量,在叶中富集。
2.5 矮桃各营养器官中硒及矿质元素含量之间的相关性
2.5.1 矮桃根中硒及矿质元素含量之间相关性分析
由表6可以看出,在安徽省3个地方的矮桃中,根中的Zn和Cu、Ca和Mg含量具显著差异,存在正相关性,相关系数都为0.998;Ca和Mn、Se和K含量具显著差异,存在负相关性,相关系数分别为-0.915、-0.997。
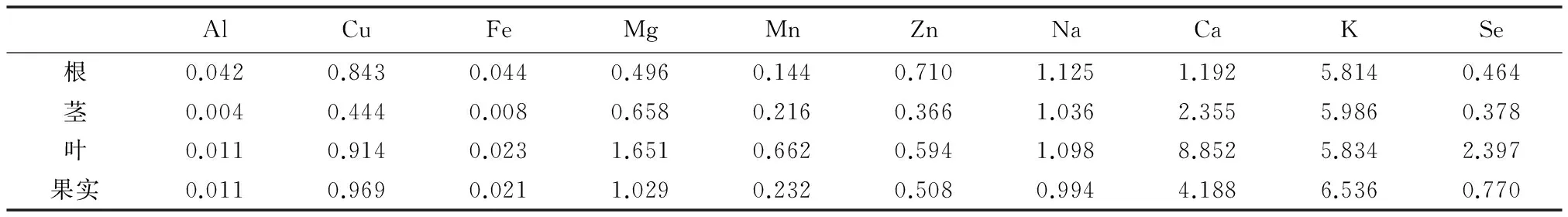
表4 矮桃不同器官对硒及矿质元素的吸收系数Table 4 The absorption coefficients of selenium and mineral elements in L. clethroides′ different organs

表5 矮桃不同器官硒及矿质元素累积量Table 5 The accumulation of mineral elements and selenium in L. clethroides′ different organs
2.5.2 矮桃茎中硒及矿质元素含量之间相关性分析
由表7可以看出,在安徽省3个地方的矮桃中,茎中的Fe和Zn、 Mn和Zn、Na和Zn的含量具显著差异,存在负相关性,其相关系数分别为-0.997、-0.999、-0.998,Na和Mn具极显著差异,存在正相关性,其相关系数为0.99。
2.5.3 矮桃叶中硒及矿质元素含量之间相关性分析
由表8可以看出,在安徽省3个地方的矮桃中,叶中各矿质元素之间,以及硒和矿质元素含量之间无相关性,这可能与矮桃不同器官对矿质元素的吸收作用不同以及生理需求不同有关。
2.5.4 矮桃果实中硒及矿质元素含量之间相关性分析
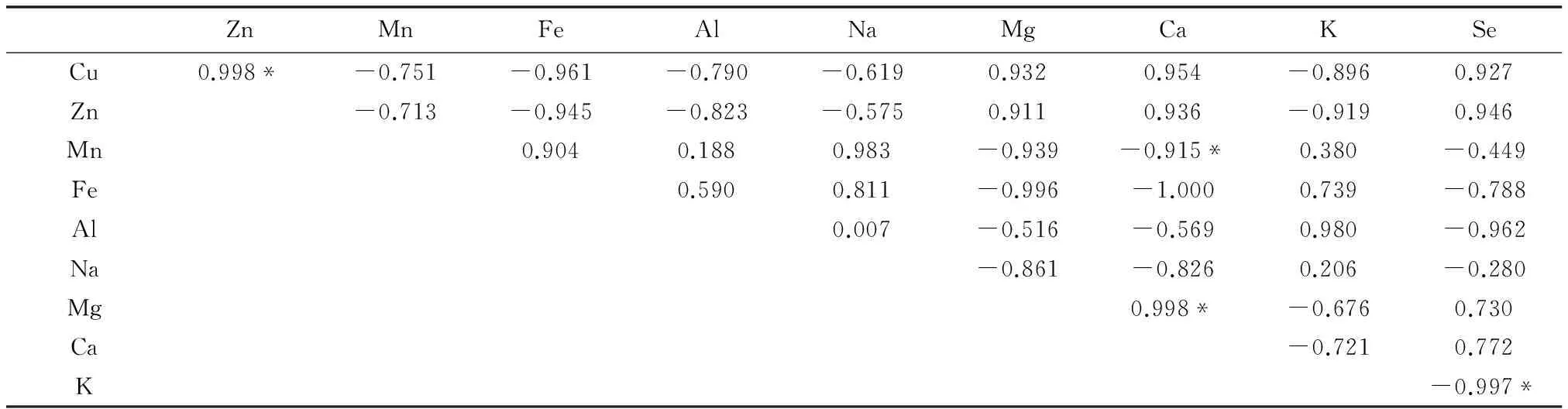
表6 矮桃根中硒及矿质元素含量之间相关性Table 6 The content correlation between the selenium and mineral elements in the L. clethroides′ roots
*:0.05水平上差异显著
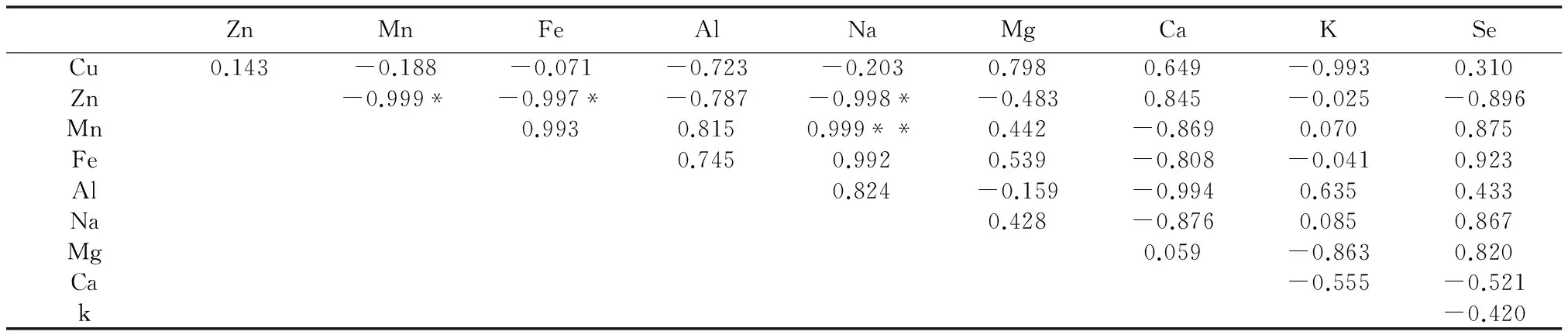
表7 矮桃茎中硒及矿质元素含量之间相关性Table 7 The content correlation between the selenium and mineral elements in the L. clethroides′ stem
*:0.05水平上差异显著;**:0.01水平上差异显著
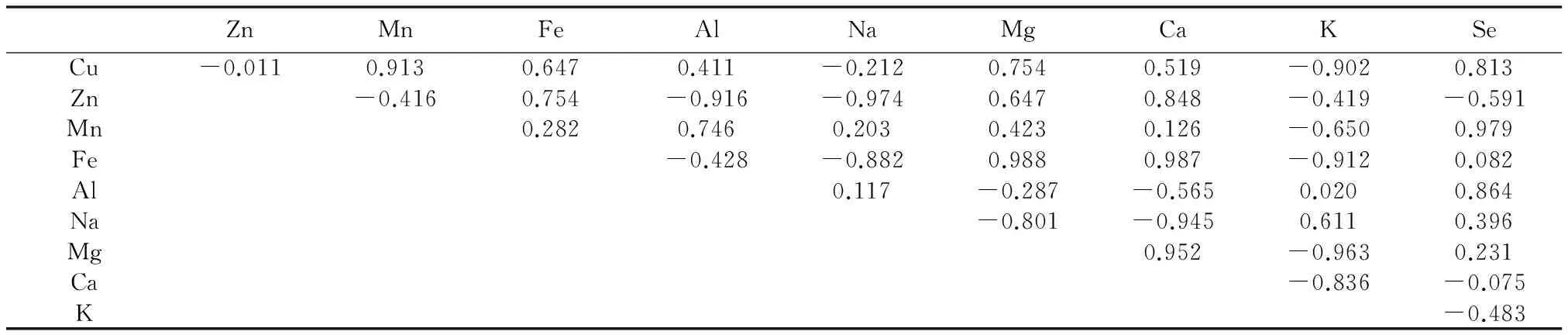
表8 矮桃叶中硒及矿质元素含量之间相关性Table 8 The content correlation between the selenium and mineral elements in the L.clethroides′ leaves
由表9可以看出,在安徽省3个地方的矮桃中,果实中的Zn和Cu,Na和Al含量具显著差异,存在正相关性,相关系数分别为0.997、0.998;Se和K含量具显著差异,存在负相关性,相关系数为-0.999。
通过表6~9的数据可以看出,矮桃根中的Zn和Cu、Ca和Mg含量具显著正相关性,Ca和Mn含量具显著负相关性;茎中的Fe和Zn、 Mn和Zn、Na和Zn的含量具显著负相关性,Na和Mn具极显著正相关性; 果实中的Zn和Cu,Na和Al含量具显著正相关性;根和果实中的Se和K含量有显著负相关性。由数据可知,Zn、Cu、Mn在矮桃植株中含量虽偏低,但与其他矿质元素吸收具相关性,说明它们在维持矿质元素吸收的化学特性上发挥重要的作用,在矮桃植物的生长发育过程中起积极作用;矮桃中的K元素对Se元素吸收关系密切,对矮桃作为富硒植物的开发上需进一步研究。

表9 矮桃果实中硒及矿质元素含量之间相关性Table 9 The content correlation between the selenium and mineral elements in the L. clethroides fruits
*:0.05水平上差异显著
3 讨论与总结
3.1 矮桃的生物量特征
不同地区矮桃单株总生物量无显著差异;不同地方产矮桃植株根冠比表现出显著差异,表明矮桃在资源分配上具有一定可塑性,以增加其在不同生境条件下的生态适应性。不同地方矮桃植株的根、茎、叶及果实生物量分布有显著差异。从营养器官生物量与繁殖器官生物量比较看,矮桃对营养器官的资源投入明显大于对繁殖器官的资源投入。矮桃在对营养器官的资源投入上具有一定可塑性,但对繁殖器官的资源投入却表现出一定程度的稳定性,这是矮桃生活史对策中的一种生殖资源保障对策。从整体而言,矮桃叶的生物量分配率为最高,其次是茎,两者生物量分配率之和达到65.9%,占较大比例。作为利用叶类资源植物开发对象,其在生物量指标上具有一定优势。
3.2 矮桃对矿质元素的吸收与分布特征
不同地方矮桃的各器官矿质元素含量中,K的累积量最高(叶除外,叶中Ca的累积量最高,其次是K),其次是Ca,Al、Mg、Na和Fe的累积量较高,Mn、Zn、Cu的含量较低,在矮桃各器官中, Al、Fe元素在根中的累积量最高,Cu、Mg、Mn、Zn、Na、Ca和K元素在叶中的累积量最高。这与蓝芙宁等[19]对岩溶山区岩石-土壤-牧草系统矿质元素的分布、迁移、富集特征的研究基本一致。这可能与叶片既是矮桃植物的光合器官,又是消耗能量的营养器官有关,某些矿质元素分配偏高,以满足其生理作用。表明矮桃植物在生长发育过程中需要多种大量的矿质元素营养,元素在矮桃体内的分布和富集,不仅取决于元素自身在矮桃体内的移动性能,还与矮桃各部位生理需要以及元素在其体内相互作用有关。
3.3 矮桃对硒及矿质元素的利用特征
不同地方矮桃植株体内不同器官硒元素含量存在明显差别。但从形态学下端向形态学上端迁移中,一般都表现出先增加后减少的趋势。其中,叶中的硒元素总含量较高。
通过数据分析可知,矮桃根中的Zn和Cu、Ca和Mg含量具显著正相关性,Ca和Mn含量具显著负相关性;茎中的Fe和Zn、 Mn和Zn、Na和Zn的含量具显著负相关性,Na和Mn具极显著正相关性; 果实中的Zn和Cu,Na和Al含量具显著正相关性;根和果实中的Se和K含量有显著负相关性。由数据可知,Zn、Cu和Mn在矮桃植株中含量虽偏低,但与其他矿质元素吸收具相关性,在矮桃植物生长发育中有积极作用;矮桃中的K元素对其Se元素吸收关系密切,在引进矮桃人工栽植中,利于Se元素在矮桃体内富集的土壤钾肥和硒肥的最适比例有待进一步研究。
本研究结果表明,常量与微量营养元素、微量有益元素之间、微量营养元素与微量有益元素呈正相关,微量非营养元素与常量营养、微量营养和微量有益元素常呈负相关。这与杜占池等[20]对红三叶与鸭茅对矿质元素吸收的相关性研究结果一致。
从对矮桃各器官的生物量分布研究来看,矮桃叶中的生物量分配率最大,作为利用叶类资源植物开发对象,其在生物量指标上具有一定优势。硒是人体和动物必需的营养元素,叶中硒元素和多数矿质元素的累积量最高,在矮桃根与叶中的硒与钾元素的含量具有负相关性,在以后开发矮桃作为富硒植物的研究中可以具体研究硒肥与钾肥的合适比例。总体来说,矮桃作为待开发的富硒植物是很有前景的。
[1]HU C M. Flora of China [M]. Beijing: Science Press, and St. Louis: Missouri Botanical Garden, 1996, 15: 39-78.
[2]王新风,戴传超,蒋海龙,等.硒对栽培平菇产量及营养成分影响的研究[J]. 食品科学, 2005,26(8):91-93.
[3]刘四运, 张静平.池州野生珍珠菜中 Zn、Se含量的研究 [J]. 广东微量元素科学, 2006, 13(6): 37-39.
[4]游本刚, 唐丽华, 徐向毅,等. 大孔吸附树脂分离纯化珍珠菜总黄酮的研究 [J]. 中草药, 2007, 38(9): 1337-1340.
[5]LIU G J, LIU J Y. Analysis of volatile components fromLysimachiastenosepala[J]. J Jining Univ, 2010, 31(3): 21-24.
[6]吴 威,王春枝,李 夏,等. 珍珠菜抗肿瘤有效部位化学成分研究 [J]. 中草药, 2011, 42(1): 38-41。
[7]陈历程, 杨方美, 胡秋辉. 南京市主要食物含硒量分析及居民硒营养水平评价 [J]. 食品科学, 2000, 21(10) : 57-59.
[8]MARTIN A L. Toxicity of selenium to plants and animals [J] . Amer J Bot, 1936, 23(7): 47-483.
[9]李继云, 任尚学, 陈代中. 陕西省环境中的硒与大骨节病关系的研究 [J]. 环境科学学报, 1982, 2 (2): 91-101.
[10]吴求亮, 杨玉爱, 谢正苗. 微量元素与生物健康 [M]. 贵阳:贵州科技出版社, 2000:1-20.
[11]杜振宇, 史衍玺, 王清华. 蔬菜对硒的吸收及适宜补硒食用量 [J]. 生态环境, 2004, 13(2): 230-231.
[12]邵剑文, 张小平, 郭新弧. 珍珠菜属(报春花科)一新种[J]. 植物研究, 2004, 24(4): 389-391.
[13]邵剑文, 张小平, 郭新弧. 中国珍珠菜属(报春花科)一新种——右旋过路黄[J]. 植物分类学报, 2006, 44 (5): 589-594.
[14]郭宝林, 肖培根, 杨世林. 中国珍珠菜属植物药用种类和研究概况 [J]. 国外医药-植物药分册, 1995, 10(4): 159-162.
[15]顾丽贞. 四川大金钱草与广金钱草抗炎作用的研究 [J]. 中药通报, 1988, 13(7): 40.
[16]张德权, 台建祥, 付 勤,等. 生物类黄酮的研究及应用概况 [J]. 食品与发酵工业, 1999, 25 (6) : 52-56.
[17]BEATE B. Isolation and characterzation of novel benzoates, cinamates, flavonoids, and lingmans from riesling wine and screening for antioxidant activity [J]. J Agric Food Chem, 2001, 49 (6): 2788- 2798.
[18]徐向毅, 唐丽华, 梁中琴,等. 珍珠菜提取物抗肿瘤作用的初步研究 [J]. 中国野生植物资源, 2003, 22(2): 31-34.
[19]蓝芙宁, 蒋忠诚, 谢运球,等. 岩溶山区岩石-土壤-牧草系统中矿质元素的分布、迁移和富集特征 [J].河南农业科学,2011,40(7):67-72.
[20]杜占池, 樊江文, 钟华平. 红三叶和鸭茅矿质元素含量的相关性研究 [J], 草业学报, 2005, 14(6):34-40.
[21]管东生,罗 琳.海南热带植物叶片化学元素含量特征[J].林业科学,2003,39(2):28-30.
[22]张西露,汤小明,刘明月,等.NPK对马铃薯生长发育·产量和品质的影响及营养动态[J].安徽农业科学,2010,38(18):9466-9469.
[23]杜占池,钟华平.红三叶人工草地营养元素的生物循环[J].植物生态学报,1998,22(2):149-156.
[24]孔令韶,李渤生,郭 柯,等.喀喇昆仑、昆仑山地区植物中一些元素的自然含量特征[J].植物生态学报,1995,19(1):13-22.
[25]高 熙,武华文.硅对草莓品质和产量的影响研究[J].现代农业科技,2010(16):122-124.
[26]邓 超,王能如,王东胜,等.烟草镁素营养研究进展[J].安徽农业科学,2009,40(1):60-62.
[27]徐明岗,张久权,文石林;南方红壤丘陵区牧草的肥料效应与施肥[J].草业科学,1997,14(6):21-23.
[28]晁德林,王俊梅.岷山红三叶引种试验[J].草原与草坪,2006(3):67-69.
[29]刘晓玲,杜文华,宋 超.氮磷肥施用量对红三叶中异黄酮含量的影响[J].西北农业学报,2010,19(7):159-163,180.
[30]蔡祖国,周会萍,李红运,等.濒危植物矮牡丹矿质元素含量研究[J].西北林学院学报,2010,25(1):43-46.
收稿日期:2016-12-18;修回日期:2016-12-26
作者简介:刘智敏,博士,研究方向为高温厌氧发酵有机废物生产沼气、污水处理,E-mail: zliu14@ncsu.edu
doi∶10.3969/j.issn.2095-1736.2017.01.058
Abstract With the increasing concerns of global climate change caused by the consumption of fossil fuels, much efforts have been spent on the exploration of renewable energy sources in the last couple of decades. Biogas produced from the treatment of organic waste materials is one of the renewables that have attracted a lot of attentions. This paper provides a summary of the studies on thermophilic anaerobic digestion or co-digestion of various organic waste materials for biogas production. Discussions in this paper include main advantages of thermophilic anaerobic digestion technology over mesophilic one, factors that critically impact the efficiency of the anaerobic digestion such as temperature, pH, total solids, carbon/nitrogen ratio, and hydraulic retention time, the effect of the pretreatment of lignocellulosic materials on the biogas production from anaerobic co-digestion of animal manure and agricultural residues, and mathematical models describing the anaerobic digestion processes.
Keywords anaerobic digestion; biogas; organic wastes; renewable energy; thermophilic
摘 要 随着化石燃料消耗导致越来越严重的全球气候变化问题,可再生能源在近20年被人们大量的研究和探索。通过厌氧发酵处理有机废物产生的沼气作为一种可再生能源得到了广泛的关注。汇总了高温混合厌氧发酵有机废物生产沼气的相关研究, 探讨了高温厌氧发酵相比中温厌氧发酵的优点,影响厌氧发酵效率的因素(例如温度、pH值、总固体量、碳氮比和水力停留时间),以及木质纤维素原料的预处理方法对动物粪便和农作物废弃物混合厌氧发酵生产沼气的影响,并描述了厌氧发酵产生沼气过程的数学模型。
关键词 厌氧发酵;沼气;有机废物;可再生能源;高温发酵
1 Introduction
With the rapid development of industry and agriculture, large volume of organic wastes is generated. Organic wastes can be treated using physical, chemical and biological treatment methods. Among different types of biological treatment methods, anaerobic digestion (AD) is regarded as the most cost-effective one because of its environment friendly operations, net high energy recovery and carbon neutral impact[1].
AD involves the breakdown of complex organic wastes and produces biogas by a consortiumof anaerobic microorganisms[2]. Biogas is mainly composed of 48%-70% methane, 30%-49% carbon dioxide, 100-2 000 ppm hydrogen sulfide and traces of other gases[3]. In comparison with other biological treatment methods, AD offers signification advantages including:
● Biogas produced can be used for heat and electricity generation;
● Thedigestate can be used as fertilizer due to its high nutrients content;
● AD reduces disposed waste volume and weight;
● AD eliminates odor;
● AD is a low cost and low technology system to provideenergy for rural areas.
In order to create a stable and optimized AD process, there are many factors that need to be taken into consideration. Among them, temperature is the most important one because it alone can affect the rate of biochemical reactions in the AD process[1]. AD can be carried out under psychrophilic (15-25℃), mesophilic (30-40℃), and thermophilic (50-60℃) conditions, of which mesophilic and thermophilic conditions are commonly used in applications. In comparison with mesophilic digestion, thermophilic digestion offers many advantages such as higher specific growth rate for the anaerobic microorganisms, higher kinetic advantage in fermentation, higher percentage destruction of pathogens and weed seeds, improved solid-liquid separation and more stability of organic wastes[1]. Thermophilic AD is attracting more and more attentions because of the advantages. In this paper a critical review is presented on themophilic anaerobic digestion of organic wastes including the characteristics of the microorganisms, critical factors, applications, challenges and modeling of the process.
2 Basic Scheme of Thermophilic Anaerobic Digestion Process
Under thermophilic conditions, the basic scheme of digestion process remains almost the same as that undermesophilic conditions. The thermophilic AD is mainly comprised of four steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. A schematic diagram of the AD process is shown in Figure 1.
3 Critcal Factors in Maintaining a Stable Thermophilic Anaerobic Digestion Process
3.1 Temperature
The temperature range for thermophilic AD is considered to be between 48-60℃, with the optimum temperature around 55℃[4]. Compared with mesophilic AD, thermophilic AD has higher rates in biogas production and pathogen destruction. However, thermophilic AD is more sensitive to temperature fluctuations and requires a longer time to adapt to a new environment[5]. Zinder et al.[6]found that only a few degrees increase in temperature resulted in a completely irreversible deterioration of the thermophilic process. This might be attributed to the temperature limitation of aceticlastic methanogens[7]. A fluctuation in temperature results in low biogas production, VFA accumulation and a decrease in pH values[4]. In order to maintain the stability of the digester performance, keeping the temperature at the optimum level is very important. De la Rubiaet et al.[4]indicated that the reactor temperature fluctuations in modern thermophilic digesters should be 0.1-0.2℃ on a daily basis.
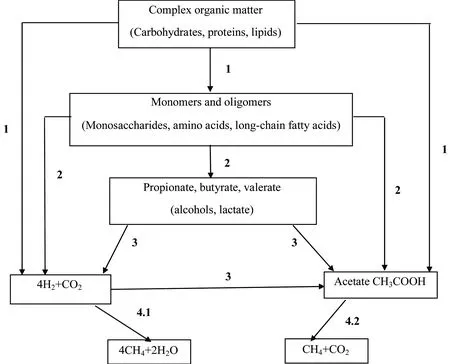
Fig 1 Anaerobic digestion of organic materials into biogas 图1 有机物质通过厌氧发酵转化为沼气的过程
1. Hydrolysis; 2. Acidogenesis; 3. Acetogenesis; 4. Methanogenesis, 4.1.Hydrogenotrophicmethanogenesis, 4.2.Aceticlasticmethanogenesis
1: 水解;2: 酸化;3: 乙酸化;4: 甲烷化,4.1:氢营养型甲烷化,4.2:乙酸发酵型甲烷化
3.2 pH
A pH range of 6.8 to 7.2 is ideal for the AD[3]. However, the optimum pH of methangenesis and hydrolysis are different. The optimal pH for the methanogenesis is at 6.5-8.2 while the optimum pH for the hydrolysis and acidogenesis is between 5.5 and 6.5[8-9].Liu et al.[10]found that the maximum biogas yield was achieved when the pH was between 7.2 and 7.3 for the treatment of organic fraction of municipal solid waste under thermophilic condition.
3.3 Total Solids (TS)
AD can be categorized into wet, semi-dry and dry digestion based on the TS content in the feed material. The wet digestion processes are operated with TS concentration below 10% which is quite applicable for conventional anaerobic digesters such as continuously stirred tank reactors(CSTRs). The TS content in semi-dry process is usually between 10% and 20%, while the dry digestion is operated with a TS content inside the reactor between 20% and 40%[5]. Compared with the wet AD, dry AD has several advantages such as higher organic loading rate and more efficient energy performance[11]. Many studies have been conducted by researchers investigating the influence of TS content on the performance of AD. Desai et al.[12]found that the biogas production was enhanced with the increased TS reaching a maximum at 6% (w/v) in the biomethanation of the mixture of cattle dung, poultry waste and whey. However, contrary result was found when Itodo et al.[13]tested the effect of different TS concentrations (5%, 10%, 15% and 20% TS) of poultry, cattle and piggery waste slurries on the biogas yield in anaerobic batch digesters. The result indicated that the gas yield increased with the decreasing TS concentration of the slurries. In this case, higher gas yield was obtained from the lower TS.This is because when the TS was too high, the slurry became too thick to digest[13].
3.4 Carbon/Nitrogen (C/N) Ratio
Methane production would be enhanced if the C/N ratio in the feed to an anaerobic digester was balanced in the optimum range. Hills[14]investigated the effects of C/N ratio on the AD of dairy manure. The C/N ratio in the study was determined using available carbon (total organic carbon minus the lignin carbon) tototal Kjeldahl nitrogen (TKN). Different C/N ratios between 8.0 and 51.7 was studied by combining the dairy manure with glucose. The result showed that the highest methane production was achieved when the C/N of the feed was 25. Backus et al.[15]tested four C/N ratios of 8.4, 13.9, 22.2 and 27.6 on the performance of AD of raw sweet cheese whey in a semi-batch, fixed film anaerobic digester. The C/N ratio was defined as TOC/TKN in their study. It was observed that the highest percentage of methane and methane production rate occurred when the C/N ratio was between 22 and 28.
3.5 Hydraulic Retention Time (HRT)
HRT is an important design parameter since it determines the microbe/substrate reaction and further influences the consumptionefficiency of the substrate[16]. Typical HRT for mesophilic anaerobic digestion is 20-25 days, while the HRT for thermophilic anaerobic digestion is 10-15 days.
4 Applications of Thermophilic Anaerobic Digestion/Co-Digestion
Thermophilic AD has been widely applied in the past decades because of its efficient hygienic treatment and higher biogas production rate. Compared with traditional mesophilic AD process which has lower energy requirements, the operational cost of thermophilic AD isslightly higher. However, the real benefit of thermophilic AD is total (99.9%) reduction of pathogens and thereby reducing the risk of disease transmission[17]. Substrates that can be used for thermophilic AD are sewage sludge, municipal solid waste, organic fraction of municipal solid waste, animal manure, energy crops and crop residues. Previous studies on thermophilic AD are summarized in Table 1.
Anaerobic co-digestion (AcoD) is defined as a waste treatment method in which two or more substrates are mixed and treated together, so the biogas production is improved through their joint treatment[25]. The potential benefits of AcoD include dilution of toxic compounds, increased load of biodegradable matter, improved nutrients balance, synergistic effect of microorganisms and better biogas yield[25]. AcoD of animal manure with agricultural residues had been extensively investigated in the past decades[26-27]. Cuetos et al.[26]studied the AcoD of swine manure with maize, rapseed and sunflower residues under batch and semi-continuous conditions. The results indicated that the AcoD system resulted in a major increase in the amount of daily biogas production. Similar results were achieved by Wu et al.[27]when they co-digested swine manure with corn stalks, oat straw, and wheat straw. They found the daily maximum biogas production increased by 11.4-fold when the swine manure is co-digested with corn stalks in comparison with the only manure as the substrate. These studies supported that the idea of AcoD of animal manure/wastewater with corn stover could be an effective method in converting organic wastes into biogas.

Table 1 Summary of previous studies on thermophilic anaerobic digestion (AD) of organic wastes表1 有机废物高温厌氧发酵研究汇总
5 Application of Pretreatment on Lignocellulosic Materials in Enhancing Biogas Production in Anaerobic Co-Digestion
One of the main challenges in converting lignocellulosic materials such as agricultural residues into biogas is the recalcitrant compact structure of the materials. Lignocellulosic materials mainly consist of three components: cellulose, hemicellulose and lignin. Cellulose chains are embedded in a cross-linked matrix of hemicellulose surrounded by lignin. The lignin barrier prevents the enzymes from accessing into the cellulose fraction, making the hydrolysis process difficult. The objective of pretreatment is to break the lignin seal and disrupt the crystalline structure to make cellulose more accessible to the enzymes that convert the carbohydrate polymers into fermentable sugars[28].
Pretreatment can be categorized into physical pretreatment, physical-chemical pretreatment, chemical pretreatment and biological pretreatment:
5.1 Physical Pretreatment
The objective of physical pretreatment is to reduce the particle size of the influent substrate. Physical pretreatment process can not only increase the available surface area but also decrease the crystallinity and degrees of polymerization of cellulose.
5.2 Physical-Chemical Pretreatment
Physical-chemical pretreatment combines both the physical and chemical processes. Typical form of physical-chemical pretreatment includes steam explosion, ammonia fiber explosion, carbon dioxide explosion. In comparison with the untreated wheat straw, pretreated wheat straw using wet explosion method can produce 20% more methane production[29].
5.3 Chemical Pretreatment
Chemical pretreatment methods include ozonolysis, acid hydrolysis, alkaline hydrolysis and oxidative dilignification. Among all these methods, alkaline pretreatment proves to be a particularly advantageous method in treating agricultural residuesin anaerobic digestion due to its low cost and effectiveness. NaOH and lime are commonly used in the alkaline pretreatment. Pretreated corn stover with 2% of NaOH would increase the accessibility and digestibility of cellulose[30]. Pang et al.[31]showed that NaOH pretreatment can improve the biodegradability of corn stover and improve the biogas yield. When the NaOH dose was 6% and the loading rate was 65 g/L, 48.5%, more biogas were produced compared with the untreated ones. Corn stover which is pretreated by NaOH, resulted in 72.9% and 73.4% increase of biogas and methane production, respectively[32].
5.4 Biological Pretreatment
Biological pretreatment process uses microorganisms to degrade lignocellulosic materials. The microbes include brown-, white-, and soft-rot fungi. Compared with chemical pretreatment processes, biological pretreatment process needs less energy and no requirement of chemicals. Biological pretreatment can not only degrade the lignin, but also hemicelluloses and cellulose. Romano et al.[33]studied the cellulase, hemicellulase and glucosidase effect in the ananerobic digestion process of wheat grass. They found that the solubility of the wheat grass increased; however, the biogas and methane production was similar compared with the control.
6 Mathematical Modeling of Anaerobic Digestion Process
Considerable effort has been put into the mathematical modeling of anaerobic digestion in the past decades. The development of mathematical models results in a better understanding of the process dynamics, reveals optimization opportunities and is an overall prerequisite for improvement of digester performance[34]. The first anaerobic digestion models were simple kinetic models that describe only the rate-limiting step of the biological process, i.e., the slowest step that limits the rate of the overall process[35-36]. According to the digester operating conditions and influent characteristics, the limiting step of the anaerobic digestion process is different. Hydrolysis, being the first step in overall process, is normally the rate-limiting step of the overall anaerobic digestion process[37]. Several kinetic equations for hydrolysis are reported in the literature. Vavilin et al.[38]used traditional first-order kinetics (Equations (1) and (2)) and Contois kinetics (Equation (3)) to describe the hydrolysis of biodegradable solids.
(1)
(2)
(3)
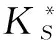
(4)
whereMisthemassofsubstrate,tisthetime(days),Ksbkisthesurfacebasedhydrolysisconstant(kg/m2-day),Aisthesurfaceavailableforhydrolysis(m2).Acetogenesisandmethanogenesishadbeenreportedasrate-limitingstepsaswell[42-43].ThekineticsofthesestepsistraditionallyexpressedbyMonodtypekineticswhichconsiderasinglegrowth-limitingsubstrate(Equation(5)):
(5)
whereμ(d-1)isthespecificgrowthrate,μmax(d-1) the maximum specific growth rate, [S] (g/L) the substrate concentration andKS(g/L) the substrate saturation constant.
It has been found that several chemical compounds at certain concentration are inhibitory to the anaerobic digestion process. These chemical compounds include ammonia, sulfide, metals and some organic compounds such as long-chain fatty acids[44]. Inhibition usually results in decreased or a complete stop in methane production[44].
Organic acids accumulation at neutral pH is regarded as the most common product inhibition in the biological model[45]. Costello et al.[45]proposed a competitive and a non-competitive inhibition model to account for product inhibition by acetic acid. The concentration of the inhibitor [I] is the millimolar concentration of acetic acid in the reactor. It is assumed that the inhibition of the bacteria by the volatile acid substrates or lactic acid is insignificant. The general equation for the competitive inhibition of the acetogenic bacteria is written as follows:
(6)
A noncompetitive model for substrate uptake was used to model product inhibition, the equation is presented in Equation (7):
(7)
In 2002, the International Water Association (IWA) Task Group for Mathematical Modeling of Anaerobic Digestion Processes developed a comprehensive mathematical model known as Anaerobic Digestion Model no.1 (ADM1)[46]. The model includes both biochemical and physicochemical processes. The biochemical steps include extracellular disintegration that coverts homogeneous particulate into carbohydrates, proteins and lipids; extracellular hydrolysis that converts these particulate substrates into sugars, amino acids and long chain fatty acids (LCFA); acidogenesis or fermentation that converts sugars and amino acids into volatile fatty acids (VFA) and hydrogen; acetogenesis that converts LCFA and VFA into acetic acid; and acetoclastic and hydrogenotrophicmethanogenesis[34]. The physicochemical process include ion association and dissociation and gas-liquid transfer[46].
The structured ADM1 model had been successfully applied to simulate the behaviour of a bioreactor for anaerobic digestion of a wide variety of substrates such as municipal waste mixed with activated sludge[47], olive mill wastewater mixed with solid waste[48]and manure mixed with vegetable waste[49].
Recently, some effort has been made to model the solid waste digestion. Esposito et al.[50], for instance, extended the ADM1 to simulate the organic solid particle disintegration and the effect of LCFA production on pH for a sewage sludge and organic fraction of municipal solid waste co-digestion system. In the future, considerable effort will put into the biological aspect of the modelling that is how to mathematically model the anaerobic digestion performance based on microbial diversity and activity[34].
[1]SURYAWANSHI P C, CHAUDHARI A B, KOTHARI R M. Thermophilic anaerobic digestion: the best option for waste treatment [J]. Critical Reviews in Biotechnology, 2010, 30(1): 31-40.
[2]CANTRELL K B, DUCEY T, RO K S, et al. Livestock waste-to-bioenergy generation opportunities [J]. Bioresource Technology, 2008, 99(17): 7941-7953.
[3]WARD A J, HOBBS P J, HOLLIMAN P J, et al. Optimisation of the anaerobic digestion of agricultural resources [J]. Bioresource Technology, 2008, 99(17): 7928-7940.
[4]DE LA RUBIA M A, RIAU V, RAPOSO F, et al. Thermophilic anaerobic digestion of sewage sludge: focus on the influence of the start-up. A review [J]. Critical Reviews in Biotechnology, 2013, 33(4): 448-460.
[5]WEILAND P. Biogas production: current state and perspectives [J]. Applied Microbiology and Biotechnology, 2010, 85(4): 849 860.
[6]ZINDER S H, ANGUISH T, CARDWELL S C. Effects of temperature on methanogenesis in a thermophilic (58 ℃) anaerobic digestor [J]. Applied and Environmental Microbiology, 1984, 47(4): 808-813.
[7]WILSON C A, MURTHY S M, FANG Y, et al. The effect of temperature on the performance and stability of thermophilic anaerobic digestion [J]. Water Science and Technology, 2008, 57(2): 297-304.
[8]KIM J, PARK C, KIM T H, et al. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge [J]. Journal of Bioscience and Bioengineering, 2003, 95(3): 271-275.
[9]LEE D H, BEHERA S K, KIM J W, et al. Methane production potential of leachate generated from Korean food waste recycling facilities: a lab-scale study [J]. Waste Management, 2009, 29(2): 876-882.
[10]LIU C F, YUAN X Z, ZENG G M, et al. Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste [J]. Bioresource Technology, 2008, 99(4): 882-888.
[11]FORSTER-CARNEIRO T, P REZ M, ROMERO L I. Influence of total solid and inoculum contents on performance of anaerobic reactors treating food waste [J]. Bioresource Technology, 2008, 99(15): 6994-7002.
[12]DESAI M, PATEL V, MADAMWAR D. Effect of temperature and retention time on biomethanation of cheese whey-poultry waste-cattle dung [J]. Environmental Pollution, 1994,83(3): 311-315.
[13]ITODO I N, AWULU J O. Effects of total solids concentrations of poultry, cattle, and piggery waste slurries on biogas yield [J]. Transactions of the ASAE, 1999, 42(6): 1853-1855.
[14]HILLS D J. Effects of carbon:nitrogen ratio on anaerobic digestion of dairy manure [J]. Agricultural Wastes, 1979, 1(4): 267-278.
[15]BACKUS B D, CLANTON C J, GOODRICH P R, et al. Carbon-to-nitrogen ratio and hydraulic retention time effect on the anaerobic digestion of cheese whey [J]. Transactions of the ASAE, 1988, 31(4): 1274-1282.
[16]AGUILAR M A R, FDEZ-G ELFO L A, LVAREZ-GALLEGO C J, et al. Effect of HRT on hydrogen production and organic matter solubilization in acidogenic anaerobic digestion of OFMSW [J]. Chemical Engineering Journal, 2013, 219: 443-449.
[17]SAHLSTR M L. A review of survival of pathogenic bacteria in organic waste used in biogas plants [J]. Bioresource Technology, 2003, 87(2): 161-166.
[18]NIELSEN H B, MLADENOVSKA Z, WESTERMANN P, et al. Comparison of two-stage thermophilic (68 ℃ /55℃) anaerobic digestion with one-stage thermophilic (55℃) digestion of cattle manure [J]. Biotechnology and Bioengineering, 2004, 86(3): 291-300.
[19]FORSTER-CARNEIRO T, FERNANDEZ L A, P REZ M, et al. Optimization of Sebac start up phase of municipal solid waste anaerobic digestion [J].Chemical and Biochemical Engineering Quarterly, 2004, 18(4): 429-439.
[20]ONODERA M, OOTSU T, SATO E, et al. Biogas production from waste milk by thermophilic anaerobic digestion [J]. Journal of BioTechnology, 2007, 131(2): S176.
[21]BOUALLAGUI H, RACHDI B, GANNOUN H, et al. Mesophilic and thermophilic anaerobic co-digestion of abattoir wastewater and fruit and vegetable waste in anaerobic sequencing batch reactors [J]. Biodegradation, 2009, 20(3): 401-409.
[22]FORSTER-CARNEIRO T, P REZ M, ROMERO L I, et al. Dry-thermophilic anaerobic digestion of organic fraction of the municipal solid waste: focusing on the inoculum sources[J]. Bioresource Technology, 2007, 98(17): 3195-3203.
[23]YILMAZ T, YUCEER A, BASIBUYUK M. A comparison of the performance of mesophilic and thermophilic anaerobic filters treating papermill wastewater [J]. Bioresource Technology, 2008, 99(1): 156-163.
[24]KAPARAJU P, ANGELIDAKI I. Effect of temperature and active biogas process on passive separation of digested manure [J]. Bioresource Technology, 2008, 99(5): 1345-1352.
[25]KHALID A, ARSHAD M, ANJUM M, et al. The anaerobic digestion of solid organic waste [J]. Waste Management, 2011, 31(8): 1737-1744.
[26]CUETOS M J, FERN NDEZ C, G MEZ X, et al. Anaerobic co-digestion of swine manure with energy crop residues [J]. Biotechnology and Bioprocess Engineering, 2011, 16(5): 1044-1052.
[27]WU X, YAO W, ZHU J, et al. Biogas and CH4productivity by co-digesting swine manure with three crop residues as an external carbon source [J]. Bioresource Technology, 2010, 101(11): 4042-4047.
[28]MOSIER N, WYMAN C, DALE B, et al. Features of promising technologies for pretreatment of lignocellulosic biomass [J]. Bioresource Technology, 2005, 96(6): 673-686.
[29]BAUER A, B SCH P, FRIEDL A, et al. Analysis of methane potentials of steam-exploded wheat straw and estimation of energy yields of combined ethanol and methane production[J]. Journal of Biotechnology, 2009, 142(1): 50-55.
[30]CHEN M, ZHAO J, XIA L. Comparison of four different chemical pretreatments of corn stover for enhancing enzymatic digestibility [J]. Biomass and Bioenergy, 2009, 33(10): 1381-1385.
[31]PANG Y Z, LIU Y P, LI X J, et al. Improving biodegradability and biogas production of corn stover through sodium hydroxide solid state pretreatment [J]. Energy& Fuels, 2008, 22(4): 2761-2766.
[32]ZHENG M, LI X, LI L, et al. Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment[J]. Bioresource Technology, 2009, 100(21): 5140-5145.
[33]ROMANO R T, ZHANG R, TETER S, et al. The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass[J]. Bioresource Technology, 2009, 100(20): 4564-4571.
[34]LAUWERS J, APPELS L, THOMPSON I P, et al. Mathematical modelling of anaerobic digestion of biomass and waste: Power and limitations [J]. Progress in Energy and Combustion Science, 2013, 39(4): 383-402.
[35]ANDREWS J, PEARSON E A. Kinetics and characteristics of volatile fatty acid production in anaerobic fermentation processes [J]. International Journal of Air Water Pollution, 1965, 9: 439-461.
[36]ANDREWS J. Dynamic model of the anaerobic digestion process [J] . Journal of the Sanitary Engineering Division, 1969, 95(1): 95-116.
[37]ANGELIDAKI I, SANDERS W. Assessment of the anaerobic biodegradability of macropollutants [J]. Reviews in Environmental Science and Bio/Technology, 2004, 3(2): 117-129.
[38]VAVILIN V A, RYTOV S V, LOKSHINA L Y A, et al. Simplified hydrolysis models for the optimal design of two-stage anaerobic digestion [J]. Water Research, 2001, 35(17): 4247-4251.
[39]HILLS D J, NAKANO K. Effects of particle size on anaerobic digestion of tomato solid wastes [J]. Agricultural Wastes, 1984, 10(4): 285-295.
[40]VAVILIN V A, RYTOV S V, LOKSHINA L Y A. A decription of hydrolysis kinetics in anaerobic degradation of particulate organic matter [J]. Bioresource Technology, 1996, 56(2): 229-237.
[41]SANDERS W T M, GEERINK M, ZEEMAN G, et al. Anaerobic hydrolysis kinetics of particulate substrates [J]. Water Science and Technology, 2000, 41(3): 17-24.
[42]SIEGRIST H, RENGGLI D, GUJER W. Mathematical modelling of anaerobic mesophilic sewage sludge treatment [J]. Water Science and Technology, 1993, 27(2): 25-36.
[43]MOLETTA R, VERRIER D, ALBAGNAC G. Dynamic modelling of anaerobic digestion[J]. Water Research, 1986, 20(4): 427-434.
[44]CHEN Y, CHENG J J, CREAMER K S. Inhibition of anaerobic digestion process: a review[J]. Bioresource Technology, 2008, 99(10): 4044-4064.
[45]COSTELLO D J, GREENFIELD P F, LEE P L. Dynamic modelling of a single-stage high-rate anaerobic reactor-I. model derivation [J]. Water Research, 1991, 25(7): 847-858.
[46]BATSTONE D J, KELLER J, ANGELIDAKI I, et al. The IWA anaerobic digestion model No 1 ( ADM1 ) [J]. Water Science & Technology, 2002, 45(10): 65-73.
[47]DERBAL K, BENCHEIKH-LEHOCINE M, CECCHI F, et al. Application of the IWA ADM1 model to simulate anaerobic co-digestion of organic waste with waste activated sludge in mesophilic condition [J]. Bioresource Technology, 2009, 100(4): 1539-1543.
[48]FEZZANI B, CHEIKH R B. Implementation of IWA anaerobic digestion model No. 1 (ADM1) for simulating the thermophilic anaerobic co-digestion of olive mill wastewater with olive mill solid waste in a semi-continuous tubular digester [J]. Chemical Engineering Journal, 141(1-3): 75-88.
[50]ESPOSITO G, FRUNZO L, PANICO A, et al. Mathematical modelling of disintegration-limited co-digestion of OFMSW and sewage sludge [J]. Water Science and Technology, 2008, 58(7): 1513-1519.
Uncultivated lysimachia clethroides biomass distribution and its feature of utilizing celenium and mineral element
NI Kui
(Department of Journalism and Communication, Anhui News and Publishing Vocational College, Hefei 230061, China)
The research analyzed the biomass distribution of uncultivated lysimachia clethroides and the absorbing ability, migration and correlation between the content of selenium and mineral elements; the samples of uncultivated lysimachia clethroides were gathered to measure the biomass. The contents of mineral elements and selenium were determined respectively by the atomic luminescence chromatography and the atomic fluorescent method,and the correlation analysis was done meantime. The result showed the biomass distribution rates in the leaves of the lysimachia clethroides were the highest, and the content of selenium was negatively correlated to potassium in the roots and fruits. Besides, the leaves possessed the highest content of selenium.
Lysimachiaclethroides; biomass; mineral elements; selenium; correlation
Thermophilic anaerobic digestion of organic wastes for biogas production: a review
LIU Zhi-min1, ZHU Jian-hang2, CHENG Jia-yang1
(1. Biological and Agricultural Engineering Department, North Carolina State University,Raleigh, NC 27695, USA; 2. School of Environmental Resources and Chemical Engineering, Nanchang University, Nanchang 330031, China)
高温厌氧发酵有机废物生产沼气综述
刘智敏1, 朱建航2, 成家杨1
(1. 北卡罗来纳州立大学 生物与农业工程系, 美国 罗利 NC27695;2. 南昌大学 资源环境与化工学院, 中国 南昌 330031)
参考文献
2016-03-07;
2016-03-24
2015年高校人文社科重点项目“基于景观生态学的高校校园规划研究”(SK2015A688)
倪 奎,硕士,讲师,研究方向为生物工程,E-mail:ahcbxy@126.com
S662.1
A 文章编号 2095-1736(2017)01-0052-06
成家杨,博士,教授,研究方向为厌氧发酵有机废物生产沼气、利用木质纤维素制备燃料酒精、微藻生物柴油、生物废水处理,E-mail: jay_cheng@ncsu.edu
16.4 文献标识码 A
2095-1736(2017)01-0058-07
doi∶10.3969/j.issn.2095-1736.2017.01.052
