Effect of cholesterol loaded cyclodextrin (CLC) on lipid peroxidation and reactive oxygen species levels during cryopreservation of buffalo spermatozoa
2017-01-06LoneSAPrasadJKGhoshSKDasGKNarendraKumarBhureSKBalamuruganKatiyarVermaMR
Lone SA, Prasad JK, Ghosh SK, Das GK, Narendra Kumar, Bhure SK, Balamurugan B, Katiyar R, Verma MR
1Animal Reproduction, Gynaecology and Obstetrics
2Animal Reproduction Division;
3Division of LPM, ICAR-National Dairy Research Institute, Karnal, 132001, Haryana, India.
4Division of Animal Biochemistry;
5Division of Livestock Economics and Statistics, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly-243122, Uttar Pradesh, India.
Effect of cholesterol loaded cyclodextrin (CLC) on lipid peroxidation and reactive oxygen species levels during cryopreservation of buffalo spermatozoa
Lone SA1*, Prasad JK2, Ghosh SK2, Das GK2, Narendra Kumar3, Bhure SK4, Balamurugan B2, Katiyar R2, Verma MR5
1Animal Reproduction, Gynaecology and Obstetrics
2Animal Reproduction Division;
3Division of LPM, ICAR-National Dairy Research Institute, Karnal, 132001, Haryana, India.
4Division of Animal Biochemistry;
5Division of Livestock Economics and Statistics, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly-243122, Uttar Pradesh, India.
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Cholesterol loaded cyclodextrin
Lipid peroxidation
Reactive oxygen species
Cryopreservation
Buffalo spermatozoa
Objective:To assess the effect of cholesterol loaded cyclodextrin on seminal attributes (progressive motility, livability, hypo-osmotic swelling response and acrosomal integrity), lipid peroxidation (LPO) and reactive oxygen species (ROS) levels during cryopreservation of buffalo (Bubalus bubalis) spermatozoa.
1. Introduction
Due to presence of higher amounts of polyunsaturated fatty acids in buffalo spermatozoa than bull spermatozoa[1], they become more susceptible to freezing-thawing associated damages[2]. Depending upon their concentration, nature and duration of exposure, reactive oxygen species (ROS) have both beneficial and harmful effects on spermatozoa function. Moderate levels of ROS mediate various physiological functions of spermatozoa such as hyperactivation, capacitation, acrosomal reaction and zona binding[3,4]. Normally endogenous antioxidants prevent spermatozoa from ROS induced damage by converting ROS into safer byproducts. However, when the rate of ROS generation exceeds their detoxification, ROS accumulation leads to oxidative stress which may damage the spermatozoa membrane[5], affect DNA integrity[6], block oxidative metabolism[7], reduce the chances for sperm oocyte fusion[8] and sperm motility and viability[3]. The plasma membrane of spermatozoa is damaged during freezing-thawing, when spermatozoa are exposed to atmospheric oxygen which in turn increases susceptibility to lipid peroxidation (LPO)[8]. Plasma membrane integrity is essential for spermatozoa to protect them from harmful effects of cryopreservation. Adding cholesterol orits analogues to the medium reduces capacitation process[9]. Due to hydrophobic nature of cholesterol, it is insoluble in aqueous semen diluents. Cyclodextrins can be used for inserting cholesterol into cell membranes due to presence of an internal hydrophobic core, in addition to an external hydrophilic face[10]. Addition of cholesterol loaded cyclodextrin (CLC) to semen significantly increases progressive motility, livability, acrosomal integrity[11,12] and HOST responsive spermatozoa[13]. CLC improves in vitro fertilizing ability and reduces ultrastructural damages to spermatozoa plasma membrane[11]. Since CLC maintains membrane integrity and reduces number of dead spermatozoa in buffalo semen[11], it is presumed that by maintaining membrane integrity, the levels of LPO and ROS may be reduced due reduced damage to sperm plasma membrane.
In various biological membranes, the protective effect of cholesterol against oxidative stress laid down the hypothesis that reduction of oxidative stress may be another factor responsible for enhanced cryosurvival of CLC treated sperm. However, scanty information is available regarding the effect of CLC on LPO and ROS levels during semen cryopreservation. So present aim was carried out to study the effect of CLC on LPO and ROS levels during cryopreservation of buffalo spermatozoa.
2. Materials and methods
2.1. Experimental design
Semen was collected from Murrah buffalo bulls (3), 4-6 year old, maintained at the Germ Plasm Centre of Animal Reproduction Division, ICAR-Indian Veterinary Research Institute, Izatnagar. These bulls were reared under the similar feeding and management conditions during the entire duration of the study.
2.2. Preparation of CLC
Methyl-β-cyclodextrin was loaded with cholesterol as described [14]. Briefly, 200 mg of cholesterol was dissolved in 1 mL of chloroform in a glass tube. In second glass tube, 1 g of methyl-βcyclodextrin was dissolved in 2 mL of methanol. A 0.45 mL aliquot of the cholesterol solution was added to the cyclodextrin solution, and the mixture was stirred until the combined solution appeared clear. This was followed by pouring of mixture into a glass petri dish and removing of solvents using a stream of nitrogen gas. The resulting crystals were allowed to dry for an additional 24 h and then were removed from the dish and stored in a glass container at 22 ℃. A working solution of CLC was prepared by adding 50 mg of CLC to 1 mL of Tris diluent at 37 ℃ and mixing the solution briefly using a vortex mixer.
2.3. Collection of semen and its processing
Semen was collected by using an artificial vagina as per the standard method. A total of 24 ejaculates, eight from each bull (8×3= 24) were collected. Only ejaculates with mass motility ≥ 4 and progressive motility 80% were used in the study. Immediately after collection of semen, a part of each ejaculate was evaluated for various seminal attributes, LPO and ROS. Rest ejaculate was diluted with Tris-egg yolk-glycerol dilutor up to 60 106 spermatozoa/mL. Seminal attributes, LPO and ROS were estimated at pre-freeze and post-thaw stage.
2.4. Semen freezing
French midi straws (0.5 mL) were filled with the extended semen samples, sealed with polyvinyl alcohol powder and kept for 3 h at 5 ℃ for equilibration. After equilibration, straws were kept in automatic programmable biological cell freezer (IMV technology, France) until temperature of straws reached 145 ℃. Then straws were plunged into liquid nitrogen ( 196 ℃) for storage.
2.5. Semen evaluation
2.5.1 Seminal attributes
A drop of the diluted semen was kept on a clean, grease free, prewarmed glass slide, cover slip was placed and progressive motility was assessed under high power magnification (Nikon, Eclipse 80i; 400× magnification) of a phase contrast microscope. The live sperm percentage was estimated by differential staining technique using Eosin-Nigrosin stain[15]. Acrosomal intactness was determined by Giemsa stain[16]. Hypo-osmotic swelling (HOS) test was carried out according to the method described by Jeyendran, et al. [17].
2.5.2 Washing of spermatozoa
Washing of spermatozoa was done for estimation of LPO and ROS. Immediately after evaluation, fresh, pre-freeze and frozen thawed spermatozoa were washed using percoll density gradient[18] to remove egg yolk particles, dead cells and debris. Briefly 1 mL layer of 45% percoll (v/v, Sigma-Aldrich, USA) in phosphate buffer saline (PBS, NaCl 8 g, KCl 0.2 g, Na2HPO41.44 g, KH2PO40.24 g; distilled water up to 1 000 mL, pH 7.2) was pipetted carefully over a 1 mL layer of 90% percoll (v/v in PBS) in a disposable 15 mL centrifuge tube. 1 mL semen was gently layered on top of the two step percoll column and centrifuged at 400 g for 30 min. After centrifugation, the pellets were washed once again with PBS and resuspended in PBS to make desired concentration of sperm depending on parameters.
2.5.2.1 Estimation of LPO
The LPO in spermatozoa was measured based on the malondialdehyde (MDA) concentration by adopting the procedures of Buege and Aust. [19] and modified by Suleiman, et al. [20]. Briefly 2 mL of TCA-TBA reagent (Tri-chloro acetic Acid 15% (w/v), TBA 0.375% (w/v) in 0.25 N HCl was added to 1 mL of sperm suspension (109 spermatozoa). The mixture was heated in a boiling water bath for 15 min. After cooling, the suspension was centrifuged (500 g; 10 min), supernatant was separated and absorbance was measured at 535 nm using Double beam UV-VIS Spectrophotometer (DBS; Model-UV5704SS, ECIL, India). The MDA concentration was determined by the specific absorbance coefficient (1.56×105/ mol/cm3). LPO (n M MDA/109sperm) was calculated by following formula

2.5.2.2 Estimation of ROS
Estimation of ROS was done using a high-through put spectrophotometric assay as described by Hayashi et al.[21] with some modifications. The reaction mixture contained 5 µL of sperm suspension (containing 2.5×106 spermatozoa in PBS), 140 µL of 0.1 M sodium acetate buffer (pH 4.8) and 100 µL of the mixed solution prepared from R1and R2at the ratio of 1:25. The absorbance was measured at 505 nm for 2 min at 15 s interval using a spectrophotometric plate reader. R1solution contained 100 µg/mL of N, N diethyl Para-phenylendiamine sulphate (Sigma-Aldrich, USA) in 0.1 M sodium acetate buffer while R2solution was prepared by dissolving ferrous sulphate in 0.1 M sodium acetate buffer to attain a final concentration of 4.37 µM. Ten different concentrations of hydrogen peroxide solution (50, 100, 150, 200, 250, 300, 350, 400, 450, and 500 mg/L) were used as standard. A calibration curve for the standard solutions was developed by calculating slopes (absorbance increase at 505 nm/min 1 000) and the level of ROS was expressed as units of H2O2. One unit corresponded to 1 mg/L H2O2.
2.6. Statistical analysis
Data were statistically analyzed by unpaired t-test using Stastical Analysis System[22] Software Programme, version 9.3 and results were expressed as mean ± SE.
3. Results
3.1. Seminal attributes at fresh stage, pre-freeze and postthaw stage
Average of various seminal attributes at fresh stage, pre-freeze and post-thaw stage is presented in Table 1. Seminal attributes (motility, livability, acrosomal integrity and HOS response) were significantly higher in treatment group (group II) as compared to group I (control) at pre-freeze (P<0.05) and post-thaw (P<0.01) stage. About 5% higher motility, livability and acrosomal integrity were recorded in group II as compared to control at pre-freeze stage. At post-thaw stage, about 10% higher motility, 8% higher livability and 7% higher acrosomal integrity were recorded in group II as compared to group I. About 4% and 8% increase in HOS response was noticed in group II at pre-freeze and post-thaw stage than group I.
3.2. LPO and ROS at fresh stage, pre-freeze and post-thaw stage
Mean levels of LPO and ROS at fresh stage, pre-freeze and postthaw stage are presented in Table 2. Significantly higher levels of LPO were noticed in control group (group I) at pre-freeze (P<0.05) and post-thaw stage (P<0.01) as compared to treatment group (group II). ROS levels were significantly higher in control group at pre-freeze (P<0.05) and post-thaw stage (P<0.01) as compared to treatment group. An increment of 40% and 23% ROS level in control and treatment group was recorded at pre-freeze stage.
4. Discussion
4.1. Seminal attributes at fresh stage, pre-freeze and postthaw stage
The initial progressive motility of a semen sample gives a good indication of the fertility of the bull and ability of spermatozoa to withstand the stress of cryopreservation process. At fresh stage, percent progressive motility was 88.25±0.36 which was higher than reports of [13,23-26]. In our study, higher initial progressive motility may be attributed to utilization of only ≥4 grade semen and twice semen collection in a week. Livability of spermatozoa in a semen sample is significantly and positively correlated with initial motility, post-thaw motility and fertility of spermatozoa. Percent live spermatozoa in our study was higher than reports of [13,26,27]. Mean sperm livability was in agreement to reports of[11]. Percent intact acrosome observed in present study was higher than the values of[12,28,29] but lower than the values of[23,30]. Acrosome can be detached from the sperm head under the influence of different physical and chemical factors[31]. Optimum fertility depends on the acrosome being structurally and functionally intact[11]. The percent HOS positive spermatozoa in the present study was higher than the values reported by[23,32] and were lower than the values reported by[33]. Season has an influence on HOS response and there wassignificant difference in percent HOS responsive spermatozoa in winter and summer seasons being significantly higher in winter season than summer season[33].
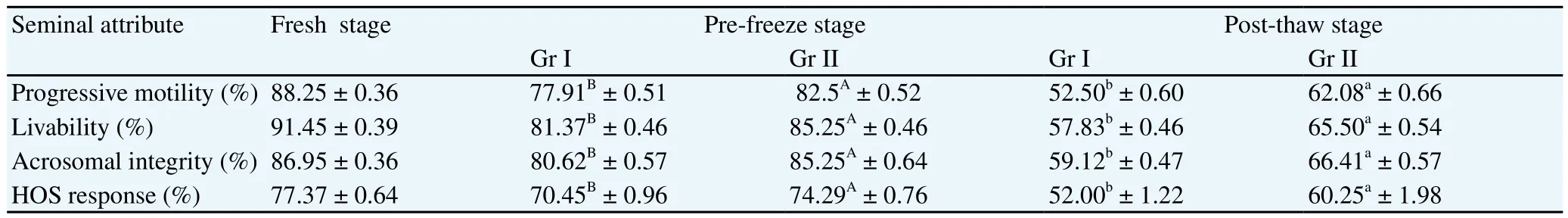
Table 1 Mean ± SE of seminal attributes at fresh, pre-freeze and post-thaw stage.

Table 2 Mean ± SE of LPO and ROS levels at fresh, pre-freeze and post-thaw stage.
At pre-freeze and post-thaw stage, progressive motility (%) was higher in group II as compared to group I. The percentage decline in progressive motility in our study was 40.5 from fresh to post-thaw stage in control group while the percentage decline in progressive motility was 29.65 in treatment group indicating beneficial effect of CLC in maintaining motility of spermatozoa. This was in agreement with Rajoriya[12] who also reported similar decline in percentage of individual progressive motility from fresh to post thaw stage. Livability was higher in group II than group I at pre-freeze and postthaw stage. In present study, decline in number of live spermatozoa from fresh to post-thaw stage was 36.70% in control and 28.31% in treatment group. In our study, we found CLC significantly increases progressive motility and livability at pre-freeze and post-thaw stage which might be due to reduced LPO and ROS levels in spermatozoa by CLC, as observed in the study. At pre-freeze and post-thaw stage, acrosomal integrity was higher in group II than group I. The reduction of acrosome intact spermatozoa from fresh to postthaw stage was 32.04% in control group and 23.66% in treatment group. This was in agreement with Rajoriya[12] who also reported decline in percentage of acrosome intact spermatozoa from fresh to post-thaw stage. HOS response was higher in treatment group as compared to control group at pre-freeze and post-thaw stage. The percent decrease in HOS response from fresh to post thaw stage was 31.80 and 20.98, respectively, in group I and group II. The higher percentage of HOS response in treatment group indicates higher percentage of membrane intact spermatozoa with intact functional status. In our study it was found that CLC increases percentage of HOS positive spermatozoa which was similar to findings of Kumar [11]. The increase in membrane intactness of spermatozoa by CLC may be due to reduced plasma membrane damage by lower levels of lipid peroxides and ROS.
4.2. LPO and ROS levels at fresh stage, pre-freeze and postthaw stage
LPO levels at fresh stage were comparable to the report of Mayuri [34] but lower than Kadirvel et al[23]. The average ROS levels recorded in fresh sperm suspension were 85.41±2.55 (Units of H2O2). Sperm mitochondria contribute to oxidative stress in damaged human sperm cells[35]. LPO is initiated when excess quantities of free radicals are produced, which results in the loss of sperm function[36]. Due to inability of spermatozoa to synthesize membrane components and low antioxidant capacity, spermatozoa become susceptible to damage by ROS [37]. An increase of about 11% and 7% in LPO level was noticed in control and treatment group after equilibration (before freezing), while as an increment of about 93% and 54% in LPO level was noticed in control and treatment group at post-thaw stage, which was in agreement with[23,24], who reported elevated levels of LPO in frozen-thawed semen. The variation in LPO levels may be due to intrinsic and extrinsic antioxidant defence mechanisms in sperm cell as well as in seminal plasma and ROS levels produced[38,39]. The reduction in LPO levels in treatment group may be due to reduced formation of ROS due to antioxidant nature of cholesterol. Previous reports have shown that peroxidation levels are higher in frozenthawed sperm due to aromatic amino oxidase enzyme activity in dead sperm and the increased number of dead sperm may be one of the attributing factors for increased levels of LPO[40]. ROS level was about 8% and 23% higher in control than treatment group at pre-freeze and post-thaw, respectively. However, at post-thaw stage, increase of about 131% and 75% ROS level was recorded in control and treatment group. An increased ROS level during equilibration at 4 ℃ was in agreement to[41] who reported increased levels of ROS during cooling at 4 ℃. The increase in the levels of ROS in frozen thawed spermatozoa in our finding was in agreement with the report of Chatdarong et al. [42] who reported increased levels of ROS in frozen-thawed spermatozoa. Various factors are responsible for the cryodamage, but ROS mediated damage is likely an important cause [43].
Conflict of interest statement
The authors declare that they have no competing interest.
Acknowledgment
The authors are thankful to the Director Indian Veterinary Research Institute for providing necessary facilities for research work of first author.
[1] Nair JS, Brar AS, Ahuja CC, Sangha SPS, Chaudhary KC. A comparative study on lipid peroxidation, activities of antioxidant enzymes and viability of cattle and buffalo bull spermatozoa during storage at refrigeration temperature. Anim Reprod Sci 2006; 96: 21-29.
[2] Dhami AJ, Kodagali SB. Freezability, enzyme leakage and fertility of buffalo spermatozoa in relation to the quality of semen ejaculates and extenders. Theriogenology 1990; 34: 853-863.
[3] Bucak MN, Atessahin A, Varish O, Yuce A, Tekin N, Akcay A. The influence of trehalose, taurine, custemine and hyaluronan on ram semen. Theriogenology 2007; 67:1060-1067.
[4] Zhang W, Yi k, Chen C, Houa X, Zhou X. Application of antioxidants and centrifugation for cryopreservation of boar spermatozoa. Anim Reprod Sci 2012; 132: 123-128.
[5] Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod update 2004; 10: 387-299.
[6] Baumber J, Sabeur K, Vo A, Ball BA. Reactive oxygen species promote tyrosine phosphorylation and capacitation in equine spermatozoa. Theriogenology 2003; 60: 1239-1247.
[7] Makker K, Agarwal A, Sharma R. Oxidative stress and male infertility.
Indian J Med Res 2009; 129: 357-367.
[8] Griveau JF, Lannou L. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl 1997; 20: 61-69.
[8] Bucak MN, Baspinar N, Tuncer PB, Çoyan K, Sanözakan S, Akalin PP, et al. Effect of curcumin and dithioerythritol on frozen-thawed bovine semen. Andrologia 2011; 10: 1111/j.1439-0272.2010.01146.x.
[9] Serin I, Aksoy M, Ceylan A. Cholesterol loaded cyclodextrin inhibits premature acrosomal reactions in liquid-stored spermatozoa. Anim Reprod Sci 2011; 123(1-2): 106-111.
[10] Dobziuk H. Molecules with holes-cyclodextrins. In: Dodziuk H editors. Cyclodextrins and their complexes. Germany: Wiley-VCH Verlag GmbH&Co, Weinheim; 2006, pp. 1-30.
[11] Kumar A. Studies on effect of cholesterol loaded cyclodextrin on freezability and in vitro fertility of buffalo Spermatozoa. 2012; Ph.D thesis submitted to Deemed University, IVRI, Izatnagar, U.P, India.
[12] Rajoriya JS. Studies on effect of cholesterol loaded cyclodextrin on cryocapacitation and in-vitro fertility of cryopreserved buffalo Spermatozoa. 2014; Ph.D thesis submitted to Deemed University, IVRI, Izatnagar, U.P, India.
[13] Lone SA, Prasad JK, Ghosh SK, Das GK, Balamurugan B, Katiyar R, et al. Effect of incubation on freezability of cholesterolloaded cyclodextrin treated buffalo (Bubalus bubalis) spermatozoa. Vet World 2016; 9(2): 182-185.
[14] Purdy PH, Graham JK. Effect of cholesterol loaded cyclodextrin on the cryosurvival of bull sperm. Cryobiology 2004; 48: 36-45.
[15] Campbell RG, Hancock JL, Rothschild L. Counting live and dead bull. J Experim Biol 1953; 30: 44-49.
[16] Watson PF. Use of Giemsa stain to detect changes in the acrosome of frozen ram spermatozoa. Vet Rec 1975; 97: 12-15.
[17] Jeyendran RS, Vander Ven HH, Parez M, Crabo BG, Zaneweld LJD. Development of an assay to assess the functional integrity of the human membrane and its relationship to other semen characteristics. J Reprod Fert 1984; 70: 219-228.
[18] Strom HB, Larson B, Linde-Forsberg C, Rodriguez-Martinez H. Evaluation of chilled and frozen-thawed canine spermatozoa using a zona pellucida binding assay. J Reprod Fert 2000; 119: 201-206.
[19] Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978; 52: 302-310.
[20] Suleiman SA, Ali ME, Zaki ZM, Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of vitamin E. J Androl 1996; 17: 530-537.
[21] Hayashi I, Morishita Y, Imai K, Nakamura M, Nakachi K, Hayashi T. High throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res 2007; 631: 55-61.
[22] SAS Institute Inc. SAS® 9.3 system options: reference. 2nd ed. Cary NC: SAS Institute Inc; 2011.
[23] Kadirvel G, Kumar S, Ghosh SK, Perumal P. Activity of antioxidative enzymes in fresh and frozen thawed buffalo (Bubalus bubalis) spermatozoa in relation to lipid peroxidation and semen quality. Asian Pac J Reprod 2014; 3(3): 210-217.
[24] Sannat C, Nair A, Sahu SB, Sahasrabudhe SA, Kuma A, Gupta AK, et al. Effect of species, breed and age on bacterial loadin bovine and bubaline semen. Vet World 2015; 8(4): 461-466.
[25] Bhakat M, Mohanty TK, Singh S, Gupta AK, Chakravarty AK, Singh P. Influence of semen collector on semen characteristics of Murrah buffalo and Crossbred bulls. Adv Anim Vet Sci 2015; 3(4):253-258.
[26] Shukla MK, Misra AK. Correlation between seminal characteristics in murrah buffaloes. Indian J Anim Sci 2005; 75: 263-266.
[27] Rajoriya JS, Prasad JK, Ghosh SK, Ramteke S, Barik NC, Das GK, et al. Cholesterol loaded cyclodextrin increases freezability of buffalo bull (Bubalus bubalis) spermatozoa by increasing cholesterol to phospholipid ratio. Vet World 2014; 7(9): 702-706.
[28] Singh M. Isolation and purification of PDC-109 protein frombuffalo seminal plasma and its relation with semen freezability. M.V.Sc. Thesis submitted to Deemed University, IVRI, Izanagar, Bareilly, U. P. India. 2012.
[29] Ramteke SS. Purification of PDC-109 and standardization of AntiPDC-109 level to minimize buffalo spermatozoa cryodamage. Ph.D thesis submitted to Deemed University, IVRI, Izatnagar, U.P. India. 2014.
[30] Lambrechts H, Nickerk FF, Coetzer WA, Clotete SWD, Horst G. The effects of cryopreservation on the survivability, viability and motility of epididymal African buffalo (Syncerus caffer) spermatozoa. Theriogenology 1999; 52: 1241-1249.
[31] Gebreselassie GA. Studies on physico-morphological characteristics and certain enzymes in freezable and non-freezable semen ejaculates of crossbred bulls. M. V. Sc thesis submitted to the Deemed University, Indian Veterinary Research Institute, Izatnagar UP. India. 2009.
[32] Karmur SD, Rana CM, Dhami AJ, Panchani VJ. Hypo-osmotic swelling test in relation to motility of fresh and frozen thawed murrah buffalo semen. Indian J Dairy Sci 2002; 55: 363-365.
[33] Rajoriya JS. Effects of seasons on enzymatic changes and cholesterol efflux in relation to freezability in Tharparkar bull semen. M.V.S.c thesis submitted to Deemed University, IVRI, Izatnagar, U.P. India. 2011.
[34] Mayuri R. Studies on oxidative damages and effects of certain antioxidants on buffalo spermatozoa during cryopreservation.M.V.Sc thesis submitted to Deemed University, IVRI, Izatnagar, U.P. India. 2006. [35] Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin A, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. Clin Endocrinol Metab 2008; 93(8): 3199-3207.
[36] Aitken RJ. The human spermatozoon - a cell in crisis. J Reprod Fertil 1999; 115(1): 1-7.
[37] Jones R, Mann T. Toxicity of exogenous fatty acid peroxides towards spermatozoa. J Reprod Fertil 1977; 50: 255-260.
[38] Griveau JF, Dumont E, Renard P, Lannou D. Reactive oxygen species, lipid peroxidation and enzymatic defence system in human spermatozoa. J Reprod Fertil 1995; 103: 17-26.
[39] Bilodeau JF, Chatterjee S, Sirard MA, Gagnon C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev 2000; 55: 282-288.
[40] Upreti GC, Jensen K, Munday R, Duganzich DM, Vishwanath R, Smith JF. Studies on aromatic amino acid oxidase activity in ram spermatozoa: role of pyruvate as an antioxidant. Anim Reprod Sci 1998; 51: 275-287.
[41] Wang C, McDonald V, Leung A, Superlano L, Berman N, Hull L, et al. Effect of Increased scrotal temperature on sperm production in normal men. Fertil Steril 1997; 68(2): 334-339.
[42] Chatdarong K, Chaivechakarn A, Thuwanut P, Ponglowhapan S. Effects of cold storage prior to freezing on superoxide dismutase, glutathione peroxidase activities, level of total reactive oxygen species and sperm quality in dogs. Reprod Dom Anim 2012; 47(6): 274-277.
[43] Chatterjee S, Gagnon C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing and thawing. Mol Reprod Dev 2001; 59: 451-458.
ment heading
10.1016/j.apjr.2016.10.003
*Corresponding author: *Shabir Ahmad Lone, Animal Reproduction, Gynaecology and obstetrics, ICAR- National Dairy Research Institute, Karnal 132001, India. E- mail: drloneshabir@gmail.com
Methods:A total of 24 ejaculates (mass motility≥4; progressive motility≥80%) were collected from three Murrah buffalo bulls (8 from each bull) through artificial vagina twice a week. One part of semen was diluted with TEYC extender (group I) and other part was treated with cholesterol loaded cyclodextrin before final dilution with TEYC extender (group II). Semen samples were evaluated for various seminal attributes, LPO and ROS levels at fresh, pre-freeze and post-thaw stage in group I and group II.
Results: Seminal attributes (progressive motility, livability, hypo-osmotic swelling response and acrosomal integrity) were significantly higher in group II than group I at pre-freeze (P<0.05) and post-thaw (P<0.01) stage. LPO and ROS levels were significantly higher in group I as compared to group II at pre-freeze (P<0.05) and post-thaw (P<0.01) stage.
Conclusion:It is concluded that cholesterol loaded cyclodextrin reduces LPO and ROS levels during cryopreservation of buffalo spermatozoa.
杂志排行
Asian Pacific Journal of Reproduction的其它文章
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- Substitution of egg yolk with different concentrations of soybean lecithin in trisbased extender during bulls' semen preservability
- Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
