The consensus on liver autotransplantation from an international panel of experts
2017-02-10
The consensus on liver autotransplantation from an international panel of experts
Qi-Fa Ye and Norbert Senninger
Introduction
More than 1.0 million patients worldwide are diagnosed with space-occupying lesions in the liver every year, with the number approaching 0.5 million per year in China, and only 20% of the lesions are resectable.[1,2]Due to a lack of available donors, only a limited number of patients underwent allogeneic liver transplantation, the remaining patients simply receive palliative care. Therefore, discovering new options for treating these patients is a high priority. Liver autotransplantation (LAT) is a surgical technique that adopts liver transplantation skills to radically treat spaceoccupying hepatic lesions, benign or malignant, parasitic or severe liver trauma that cannot be treated via conventional liver surgery. With the LAT approach, the lesion is resectedante situmorex situunder hepatic perfusion and veno-venous bypass conditions.In situhepatectomy under hypothermic perfusion is excluded because it does not include the vascular transection and anastomosis. Compared to allo-transplantation, LAT technique preserves the autologous liver and eliminates the need for allogeneic liver and subsequent immunosuppressive therapy, along with the related complications. Further, the medical costs of the surgery are reduced, adding the potential for attractive social and economic benefts. LAT was frst reported by Pichlmayr et al in 1988 in Germany.[3]The effcacy and safety of LAT is directly affected by the proportion of liver removed, the preoperative liver function, and the methods of liver perfusion during the procedure. No paradigm or standard has yet been established regarding these factors which may limit the clinical application.
Indications and contraindications
LAT is a technique with high-risk of mortality, hence indications and contraindications should be strictly emphasized.
Indications to LAT
Space-occupying hepatic lesions that cannot be treated via conventional liver surgery.
Contraindications to LAT
1) Lesions in major organs such as the brain, heart, lungs, and kidneys (i.e. patient having a low tolerance for surgery);
2) Currently active tuberculosis;
3) Gastrointestinal ulcer currently active;
4) Severe diabetes that has not been well controlled;
5) Liver functions with Child-Pugh class C, intermediate liver cirrhosis, or fatty liver with fatty proportion >50%;
6) Possibility of small-for-size syndrome;
7) Preoperative primary liver tumor assessment indicates poor prognosis or primary lesion of a metastatictumor not radically cured;
8) Mental diseases or poor surgical compliance;
9) Infective lesion, epidemic diseases, AIDS, history of drug or alcohol abuse, or severe drug dependence;
10) Patient is during replicative or progressive phases of any hepatitis virus(es) (A, B, C, D, E, F or G).
Individualized preoperative assessment system for LAT
This system generates an overall score based on the patients' results of the Child-Pugh score, model of endstage liver disease (MELD) score and indocyanine green (ICG) clearance test. The score can be used to assess patients and to reduce risks of operation, including smallfor-size syndrome. Flowchart of assessment for safe resection volume is described in Fig. 1.
Child-Pugh score
The Child-Pugh classifcation has been considered as the most widely accepted measure to evaluate liver function prior to hepatectomy.[4]The highly impaired liver function (Child-Pugh class C or advanced Child-Pugh class B) resulted in poor outcomes of patients referred to hepatectomy.[5]Therefore, the patients with Child-Pugh class C or advanced Child-Pugh class B are not eligible for LAT.
MELD score

Fig. 1. Individualized decision-making system for safe resection volume in LAT. R: residual functional liver volume; S: estimated standard liver volume; SC: surgical contraindication; LC: liver cirrhosis.
Similar to Child-Pugh score, the MELD score has been demonstrated as another effective index to predict the outcomes of patients with liver cirrhosis after hepatectomy. It has been reported that the patients with MELD scores equal to or above 9 prior to hepatectomy had a very high incidence of postoperative complications and liver failure compared with patients with lower MELD scores.[6,7]Hence, in patients with MELD score equal to or above 9, LAT should be avoided.
Indocyanine green retention rate at 15-minute (ICGR15)
The decision for hepatectomy has been based on the Child-Pugh classifcation for years,[8]however, the strategy does not always have the consistent predictive value. In fact, some individuals with Child-Pugh class A already have impaired liver function.[9]ICGR15 can provide more refned assessment of the patients' liver function reserve even they are in the absence of hyperbilirubinemia and ascites.[10]Many reports[10,11]showed that the cirrhotic patients with an ICGR15 between 10% to 20% are able to tolerate major hepatectomy safely. Therefore, we advise that the patients can have LAT if the result of ICGR15 is below 20%.
Residual liver volume assessment
Several studies[11,12]have reported a relationship between the residual liver volume and liver function reserve in patients referred for liver surgery. Individuals who have normal liver function without tumors can tolerate up to 60% of the liver resection.[13]To attenuate the risks-related with LAT, our experience is that residual liver volume after resection must be at least more than 60% by the assessment of preoperative 3-D volumetric CT reconstruction (Fig. 2).

Fig. 2. Assessment of preoperative 3-D volumetric CT reconstruction. Lesion manifestation in CT (A, B) and 3-D space-occupying lesion reconstruction (C, D).
Veno-venous bypass in LAT
In view of the complexity of LAT and the long anhepatic phase (always last for several hours), the utilization of veno-venous bypass is mandatory in maintaining the function of the kidneys and the homeostasis of the hemodynamics and the internal environment;[14-18]the lower limit of bypass fow required is 1000 mL/min.[19]The utilization of a heat exchanger is mandatory to protect against severe hypothermia.[20,21]In general condition, the bypass is from portal vein and iliac vein to axillary vein as shown in Fig. 3. The application of heparin-coated cannulae may reduce the dosage of systemic heparinization, thus alleviate the infuence on coagulation system.[22]Further safety measures such as ultrasoundguided placement of the cannula can be taken into consideration.[23,24]
The procedure without veno-venous bypass as a substitute approach for bypass to maintain the hemodynamic stability was reported.[25]The cost is reduced because this technique shortens the total operation time. An alternative approach can also reduce hemorrhage during operation and alleviate the ischemia/reperfusion injury.En-blocliver is removed after establishment of total vascular exclusion and then a 20-mm ringed polytetrafuoroethylene (PTFE) graft is used to replace the retrohepatic inferior vena cava for bridging suprahepatic inferior vena cava and infrahepatic vena cava.
Perfusion in LAT
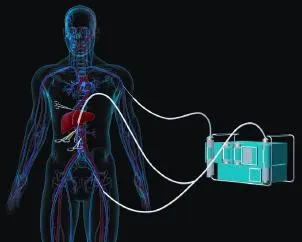
Fig. 3. Scheme of veno-venous bypass from portal vein and iliac vein to axillary vein.
Traditional hypothermic perfusion by gravity is performed using histidine-tryptophan-ketoglutarate (HTK) with 6 L intraportally and 0.5 L intra-arterially initially. Perfusion is repeated at an interval of 30-60 minutes with 1 L and 0.2 L as above, respectively.[15]It is commonly used in LAT to reduce cell metabolism and to lessen cellular edema, therefore minimizing damage to the liver. However, cellular anaerobic metabolism continues even at lower temperatures, causing further damage due to the exhaustion of ATP and the accumulation of anaerobic products. As a result, ATP is depleted, cytosolic ion concentrations are changed, and cellular membranes become instable.[26,27]Hepatic sinusoidal endothelium cells are particularly vulnerable to ischemia/reperfusion injury and develop serious alterations during the period without perfusion, such as cell body detachment and apoptosis.[28,29]
Hypothermic machine perfusion with HTK is a more effective method compared to intermittent hypothermic perfusion by gravity in LAT. Perfusate through the hepatic artery and portal vein can continuously remove metabolic products, reduce the accumulation of reactive oxygen species,[30]protect the endothelium,[27]alleviate cold ischemia injury (although cold ischemia injury cannot be completely avoided), and mitigate some warm ischemia injuries.[31,32]During hypothermic machine perfusion, the high portal vein pressure (>4 mmHg) damages the liver.[33]Thus, hepatic artery and portal vein pressures should be set at 20-25 mmHg and 2-4 mmHg, which are 1/4 of the physiological values, aiming to decrease the physical injuries caused by the hydrodynamic shear stress.[34]Temperature of the perfusate should be maintained at 0-4 ℃. Oxygenation of perfusate has a potential beneft against warm and cold ischemia in various organs and achieved better organ quality.[35]Solutions with no starch and low potassium appear to be advantageous, as low potassium concentration decreases vascular resistance in the cold temperature and avoids cardiac arrhythmia after reperfusion, while the presence of starch increases viscosity.[36]HTK solution is usually used for hypothermic machine perfusion during back-table work of LAT.
Furthermore, normothermic and subnormothermic machine perfusion protects the liver from damage, increasing the number of donor livers and improves graft function in LAT, which would be used in LAT in the future.[37,38]
Operation procedure of LAT
In liver transplantation, grafts are procured with long enough vessels, constantly with an aortic patch, in contrast, long vessels or patches are not available to theante situmorex situprocedure. Meticulous operation including dissection and reanastomosis of vessels is mandatory.[15]The intrahepatic lesion is assessed by palpation and intraoperative ultrasound when the liver has been completely freed from its ligamentous attachments.[38]Anatomical locations of lesions are shown based on the classifcationof hepatic veins[39]and the relationship between intrahepatic vessels/inferior vena cava with the lesions (Fig. 4). In principle, malignant lesions with extrahepatic manifestations and vascular invasion are deemed as contraindication.
Ante situm
For cases in which complex major hepatectomy is required, including hepatic vein dissection and reconstruction, a technique was developed allowing the suprahepatic area to be exteriorized without dividing the porta hepatic.[21,40-44]The infrahepatic vena cava can be transected as well if it is necessary, which is also appropriate for treating retrohepatic lesions.[45]One indication forante situmresection and autotransplantation is that the lesions invade the hepatic veins close to the inferior vena cava confuence or even wrap the inferior vena cava, which have a high-risk of hemorrhage and air embolismin situdisection. After establishment of total vascular exclusion, the portal vein and arteriae gastroduodenalis should be isolated and opened, followed by the insertion of the perfusion tubes. During the continuous hypothermic machine perfusion of hepatic artery and portal vein with 0-4 ℃ HTK solution, hepatic veins are transected at their entry into the vena cava. If the lesion has invaded one of the hepatic veins, part of the inferior vena cava (Fig. 4A, B, C, J, K) is resected and closed with polypropylene sutures or a vascular patch if required. The liver remains attachedin vivoby the porta hepatis, but can be completely exteriortizedex situand placed on temperature control plates for cooling down. The Cavitron ultrasound surgical aspirator is used to excise the spaceoccupying lesion and No. 0 sutures are used to ligate the tubular structure on the section. The cooling system is removed after completion of the tumor resection and tubular reconstruction. The reconstructed hepatic vein is reimplanted onto its original inferior vena cava orifce if possible and the orifce of the hepatic vein that is resected with the tumor is closed with 4/0 polypropylene sutures. If reanastomosis onto the original orifce is diffcult, then this is also closed and reimplantation is performed using a cavotomy.[3]
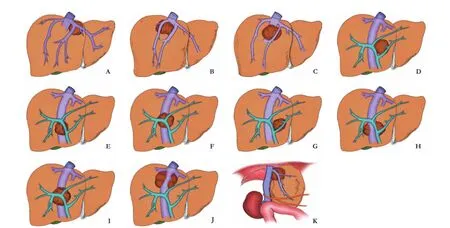
Fig. 4. Anatomical locations of space-occupying lesions based on the classifcation of hepatic veins and the relationship between intrahepatic vessels/inferior vena cava with the lesions. A: Lesion within the triangular area between left hepatic vein, middle hepatic vein & retrohepatic inferior vena cava; B: Lesion within the triangular area between right hepatic vein, middle hepatic vein & retrohepatic inferior vena cava; C: Lesion within the triangular area between middle hepatic vein & retrohepatic inferior vena cava; D: Lesion within the triangular area between left branch and right branch of portal vein & retrohepatic inferior vena cava; E: Lesion within the triangular area between right branch of portal vein & retrohepatic inferior vena cava; F: Lesion within the triangular area between secondary branch under right branch of portal vein & retrohepatic inferior vena cava; G: Lesion within the triangular area between left branch of portal vein, main trunk of portal vein & retrohepatic inferior vena cava; H: Lesion surrounding inferior vena cava within the caudate lobe; I: Lesion within the triangular area between left branch of portal vein, middle branch of portal vein & retrohepatic inferior vena cava; J: Lesion near the third porta hepatis, with lesion surrounding retrohepatic inferior vena cava; K: Lesion near the second porta hepatis, with lesion surrounding hepatic vein of the second porta hepatis.
Ex situ
Ex situresection and autotransplantation was selected for these lesions can not be resected byante situmprocedure.[3,21,46-54]All afferent and efferent structures have to be divided excepted the retrohepatic inferior vena cava, followed by the insertion of the perfusion tubes. Hypothermic liver perfusion commencedin situafter clamping of the suprahepatic inferior vena cava, infrahepatic vena cava. During the continuous hypothermic perfusion of hepatic artery and portal vein, suprahepatic inferior vena cava, infrahepatic vena cava and porta hepatis are transected meticulously. During theex situhepatectomy, the borders of the liver excision should be determined based on the plan and actual status of the operation combined with the preoperative 3-D liver reconstruction, virtual operation design and preserved liver volume assessment. After isolating the essential structures which must be preserved, hepatectomy can be performed in a manner of conventional liver surgery. During continuous perfusion, the Cavitron ultrasound surgical aspirator is used to excise the space-occupying lesion and No. 0 sutures are used to ligate the tubular structure on the section. After the completion of lesions resection, unligated and opened vessels or bile ducts can be identifed by perfusion. Large vessels should be sewn or ligated to avoid severe bleeding after implantation. Methylene blue can be used to check the closure after repair of all small leaks, especially the tiny holes along larger veins. The reconstruction of vessels required for reanastomosis should be doneex situ. The implementation of reanastomosis is commenced in the normal sequence: suprahepatic inferior vena cava, infrahepatic vena cava, hepatic artery and portal vein. Finally, reconstruction of the bile duct is carried out according to the individual situation, either by an end-to-end anastomosis of the common bile duct or Roux-en-Y loop of jejunum.[20]
Points for attention
The success of LAT is based on: (1) When restoring blood fow to the liver, calcium and sodium bicarbonate solution should be rapidly administered through an intravenous drip to prevent arrhythmia and cardiac arrest caused by disturbance of the internal environment; (2) Prior to the opening of the hepatic blood fow, 350-500 mL of blood should be released from the liver via the suprahepatic inferior vena cava or the right hepatic vein to prevent the sudden release of potassium and an acute cardiac hyperkalemia with the risk of cardiac arrest; (3) Severe metabolic, coagulation and internal environment disturbances may occur 2-3 hours after establishment of total vascular exclusion. Marked fbrinolysis and the depletion of hemostatic factors result in coagulopathy which can be prevented by early and prophylactic substitution of fresh frozen plasma and platelets; (4) The hypothermic perfusion of the portal veins and hepatic arteries should be sustained prior to the opening of the hepatic blood fow; (5) The openings in the portal and inferior veins should be incised and sutured longitudinally to prevent possible postoperative stenosis; (6) It is mandatory to perform an intraoperative ultrasound for checking the vessels; (7) At the end of the procedure, the portal vein pressure should be measured. In the case of a higher pressure than normal, caution should be taken regarding small-for-size syndrome; (8) Finally, the outfow tract should be checked to prevent blockage and subsequent swelling and overstressing of the liver.
Funding:This study was supported by the National Natural Science Foundation of China: United Foundation with Xinjiang (U1403222) and National Natural Science Foundation of China (81570079).
Competing interest:No benefts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66: 115-132.
2 Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108.
3 Pichlmayr R, Bretschneider HJ, Kirchner E, Ringe B, Lamesch P, Gubernatis G, et al. Ex situ operation on the liver. A new possibility in liver surgery. Langenbecks Arch Chir 1988;373:122-126.
4 Child CG, Turcotte JG. Surgery and portal hypertension. In: Child CG, ed. The liver and portal hypertension. Philadelphia, PA: Saunders;1964:50-64.
5 Nagasue N, Kohno H, Tachibana M, Yamanoi A, Ohmori H, El-Assal ON. Prognostic factors after hepatic resection for hepatocellular carcinoma associated with Child-Turcotte class B and C cirrhosis. Ann Surg 1999;229:84-90.
6 Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl 2006;12:966-971.
7 Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival beneft of liver transplantation. Am J Transplant 2005;5:307-313.
8 Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-649.
9 D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-231.
10 Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand ffty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198-1206.
11 Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176-1181.
12 Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999;188:304-309.
13 Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg 2005;12:31-37.
14 Shaw BW Jr, Martin DJ, Marquez JM, Kang YG, Bugbee AC Jr, Iwatsuki S, et al. Venous bypass in clinical liver transplantation. Ann Surg 1984;200:524-534.
15 Chari RS, Gan TJ, Robertson KM, Bass K, Camargo CA Jr, Greig PD, et al. Venovenous bypass in adult orthotopic liver transplantation: routine or selective use? J Am Coll Surg 1998;186:683-690.
16 Johnson SR, Marterre WF, Alonso MH, Hanto DW. A percutaneous technique for venovenous bypass in orthotopic cadaver liver transplantation and comparison with the open technique. Liver Transpl Surg 1996;2:354-361.
17 Ozaki CF, Langnas AN, Bynon JS, Pillen TJ, Kangas J, Vogel JE, et al. A percutaneous method for venovenous bypass in liver transplantation. Transplantation 1994;57:472-473.
18 Delrivière L, Hannoun L. In situ and ex situ in vivo procedures for complex major liver resections requiring prolonged hepatic vascular exclusion in normal and diseased livers. J Am Coll Surg 1995;181:272-276.
19 Grande L, Rimola A, Cugat E, Alvarez L, García-Valdecasas JC, Taurá P, et al. Effect of venovenous bypass on perioperative renal function in liver transplantation: results of a randomized, controlled trial. Hepatology 1996;23:1418-1428.
20 Pichlmayr R, Grosse H, Hauss J, Gubernatis G, Lamesch P, Bretschneider HJ. Technique and preliminary results of extracorporeal liver surgery (bench procedure) and of surgery on the in situ perfused liver. Br J Surg 1990;77:21-26.
21 Katirji MB. Brachial plexus injury following liver transplantation. Neurology 1989;39:736-738.
22 Sakai T, Planinsic RM, Hilmi IA, Marsh JW. Complications associated with percutaneous placement of venous return cannula for venovenous bypass in adult orthotopic liver transplantation. Liver Transpl 2007;13:961-965.
23 Sakai T, Gligor S, Diulus J, McAffee R, Wallis Marsh J, Planinsic RM. Insertion and management of percutaneous veno-venous bypass cannula for liver transplantation: a reference for transplant anesthesiologists. Clin Transplant 2010;24:585-591.
24 Zhang KM, Hu XW, Dong JH, Hong ZX, Wang ZH, Li GH, et al. Ex-situ liver surgery without veno-venous bypass. World J Gastroenterol 2012;18:7290-7295.
25 Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol 2013;10:79-89.
26 Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innateadaptive immune-mediated tissue infammation. Am J Transplant 2011;11:1563-1569.
27 Russo L, Gracia-Sancho J, García-Calderó H, Marrone G, García-Pagán JC, García-Cardeña G, et al. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology 2012;55:921-930.
28 Huet PM, Nagaoka MR, Desbiens G, Tarrab E, Brault A, Bralet MP, et al. Sinusoidal endothelial cell and hepatocyte death following cold ischemia-warm reperfusion of the rat liver. Hepatology 2004;39:1110-1119.
29 Tasoulis MK, Douzinas EE. Hypoxemic reperfusion of ischemic states: an alternative approach for the attenuation of oxidative stress mediated reperfusion injury. J Biomed Sci 2016;23:7.
30 Dutkowski P, Schlegel A, de Oliveira M, Müllhaupt B, Neff F, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol 2014;60:765-772.
31 Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, et al. First Comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an International-matched case analysis. Ann Surg 2015;262:764-771.
32 Schlegel A, Kron P, Dutkowski P. Hypothermic machine perfusion in liver transplantation. Curr Opin Organ Transplant 2016;21:308-314.
33 ‘t Hart NA, van der Plaats A, Leuvenink HG, van Goor H, Wiersema-Buist J, Verkerke GJ, et al. Hypothermic machine perfusion of the liver and the critical balance between perfusion pressures and endothelial injury. Transplant Proc 2005;37:332-334.
34 Minor T, Koetting M, Koetting M, Kaiser G, Efferz P, Lüer B, et al. Hypothermic reconditioning by gaseous oxygen improves survival after liver transplantation in the pig. Am J Transplant 2011;11:2627-2634.
35 Dutkowski P, de Rougemont O, Clavien PA. Machine perfusion for ‘marginal' liver grafts. Am J Transplant 2008;8:917-924.
36 Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (frst-in-man) clinical trial. Am J Transplant 2016;16:1779-1787.
37 Bruinsma BG, Yeh H, Ozer S, Martins PN, Farmer A, Wu W, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant 2014;14:1400-1409.
38 Raab R, Schlitt HJ, Oldhafer KJ, Bornscheuer A, Lang H, Pichlmayr R. Ex-vivo resection techniques in tissue-preserving surgery for liver malignancies. Langenbecks Arch Surg 2000;385:179-184.
39 Ming YZ, Niu Y, Shao MJ, She XG, Ye QF. Hepatic veins anatomy and piggy-back liver transplantation. Hepatobiliary Pancreat Dis Int 2012;11:429-433.
40 Hannoun L, Panis Y, Balladur P, Delva E, Honiger J, Levy E, et al. Ex-situ in-vivo liver surgery. Lancet 1991;337:1616-1617.
41 Boggi U, Vistoli F, Del Chiaro M, Signori S, Sgambelluri F, Roncella M, et al. Extracorporeal repair and liver autotransplantation after total avulsion of hepatic veins and retrohepatic inferior vena cava injury secondary to blunt abdominal trauma. J Trauma 2006;60:405-406.
42 Nuzzo G, Giordano M, Giuliante F, Lopez-Ben S, Albiol M, Figueras J. Complex liver resection for hepatic tumours involving the inferior vena cava. Eur J Surg Oncol 2011;37:921-927.
43 Azoulay D, Maggi U, Lim C, Malek A, Compagnon P, Salloum C, et al. Liver resection using total vascular exclusion of the liver preserving the caval fow, in situ hypothermic portal perfusion and temporary porta-caval shunt: a new technique for central tumors. Hepatobiliary Surg Nutr 2014;3:149-153.
44 Forni E, Meriggi F. Bench surgery and liver autotransplantation. Personal experience and technical considerations. G Chir1995;16:407-413.
45 Sauvanet A, Dousset B, Belghiti J. A simplifed technique of ex situ hepatic surgical treatment. J Am Coll Surg 1994;178:79-82.
46 Mukaiya M, Hirata K, Yamashiro K, Katsuramaki T, Denno R. A case of partial autotransplantation of the liver in advanced hepatocellular carcinoma. Hepatogastroenterology 1999;46: 2532-2534.
47 Oldhafer KJ, Lang H, Schlitt HJ, Hauss J, Raab R, Klempnauer J, et al. Long-term experience after ex situ liver surgery. Surgery 2000;127:520-527.
48 Brekke IB, Line PD, Mathisen Ø, Osnes S. Extracorporeal surgery and liver autotransplantation. Tidsskr Nor Laegeforen 2003;123:3210-3212.
49 Arii S. Perspective of standardization of surgical treatment for hepatocellular carcinoma. Nihon Geka Gakkai Zasshi 2003;104:399-403.
50 Sarmiento JM, Bower TC, Cherry KJ, Farnell MB, Nagorney DM. Is combined partial hepatectomy with segmental resection of inferior vena cava justifed for malignancy? Arch Surg 2003;138:624-631.
51 Chui AK, Island ER, Rao AR, Lau WY. The longest survivor and frst potential cure of an advanced cholangiocarcinoma by ex vivo resection and autotransplantation: a case report and review of the literature. Am Surg 2003;69:441-444.
52 Gruttadauria S, Marsh JW, Bartlett DL, Gridelli B, Marcos A. Ex situ resection techniques and liver autotransplantation: last resource for otherwise unresectable malignancy. Dig Dis Sci 2005;50:1829-1835.
53 Gringeri E, Polacco M, D'Amico FE, Bassi D, Boetto R, Tuci F, et al. Liver autotransplantation for the treatment of unresectable hepatic metastasis: an uncommon indication-a case report. Transplant Proc 2012;44:1930-1933.
54 Baker MA, Maley WR, Needleman L, Doria C. Ex vivo resection of hepatic neoplasia and autotransplantation: a case report and review of the literature. J Gastrointest Surg 2015;19:1169-1176.
Received October 21, 2016
Accepted after revision January 10, 2017
Cease to struggle and you cease to live.
—Thomas Carlyle
Author Affliations: Zhongnan Hospital of Wuhan University, Institute of Hepatobiliary Diseases of Wuhan University, Transplant Center of Wuhan University, Hubei Key Laboratory of Medical Technology on Transplantation, Wuhan 430071, China; Research Center of National Health Ministry on Transplantation Medicine Engineering and Technology, The 3rd Xiangya Hospital of Central South University, Changsha 410013, China (Ye QF); Department of General Surgery, University Clinics of Münster, Münster 48249, Germany (Senninger N)
Qi-Fa Ye (Email: yqf_china@163.com); Norbert Senninger (Email: Senning@ukmuenster.de)
In addition to the authors, this experts group consists of Jeremy R Chapman (University of Sydney), Ke Cheng (The 3rd Xiangya Hospital of Central South University), Francis Delmonico (Harvard Medical School), Lin Fan (Zhongnan Hospital of Wuhan University), Xiao-Li Fan (Zhongnan Hospital of Wuhan University), Zhen Fu (The 3rd Xiangya Hospital of Central South University), Yasuko Iwakiri (Yale University), Ying-Zi Ming (The 3rd Xiangya Hospital of Central South University), Philip J O'Connell (University of Sydney), Gui-Zhu Peng (Zhongnan Hospital of Wuhan University), Zhi-Hai Peng (Shanghai Jiaotong University), Rutger Ploeg (University of Oxford), Hendrik Tevaearai Stahel (University Hospital and University of Bern), Wei-Lin Wang (Zhejiang University), Yan-Feng Wang (Zhongnan Hospital of Wuhan University), Qiang Xia (Shanghai Jiaotong University), Shao-Jun Ye (Zhongnan Hospital of Wuhan University), Shu-Sen Zheng (Zhejiang University), and Zi-Biao Zhong (Zhongnan Hospital of Wuhan University).
© 2017, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60175-3
Published online January 16, 2017.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Instructions for Authors
- Associating liver partition and portal vein ligation for staged hepatectomy: the current role and development
- Right hepatectomy in living donors with previous abdominal surgery
- Diagnosis and outcomes of collateral arterial formation after irreversible early hepatic artery thrombosis in pediatric liver recipients
- Cytokines are early diagnostic biomarkers of graft-versus-host disease in liver recipients
- Resection of T4 hepatocellular carcinomas with adjacent structures, is it justifed?
