胞壁酰二肽对体外奶牛乳腺上皮细胞生长及胞内NOD2 mRNA表达的影响
2017-02-16徐丹丹王建发张旭刘东宇许小楠王乐陈嘉单旭菲王晓雅武瑞杨彬
徐丹丹,王建发,张旭,刘东宇,许小楠,王乐,陈嘉,单旭菲,王晓雅,武瑞,杨彬
(黑龙江八一农垦大学动物科技学院,黑龙江大庆 163319)
胞壁酰二肽对体外奶牛乳腺上皮细胞生长及胞内NOD2 mRNA表达的影响
徐丹丹,王建发,张旭,刘东宇,许小楠,王乐,陈嘉,单旭菲,王晓雅,武瑞,杨彬
(黑龙江八一农垦大学动物科技学院,黑龙江大庆 163319)
【目的】奶牛乳房炎是奶牛养殖业中最为常见,同时也是带来经济损失最为严重的疾病之一,其主要病因是细菌感染。奶牛乳腺的固有免疫是抵抗病原菌入侵的第一道防线。NOD2是机体固有免疫模式识别受体核苷酸结合寡聚域(nucleotide-binding oligomerization domain, NOD)蛋白家族中的重要一员,通过识别其特异性配体胞壁酰二肽(muramyl dipeptide, MDP)——一种广泛存在于革兰氏阳性菌和革兰氏阴性菌细胞壁中的成分,而参与抵抗多种病原菌入侵。奶牛乳腺上皮细胞(bovine mammary epithelial cells, BMEC)除泌乳以外,还是奶牛乳腺的免疫屏障。本试验欲探究MDP对BMEC体外生长状态及胞内NOD2表达量的影响。【方法】选取健康泌乳中期荷斯坦奶牛乳腺腺泡为组织原材料,采用胶原酶Ⅰ消化法结合梯度浓度胰蛋白酶纯化法分离BMEC;使用上皮细胞特异性表达的角蛋白-18(cytokeratin-18,CK-18)及成纤维细胞特异性表达的波形蛋白(Vimentin)抗体,通过免疫荧光技术对纯化后获得的细胞进行鉴定;将BMEC设为6个处理组,分别添加浓度为0(空白对照组)、1、5、10、15及20 μg·mL-1的MDP,24 h后显微镜下观察细胞状态,同时提取细胞RNA并反转录为cDNA,采用实时荧光定量PCR方法检测BMEC中NOD2的表达量。【结果】(1)胶原酶Ⅰ消化法结合梯度浓度胰蛋白酶纯化法分离得到的细胞CK-18免疫荧光结果为阳性,而Vimentin反应为阴性;细胞生长状态良好。(2)空白对照组、1、5及10 μg·mL-1MDP刺激组的BMEC生长状态良好,无任何肉眼可见变化;15 μg·mL-1MDP刺激组可见少量的BMEC脱落;而20 μg·mL-1MDP刺激组可见大量BMEC脱落,漂浮,且即使仍贴壁的细胞,其形态也发生了变化。(3)与空白对照组相比,各刺激组细胞NOD2 mRNA的表达量与MDP的刺激浓度呈正相关,即刺激时间为24 h时,随着MDP浓度的增加,BMEC中NOD2受体mRNA的表达量逐渐增加(P < 0.05)。【结论】成功获得了纯度较好的BMEC,该细胞生长状态良好,可以用于后续试验;虽然BMEC中NOD2受体mRNA的表达量与MDP的刺激浓度呈正相关,但在保持BMEC生长状态正常的前提下,MDP体外刺激浓度应控制在10 μg·mL-1以下。这些结果提示我们:当病原菌入侵乳腺时,BMEC可以通过NOD2受体途径参与免疫防御反应,但这种防御能力受细菌数量或毒力强度的影响。即在一定的细菌数量或毒力范围内,随着细菌数量的增加或毒力的增强,奶牛乳腺的免疫防御反应也增强,进而清除外来病原菌;而当细菌数量或毒力强度超过一定范围时,乳腺组织受到严重损伤,免疫防御屏障也随之崩溃,奶牛乳腺局部甚至全身将呈现出明显的临床症状。
奶牛乳腺上皮细胞;MDP;NOD2
0 引言
【研究意义】奶牛乳房炎是奶牛养殖业中最为常见的疾病,其主要病因就是细菌感染,该病严重影响奶牛健康、牛奶质量与产量,给奶牛养殖业造成了极大的经济损失。近年来,随着耐药菌群的不断出现以及人们对抗生素残留问题的逐渐重视,因此笔者逐渐将奶牛乳房炎的防治重点转移到了提高奶牛机体以及乳腺组织局部免疫力的方向。【前人研究进展】NOD2是机体固有免疫模式识别受体核苷酸结合寡聚域(nucleotide-binding oligomerization domain,NOD)蛋白家族中的重要一员,其通过识别细菌细胞壁的固有成分胞壁酰二肽(muramyl dipeptide,MDP),进而激活下游的核转录因子-κB(nuclear transcription factor-κB,NF-κB)及促分裂素原活化蛋白激酶(mitogenactivated protein kinases,MAPK)信号通路,产生大量的炎性因子,从而启动机体的免疫防御反应[1-3]。【本研究切入点】除了具有泌乳功能以外,奶牛乳腺上皮细胞(bovine mammary epithelial cells,BMEC)也是乳腺组织中重要的免疫细胞[4-5]。有研究表明,奶牛乳腺中存在NOD2受体,但MDP作为刺激剂对于体外BMEC的生长以及胞内NOD2受体的表达有何影响未见报道[6]。本试验采用胶原酶Ⅰ消化法体外培养BMEC,并以不同浓度的MDP刺激BMEC,观察细胞状态,同时采用实时荧光定量 PCR的方法检测BMEC中NOD2的mRNA表达量。【拟解决的关键问题】明确MDP作为BMEC体外刺激剂的安全浓度范围,及其对BMEC中NOD2表达量的影响,为进一步揭示NOD2受体在奶牛乳腺组织局部免疫中的作用提供了理论基础和试验依据。
1 材料与方法
试验于 2014—2015年在黑龙江八一农垦大学兽医临床疾病诊断与治疗实验室完成。
1.1 材料
1.1.1 乳腺组织 取自黑龙江省某养殖场泌乳期健康荷斯坦奶牛。
1.1.2 主要试剂 DMEM F/12培养基购于 GIBCO公司;胎牛血清(FBS)购于 Hyclone公司;胶原酶Ⅰ及MDP购于Invivogen公司;胰蛋白酶购于碧云天公司;角蛋白-18(cytokeratin-18,CK-18)抗体购于Abcam公司;波形蛋白(Vimentin)抗体购于Neomarker公司;Mini BEST Universal RNA Extraction Kit及PrimeScript RT reagent Kit购于TaKaRa公司;SYBR®Green Master Mix购于Vazyme公司。
1.2 方法
1.2.1 BMEC的体外培养及分离纯化 取新鲜乳腺组织,浸于75%酒精中2—3 min后转至超净台中,用灭菌生理盐水(含青霉素100 IU·mL-1,链霉素100 μg·mL-1)冲洗数次,将组织剪成小块(约1 mm3)后加入Hank’s液洗涤数次,转移组织块至离心管,加入300 U·mL-1胶原酶Ⅰ,37℃水浴消化3—5 h,细胞筛过滤消化液后离心收集细胞,加入 DMEM F/12培养基(含10%FBS,青霉素100 IU·mL-1,链霉素100 μg·mL-1)混匀并移入培养瓶,置于CO2培养箱中培养。
待细胞初次长满至70%—80%时,根据BMEC与成纤维细胞对胰酶的耐受能力不同,先用 0.05%的胰酶对成纤维细胞进行消化分离,PBS清洗后去除成纤维细胞;再用0.25%胰酶消化BMEC,离心收集BMEC加入培养基后继续传代培养,重复以上操作数次获取试验所需的BMEC,转入含有细胞爬片的六孔板继续培养。
1.2.2 免疫荧光方法对BMEC进行鉴定 将长满至汇合的BMEC用PBS漂洗,而后加入预冷的4%多聚甲醛于4℃固定30 min,PBS漂洗;用0.5%的Trition处理10—15 min,PBS漂洗;用1%BSA封闭30 min,分别加入 CK-18(上皮细胞标志性蛋白)抗体、Vimentin(成纤维细胞标志性蛋白)抗体,4℃孵育过夜,PBS漂洗;加入相应二抗,37℃作用 1 h,PBS漂洗;加入DAPI作用5—10 min;最后用抗猝灭剂封片,荧光显微镜下观察结果。
1.2.3 实时荧光定量PCR检测BMEC中NOD2的mRNA表达量 将BMEC分为5组,在培养基中加入MDP,使其浓度依次为1、5、10、15和20 μg·mL-1,另设空白对照组。24 h后观察细胞状态,并按照MiniBEST Universal RNA Extraction Kit说明书提取各组细胞总RNA,核酸测定仪检测RNA的浓度及OD260/280值。按照PrimeScript RT reagent Kit说明书将RNA反转为cDNA。实时荧光定量PCR所用引物由上海生工生物工程股份有限公司合成,序列详见表1;反应体系为:SYBR®Green Master Mix 10 μL,Primer1、Premer2各0.4 μL,cDNA 3 μL,灭菌水6.2 μL,总体系为20 μL;扩增程序见表2。
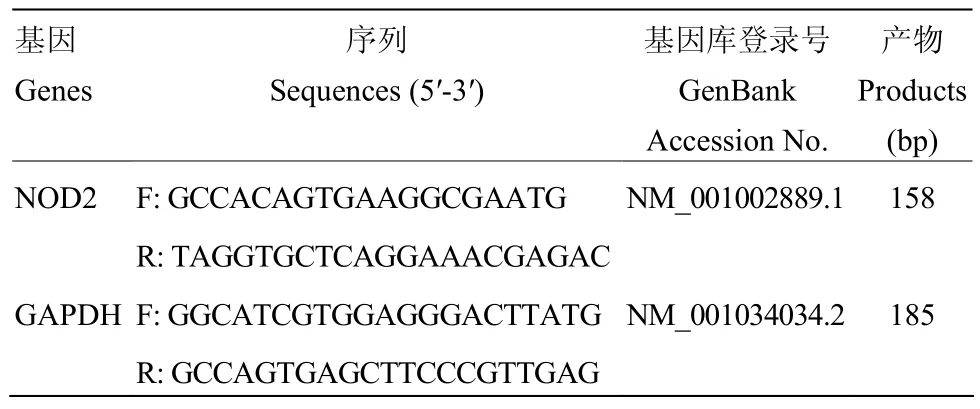
表1 引物序列Table 1 Primer sequences
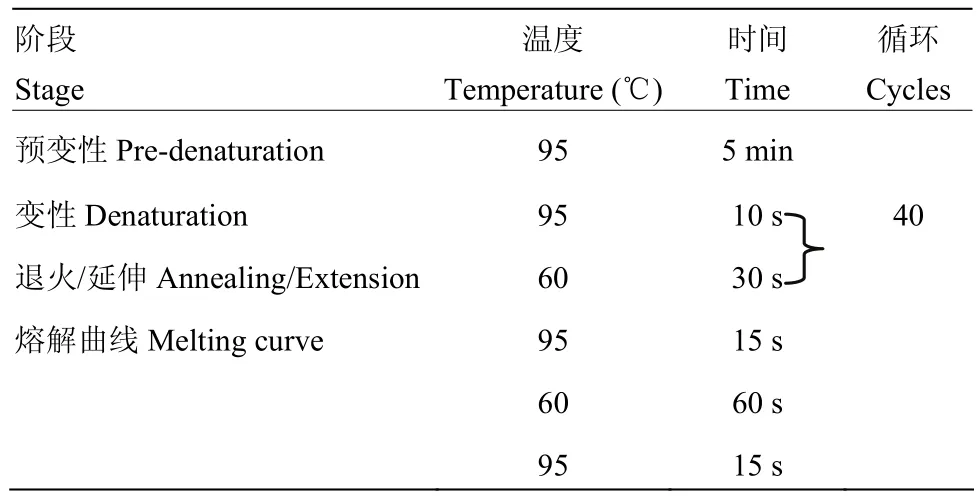
表2 荧光定量PCR扩增程序Table 2 Quantitative real-time PCR amplification procedure
1.2.4 数据分析 采用 2-△△Ct法表示实时荧光定量PCR结果,数据应用SPSS 19.0进行单因素方差分析,P<0.05为具有统计学意义,以“*”表示。
2 结果
2.1 BMEC的体外培养及分离纯化
酶消化法培养细胞数小时后就有少量的细胞先贴壁生长,培养至第3天时可见大片聚集生长的BMEC与形态不规则的成纤维细胞,但两种细胞之间界限明显并不混杂生长(图1-a)。细胞纯化后得到较为纯净的“铺路石”或“岛屿状”聚集生长的形态规则的BMEC,细胞核及核仁(多为多核仁)清晰可见,且部分细胞具有分泌乳汁的功能,形成空泡样结构(图1-b)。
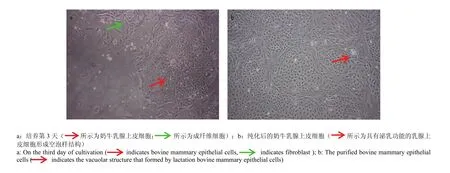
图1 奶牛乳腺上皮细胞的显微镜下观察Fig. 1 The observation of bovine mammary epithelial cells under the microscope (100×)
2.2 BMEC的免疫荧光鉴定
免疫荧光鉴定结果显示,细胞CK-18反应呈阳性,发出绿色荧光(图2-a,b,c),而细胞Vimentin反应呈阴性(图2-d,e,f)。
2.3 MDP对BMEC生长状态的影响
将添加了不同浓度MDP的BMEC于24h后置于倒置显微镜下观察细胞状态,结果如图所示,空白对照组、添加1、5及10 μg·mL-1MDP组的BMEC生长状态良好,无任何肉眼可见变化(图3-a,b,c,d);添加了15 μg·mL-1MDP组有少量的BMEC脱落(图3-e);而添加了20 μg·mL-1MDP组有大量的BMEC脱落、漂浮,且即使仍贴壁的细胞形态上也出现了变化(图3-f)。
2.4 MDP对BMEC NOD2表达量的影响
实时荧光定量 PCR方法对各组细胞 NOD2 mRNA表达量检测的结果显示,与空白对照组相比,NOD2 mRNA表达量与MDP的刺激浓度呈正相关,即随着MDP浓度的增加,BMEC中NOD2受体的mRNA表达量增加(图4)。
3 讨论
尽管有相关报道表明,酶消化法培养BMEC易破坏细胞结构,不易成功获得BMEC[7-8];且常常添加多种外源激素及表皮生长因子[9]。而本试验在尽可能减少外源激素及生长因子添加的情况下(只添加DMEM F/12,FBS,青、链霉素)采用胶原酶Ⅰ消化法成功获得了BMEC,且细胞生长状态良好,这提示要根据所使用酶的质量及组织差异等实际情况掌握好消化时间。另外,本试验所用胶原酶Ⅰ消化法相对于常用的组织块培养法具有污染风险小、培养周期短、细胞纯度高等优点。分离纯化及鉴定结果表明,本试验获得了生长状态良好且较为纯净的BMEC,该细胞外源物质干扰因素少,可以用于后续试验。
NOD2是近年来才发现的一类细胞内模式识别受体,存在于某些抗原提呈细胞和上皮细胞等细胞中[10-11]。其通过识别细菌固有成分 MDP进而参与机体固有免疫调节。据报道,NOD2参与机体抵抗葡萄球菌[12]、大肠杆菌[13]、沙门氏菌[14]、肺炎链球菌[15]、分支杆菌[16]、志贺氏菌[17]、李斯特菌[18-19]、嗜肺军团菌[20]等多种细菌甚至是衣原体[21]、病毒[22]及胞内原虫[23]的侵袭。目前对于NOD2的研究多集中于其与克罗恩病、Blau综合征、早发性结节及过敏性疾病等疾病的关系[13,24-28]。
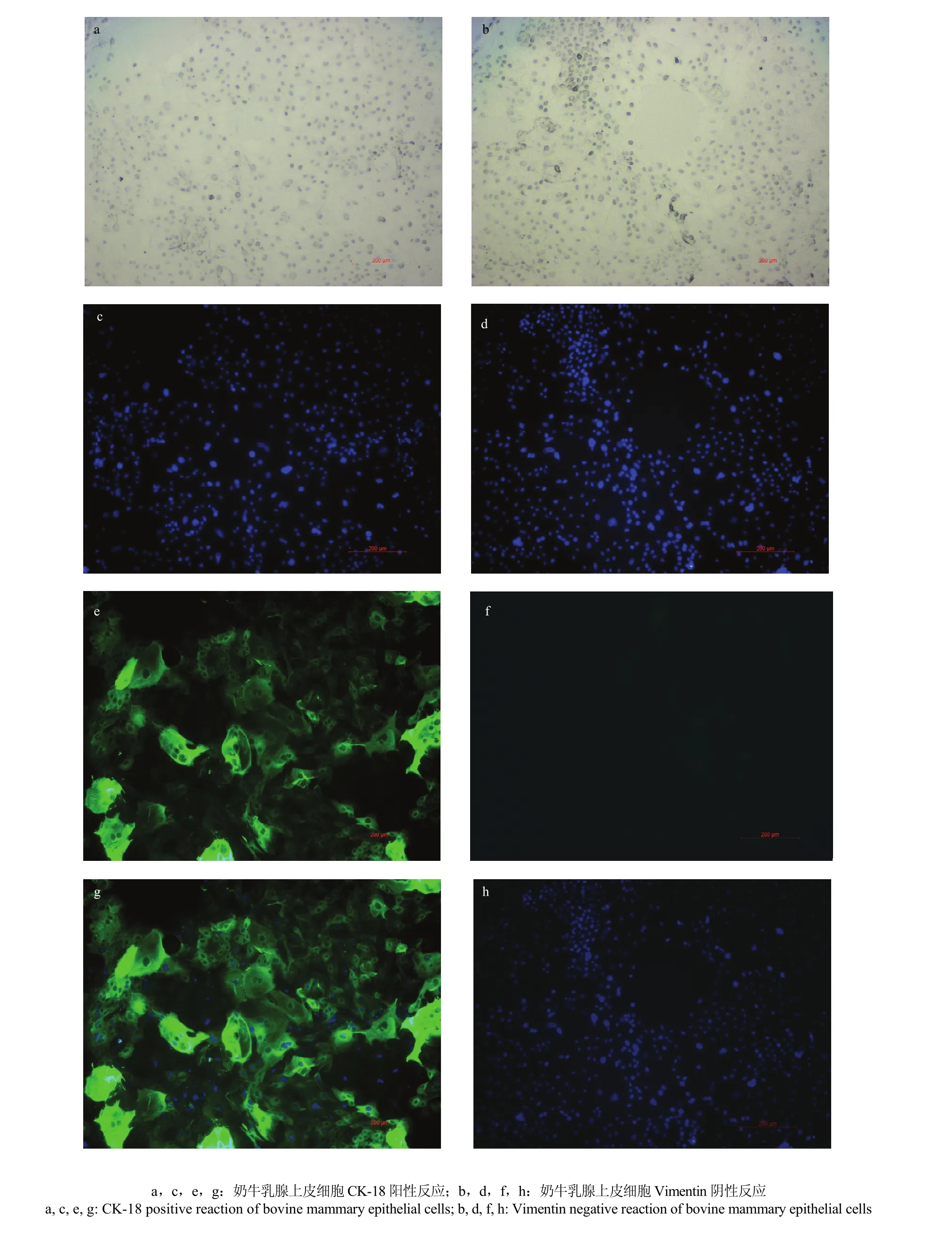
图2 奶牛乳腺上皮细胞的免疫荧光鉴定Fig. 2 Identification of bovine mammary epithelial cells by immuno- fluorescence
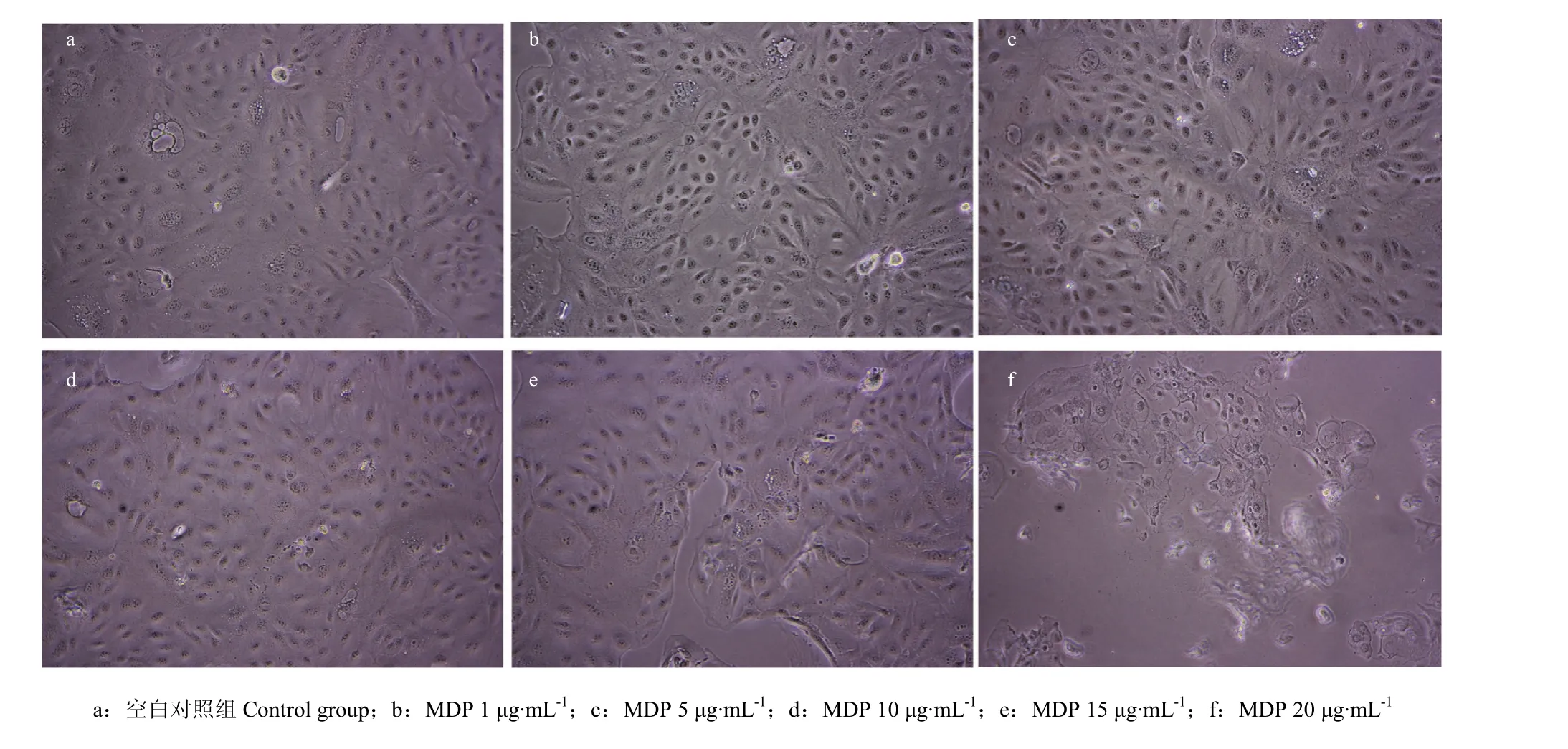
图 3 MDP刺激奶牛乳腺上皮细胞24 h后的显微镜下观察Fig. 3 Microscope observation of bovine mammary epithelial cells that stimulated by MDP after 24 h (200×)

图4 不同浓度MDP刺激奶牛乳腺上皮细胞后NOD2 mRNA的相对表达量Fig. 4 The relative expression of mRNA NOD2 in bovine mammary epithelial cells that stimulated by different concentrations of MDP
引起奶牛乳房炎的主要病因为细菌感染,而BMEC是乳腺组织兼具泌乳及免疫功能的细胞[29]。相关研究表明NOD2在奶牛乳腺腺泡、乳导管、乳池及乳头管组织以及BMEC中表达[6,30]。GILBERT等以金黄色葡萄球菌的培养上清液及LPS刺激BMEC,观察不同刺激剂对BMEC免疫反应的影响[31]。但至今MDP单独作为体外刺激剂对 BMEC的生长状态以及胞内NOD2 mRNA表达量的影响仍没有明确报道。本试验结果显示,MDP刺激BMEC后,细胞中NOD2受体mRNA表达量显著增加。这说明,当奶牛乳腺受到外来病原菌入侵时,BMEC可以通过NOD2受体途径启动乳腺免疫防御机制,协同机体其他免疫途径抵抗病原菌入侵。尽管BMEC中NOD2的表达量与MDP的刺激浓度呈正相关,但本试验对MDP刺激后的BMEC进行形态学观察的结果显示,当MDP的刺激浓度达到15 μg·mL-1与20 μg·mL-1时,贴壁的BMEC出现了不同程度的死亡、脱落现象。可以推测,在高浓度的MDP作用下,BMEC中的NOD2受体及其下游通路被过度活化,过度的免疫应答产生了大量的炎性因子,在免疫防御反应的同时对细胞产生了损伤,但其中是否有其他通路参与,还有待进一步验证。这一结果表明,如果试验中以MDP作为BMEC模型的刺激剂,可以将 MDP的刺激浓度设在 10 μg·mL-1以下,避免因 BMEC过度损伤对试验结果造成影响。这也提示,当奶牛乳腺受到外来病原菌入侵时,细菌数量或毒力强度与乳腺组织的免疫防御反应有一定的关系,即细菌数量或毒力强度在一定范围内时随着细菌数量的增加或毒力的增强,乳腺组织的免疫防御反应也增强,进而清除外来病原菌入侵,而当细菌数量或毒力强度超过一定范围时,乳腺的过度免疫应答使组织受到严重损伤,免疫防御屏障随之崩溃,奶牛乳腺局部甚至是全身将呈现出严重的临床症状。
4 结论
本试验采用胶原酶Ⅰ消化法,经分离纯化后成功获得了奶牛乳腺上皮细胞;NOD2 mRNA的表达量与胞壁酰二肽的刺激浓度呈正相关;但在保持奶牛乳腺上皮细胞生长状态正常的前提下,胞壁酰二肽的体外刺激浓度应控制在10 μg·mL-1以下。
[1] CANNING P, RUAN Q, SCHWERD T, HRDINKA M, MAKI J L, SALEH D, SUEBSUWONG C, RAY S, BRENNAN P E, CUNY G D, UHLIG H H, GYRD H M, DEGTEREV A, BULLOCK A N. Inflammatory signaling by NOD-RIPK2 Is Inhibited by clinically relevant type II kinase inhibitors. Chemistry & Biology, 2015, 22(9): 1174-1184.
[2] TAMAKI Y, SHOICHIRO K. Intracellular recognition of pathogens and autophagy as an innate immune host defence. Journal of Biochemistry, 2011, 150(2): 143-149.
[3] TRAVASSOS L H, CARNEIRO L A, GIRARDIN S, PHILPOTT D J. Nod proteins link bacterial sensing and autophagy. Autophagy, 2010, 6(3): 409-411.
[4] PORCHERIE A, CUNHA P, TROTEREAU A, ROUSSEL P, GILBERT FB, RAINARD P, GERMON P. Repertoire of Escherichia coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Veterinary Research, 2012, 43(1): 1-8.
[5] ZHU Y H, LIU P Q, WENG X G, ZHUGE Z Y, ZHANG R, MA J L, QIU X Q, LI R Q, ZHANG X L,WANG J F. Short communication: Pheromonicin-SA affects mRNA expression of toll-like receptors, cytokines, and lactoferrin by Staphylococcus aureus-infected bovine mammary epithelial cells. Journal of Dairy Science, 2012, 95(2): 759-764.
[6] WHELEHAN C J, MEADE K G, ECKERSALL P D. Experimental Staphylococcus aureus infection of the mammary gland induces region-specific changes in innate immune gene expression. Veterinary Immunology and Immunopathology, 2011, 140(3/4): 181-189.
[7] 刘晓云, 郑家珍, 王艳青, 裴建秋, 樊爱萍. 牛乳腺上皮细胞的快速分离和培养. 河北大学学报(自然科学版), 2015, 35(4): 385-389.
LIU X Y, ZHENG J Z, WANG Y Q, PEI J Q, FAN A P. Isolation and culture of bovine mammary epithelial cell. Journal of Hebei University (Natural Science Edition), 2015, 35(4): 385-389. (in Chinese)
[8] 詹康, 贡笑笑, 左晓昕, 陈银银, 占今舜, 赵国琦. 奶牛乳腺上皮细胞系的培养与鉴定. 动物营养学报, 2015(8): 2544-2550.
ZHAN K, GONG X X, ZUO X X, CHEN Y Y, ZHAN J S, ZHAO G Q. Culture and identification of the bovine mammary epithelial cell line. Chinese Journal of Animal Nutrition, 2015(8): 2544-2550. (in Chinese)
[9] WU Q, LIU M C, YANG J, WANG J F, ZHU Y H. Lactobacillus rhamnosus GR-1 ameliorates Escherichia coli-Induced inflammation and cell damage via attenuation of ASC-independent NLRP3 inflammasome activation. Applied and Environmental Microbiology, 2015, 82(4): 1173-1182.
[10] NAOHIRO I, GABRIEL N. NODs: intracellular proteins involved in inflammation and apoptosis. Nature Reviews Immunology, 2003, 3(5): 371-382.
[11] WARREN S, MURRAY P J, ATSUSHI K, TOMOHIRO W. Signalling pathways and molecular interactions of NOD1 and NOD2. Nature Reviews Immunology, 2006, 6(1): 9-20.
[12] PETR H, ZINKERNAGEL A S, GABRIELA J, BOTWIN G J, JEAN-PIERRE H, MICHAEL K, VICTOR N, LARS E. NOD2 contributes to cutaneous defense against Staphylococcus aureus through α-toxin-dependent innate immune activation. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(31): 12873-12878.
[13] CORREA R G, MILUTINOVIC S, REED J C. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Bioscience Reports, 2012, 32(6): 597-608.
[14] KAORU G, RUBINO S J, MAGALHAES J G, CATHERINE S, LIONEL L B, CHO J H, ROBERTSON S J, KIM C J, RUPERT K, PHILPOTT D J, GIRARDIN S E. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nature Medicine, 2011, 17(7): 837-844.
[15] BASTIAN O, ANJA P, BERND S, ANDREAS C H, SIMONE R, SVEN H, RALF R S, NORBERT S, STEFAN H. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. Journal of Biological Chemistry, 2004, 279(35): 36426-36432.
[16] ERAN E, TILL S, JORGE H M, RICHARD A F. Regulation of the antimicrobial response by NLR proteins. Immunity, 2011, 34(5): 665-679.
[17] NIGRO G, FAZIO L L, MARTINO M C, ROSSI G, TATTOLI L, LIPAROTI V, CRISTINA D C, MOLINARO A, DANA J P, MARIA L B. Muramylpeptide shedding modulates cell sensing of Shigella flexneri. Cellular Microbiology, 2008, 10(3): 682-695.
[18] KIM Y G, PARK J H, SHAW M H, FRANCHI L, INOHARA N,GABRIEL N. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to toll-like receptor ligands. Immunity, 2008, 28(2): 246-257.
[19] WARREN S E, MAO D P, RODRIGUEZ A E, MIAO E A, ALAN A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. Journal of Immunology, 2008, 180(11): 7558-7564.
[20] BERRINGTON W R, RAVI I, WELLS R D, SMITH K D, SKERRETT S J, HAWN T R. NOD1 and NOD2 regulation of pulmonary innate immunity to legionella pneumophila. European Journal of Immunology, 2010, 40(12): 3519-3527.
[21] KENICHI S, SHUANG C, DEMPSEY P W, ROSALINDA S, RANDA A, SLEPENKIN A V, ELLENA P, DOHERTY T M, DAVID U, CROTHER T R, MOSHE A. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. Plos Pathogens, 2009, 5(4): 1243-1248.
[22] TAKEUCHI O, AKIRA S. Pattern recognition receptors and inflammation. Cell, 2010, 140(6): 805-820.
[23] SHAW M H, REIMER T, SÁNCHEZ-VALDEPEÑAS C, WARNER N, KIM Y G, FRESNO M, NUÑEZ C. T cell intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nature Immunology, 2009, 10(12): 1267-1274.
[24] BRAIN O, ALLAN P, SIMMONS A. NOD2-mediated autophagy and Crohn disease. Autophagy, 2010, 6(3): 412-414.
[25] YAMAMOTO-FURUSHO J K, KORZENIK J R. Crohn's disease: Innate immunodeficiency? World Journal of Gastroenterology, 2006, 12(42): 6751-6755.
[26] HUGOT J P, CHAMAILLARD M, ZOUALI H, LESAGE S, CÉZARD J P, BELAICHE J, ALMER S, TYSK C, O'MORAIN C A, GASSULL M, BINDER V, FINKEL Y, CORTOT A, MODIGLIANI R, PIERRE L P, CORINE G R, MACRY J, COLOMBEL J F, SAHBATOU M, THOMAS G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature, 2001, 411(6837): 599-603.
[27] LAM C, MAGALHAES J G, TATTOLI I, PHILPOTT D J, TRAVASSOS L H. NOD-like proteins in inflammation and disease. Journal of Pathology, 2008, 214(2): 136-148.
[28] FRANCHI L, PARK J H, SHAW M H, MARINA G N, CHEN G, KIM Y G, NUNEZ G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cellular Microbiology, 2008, 10(1): 1-8.
[29] 崔新洁, 胡庆亮, 李奕平, 陶琳, 修磊, 刘秉春, 陈媛, 王潇. 金黄色葡萄球菌诱导牛原代乳腺上皮细胞的凋亡. 中国农业科学, 2013, 46(15): 3212-3219.
CUI X J, HU Q L, LI L P, TAO L, XIU L, LIU B C, CHEN Y, WANG X. The apoptosis of bovine primary mammary epithelial cells induced by Staphylococcus aureus. Scientia Agricultura Sinica, 2013, 46(15): 3212-3219. (in Chinese)
[30] BOUGARN S, CUNHA P, HARMACHE A, FROMAGEAU A, GILBERT F B, RAINARD P. Muramyl dipeptide synergizes with Staphylococcus aureus lipoteichoic acid to recruit neutrophils in the mammary gland and to stimulate mammary epithelial cells. Clinical and Vaccine Immunology, 2010, 17(11): 1797-809.
[31] GILBERT F B, CUNHA P, JENSEN K, GLASS E J, FOUCRAS G, ROBERT-GRANIÉ C, RUPP R, RAINARD P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Veterinary Research, 2013, 44(1): 1-23.
(责任编辑 林鉴非)
Growth and Expression of NOD2 mRNA in Bovine Mammary Epithelial Cells Treated with Different Concentrations of MDP in Vitro
XU DanDan, WANG JianFa, ZHANG Xu, LIU DongYu, XU XiaoNan, WANG Le, CHEN Jia, SHAN XuFei, WANG XiaoYa, WU Rui, YANG Bin
(College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, Daqing 163319, Heilongjiang)
bovine mammary epithelial cell; MDP; NOD2
2016-04-13;接受日期:2016-11-22
国家自然科学基金(31472249,31402157)、黑龙江八一农垦大学研究生创新项目(YJSCX2015-Y23)
联系方式:徐丹丹,E-mail:xudandan19910520@163.com。通信作者武瑞,E-mail:fuhewu@126.com
Abstract:【Objective】 Dairy cow mastitis is one of the most common diseases causing serious economic losses in dairy-farming industry. Bacterial infection is the main cause of mastitis. Innate immunity is the first line of defense against the invasion of pathogenic bacteria in mammary gland. NOD2 is an important member of the innate immune pattern recognition receptor of nucleotide-binding oligomerization domain (NOD) family, which defenses against various microbial invasions by recognizing its specific ligand-muramyl dipeptide (MDP), a component widely existing in gram positive bacteria and gram negative bacteria cell wall. Bovine mammary epithelial cell (BMEC) is the immune barrier of dairy cow mammary gland other than secreting milk. Here, the effect of MDP on the in vitro growth state of BMEC and the expression of NOD2 in the BMEC was explored in this experiment.【Method】 Mammary gland tissue of healthy and lactating Holstein cows was chosen as raw materials. Collagenase digestion method combined with concentration gradient of trypsin was used to separate BMEC. Cytokeratin-18 specific expression in epithelial cells and vimentin specific expression in fibroblasts were used to identify the obtained cells by immunofluorescent techniques. BMEC was set to 6 treatment groups, including MDP stimulating concentrations of 0 (control group), 1, 5, 10, 15 and 20 μg·mL-1. Twenty-four hours of poststimulation, BMEC status were observed under a microscope, meanwhile total RNA was extracted from BMEC and reverse transcribed to cDNA. Real time fluorescent quantitative PCR method was used to detect the expression of NOD2 in BMEC.【Result】Those cells separated by collagenase digestion method combined with concentration gradient of trypsin, immunofluorescence results of CK-18 reaction was positive and vimentin reaction was negative. All cells were in a good growth condition. In the control group and in the groups of MDP stimulating concentration at 1, 5 and 10 μg·mL-1, BMEC grew well without any visible abnormalities. There was a small amount of BMEC detached from bottom in the group of MDP stimulating concentration at 15 μg·mL-1. However, the group of MDP stimulating concentration at 20 μg·mL-1showed a large number of BMEC detached and floated from bottom. Even though those BMEC were still attached to the bottom, their morphology had already changed. Compared with the control group, the expression of NOD2 mRNA in BMEC was positively correlated with the stimulating concentrations of MDP. In other words, 24 h of poststimulation, the expression of NOD2 mRNA in BMEC gradually increased along with the stimulating concentrations of MDP.【Conclusion】High purity BMEC was successfully obtained. The obtained cells grew well and could be used in the following experiments. Although the expression of NOD2 mRNA was positively correlated with the stimulating concentrations of MDP, the stimulating concentrations of MDP in vitro culture BMEC should be controlled below 10 μg·mL-1in order to maintain the normal growth condition. These results suggested that BMEC could participate in the immune defense response of bovine mammary gland through the NOD2 receptor pathway. But this defense capability was influenced by the number of bacteria or the intensity of bacterial virulence. In a certain number or virulence of bacteria, the immune defense response of bovine mammary gland was enhanced along with the increasing number of bacteria or the enhancement of virulence to eliminate intramammary pathogens. While the number or virulence of bacteria exceeded to a certain range, bovine mammary gland tissue would be seriously damaged, so the immune defense barrier would be collapsed. Under this condition, the local bovine mammary gland or even all over the body would present obvious clinical symptoms.
