Synergy to discovery and innovation—Growth of nanorods
2017-01-06ZhengyngLiHnchenHung
Zhengyng Li,Hnchen Hung∗
aInstitute of Mechanics,Chinese Academy of Science,Beijing 100190,China
bDepartment of Mechanical and Industrial Engineering,Northeastern University,Boston,MA 02115,USA
Review
Synergy to discovery and innovation—Growth of nanorods
Zhengyang Lia,Hanchen Huangb,∗
aInstitute of Mechanics,Chinese Academy of Science,Beijing 100190,China
bDepartment of Mechanical and Industrial Engineering,Northeastern University,Boston,MA 02115,USA
H I G H L I G H T S
·Synergy of atomistic simulations,analytical formulations,and experiments is described and demonstrated in the study of nanorod growth.
·As one demonstration,the discovery of smallest and well-separated nanorods becomes possible.
·As another demonstration,the innovation of metallic glue technology comes to light.
A R T I C L E I N F O
Article history:
Received 29 August 2016
Received in revised form
20 October 2016
Accepted 20 October 2016
Available online 25 October 2016
Metallic glue
A synergy of computer simulations,analytical formulations,and experiments proves very effective in research and development.Using nanorod growth as an example,this letter presents the synergy of the three complementary approaches in research,and demonstrates its effectiveness.Through this synergy,a theoretical framework of nanorod growth emerges;through the direction of the theories, experimental realization of smallest well-separated nanorods becomes reality;through the realization of such nanorods,metallic glue in ambient environments becomes technical reality and commercial possibility.
©2016 The Author(s).Published by Elsevier Ltd on behalf of The Chinese Society of Theoretical and Applied Mechanics.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Contents
1. Introduction........................................................................................................................................................................................................................249
2. Synergy...............................................................................................................................................................................................................................250
3. Summary............................................................................................................................................................................................................................251
Acknowledgments.............................................................................................................................................................................................................251
...........................................................................................................................................................................................................................252
1.Introduction
Nanorods are rods,pillars,or columns with a lateral dimension on the order of tens of nanometers.Their aspect ratio is typically on the order of 10 or smaller;if the aspect ratio is larger,they are generallycallednanowires.Growthofnanorodsisfeasiblethrough various approaches,such as physical vapor deposition(PVD), chemical vapor deposition(CVD),and solution processing[1–3]. PVD is the cleanest and most controllable process.Once the PVD process is well understood,one may include additional chemical reactions and solid–fluid mechanics to understand other growth processes that are more complex.Therefore,we take the PVD process as the prototype for scientific understanding of nanorod growth.
Within the framework of PVD,nanorod growth relies on the glancing angle deposition technique or geometrical shadowing effects[4,5].Inconnectionwiththinfilmdepositions,thenanorods bear some similarities with porous films except that nanorods are better separated.In addition to geometrical shadowing,nanorod growth also relies on limited diffusion at low temperatures, typically below 0.3Tm(30%of the melting temperature).
Theories exist for thin film deposition at the continuum level[6],nucleation and growth at the atomic level[7],and growth of roughening surfaces with Ehrlich–Schwoebel(ES)barriers [8–11].However,these theories do not apply to the growth ofnanorods.Even with the ES barrier taken into account,characteristic length scales of surfaces are on the order of a micron according to the existing theories.In contrast,nanorods from PVD experiments are as small as a few nanometers and typically on the order of tens of nanometer.In order to design nanorod growth,it is essential to understand what mechanisms control the growth process and to establish a theoretical framework.Achieving this goal requires or at least benefits from the synergy of atomistic simulations,analytical formulations,and experiments.

Fig.1.Flow chart of synergy,showing the five milestones from M1 to M5.
2.Synergy
Throughout our studies of nanorod growth,five milestones of scientific discoveries have been pivotal:(1)the discovery of the large diffusion barrier over multiple-layer surface steps,(2)the identificationofanerrorintheclassicaltheorythatwhencorrected establishes the kinetic stability of multiple-layer surface steps, (3)development of theories for nanorod diameter and nanorod separation,(4)verification and validation of the theories,and (5)theory-guided design of experiments.As shown in Fig.1, these five milestones involve atomistic simulations,analytical formulations,experiments,andmoreimportantlytheirsynergy.As translation of the scientific discoveries,metallic glue technology represents an innovation.This section describes each of the five milestones and the translation,highlighting the synergy of methods.
The first breakthrough in our understanding of nanorod growth is the identification of the large diffusion barrier over multiplelayer surface steps through atomistic simulations including both classical molecular statics and density-functional-theory calculations.Onthefoundationoftheweddingcakemodel[10,11], it is possible to understand characteristic length scales of surfaces on the order of a micron or a fraction of a micron during PVD. However,the diameter of nanorods from PVD experiments is at least an order of magnitude smaller.The order-of-magnitude difference between existing theories and experiments calls for the discovery of missing mechanisms.Using interatomic potential based molecular statics calculations,we have brought to light the critical role of diffusion of adatoms over multiple-layer surface steps,which had been overlooked.According to our calculations,the diffusion over multiple-layer surface steps can be 100000 times slower than over monolayer surface steps at room temperature[12,13].As shown in Fig.2,for a Cu{111}system that contains an adatom,the total energy increases and then decreases as an adatom diffuses from start to end(diffusion coordinate from 0 to 1).The diffusion barrier of Cu adatoms increases from 0.16 eV to 0.40 eV as the steps go from monolayer to two or more layers.
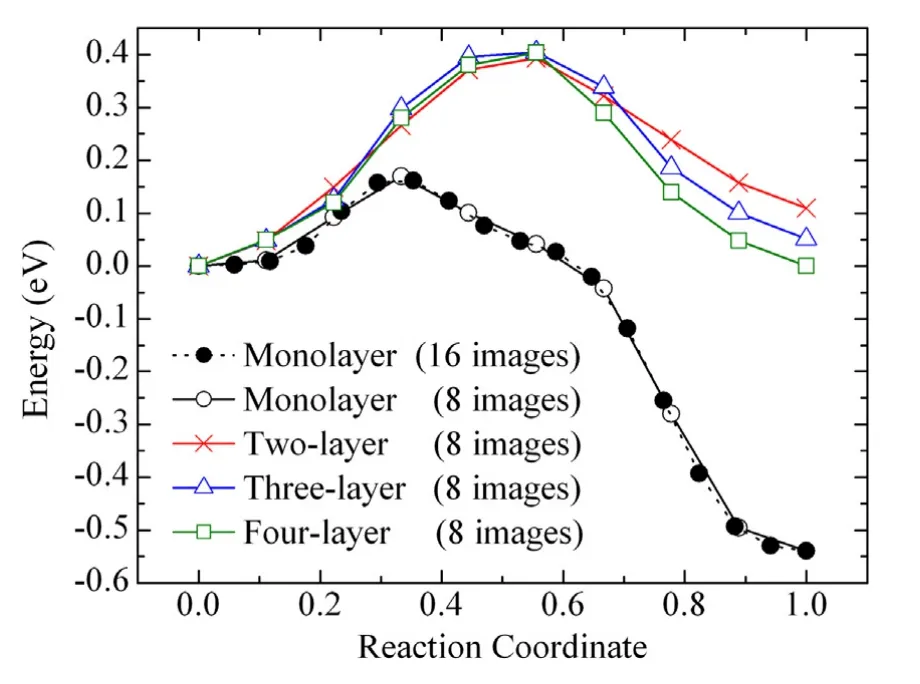
Fig.2.EnergyofaCusystemasafunctionofreactioncoordinatealongthediffusion path of an adatom.
A dilemma was realized soon after the discovery of the large diffusion barrier of adatoms over multiple-layer surface steps.On the one hand,this large diffusion barrier offers a potential explanation for the order-of-magnitude smaller diameter of nanorods in experiments.On the other hand,this large diffusion barrier cannot function according to the classical theories.In the publication in 1966[9],Schwoebel and Shipsey analytically showed that multiple-layer surfaces steps would not be stable kinetically. In nanorod growth process,thermodynamic driving forces such as strain energy are absent due to the small dimensions.If the classical theory prevails,the discovery of the large diffusion barrier is meaningless since multiple-layer surface steps are not stable.Another atomistic simulation method came to the rescue:the lattice kinetic Monte Carlo(LKMC)method.Using LKMC simulations,we have shown that a positive feedback mechanism stabilizes multiple-layer surface steps.By random fluctuations,two or more monolayer steps can bunch to form a multiple-layer step. Once formed,the large diffusion barrier of the multiple-layer step enhances its stability by reducing the chance of dissociation[14]. Geometricalshadowinginglancingangledeposition,whichisgenerally used for nanorod growth,further stabilizes multiple-layer surface steps.This advancement enriches and corrects the classical theory,and more importantly takes us further along the way of understanding nanorod growth.
Now that the large diffusion barrier of adatoms over multiplelayer surface steps is shown to exist and to function,a characteristic surface length scale should exist in connection with this barrier.The diameter of nanorods depends on the nature of steps that bound the top surface.If the steps are multiple-layer,instead of monolayer,the diffusion barrier is large and therefore the diameter is small.When the steps are 100%multiple-layer in nature, the diameter isthesmallest.Our analytical formulations[15]show that the smallest diameter of nanorodLminis
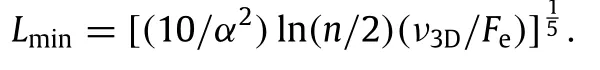
Here the diffusion jump rateν3Dis an Arrhenius function of the diffusion barrier of adatoms over multiple-layer surface steps,Feis the effective deposition rate on the top surface,αis a geometrical factor of the top cross section of the nanorod and it is on the order of one,andnis the height of nanorod that remains in cylindrical shape and typically is on the order of 2000 for Cu.It is important to recognize that the analytical formulations rely on idealized assumptions;for example,the cross section of nanorods is assumed circular,and it is rarely the case for crystalline nanorods. Using LKMC simulations,we have verified the analytical theory ofLmin,and shown that the idealized assumptions cause only minor errors.This verified theory allows us to understand why the orderofmagnitudeofnanoroddiameteristensofnanometers.However, it still does not allow us to design nanorod growth without knowing how nanorod separation varies.In fact,considering only thetheoryofnanoroddiameter,weranintonanorodmorphologies that we had to classify as an‘anomaly’[16].According to the theory,increasing the deposition rate will lead to smaller nanorod diameter.However,our experiments show that increasing the deposition rate beyond a certain point can lead to transition from nanorods to thin films.

Fig.3. LKMC simulation results under incomplete geometry shadowing as a function of(ν3D/F)1/5;the separation of nanorod nuclei Lsis included for comparison.
Tounderstandthisanomalyandtoenablethedesignofnanorod growth,we analytically formulate the separation of nanorods.The nanorod separation is related to the separation of nuclei on a substrate.Interestingly,decades of study of the nuclei separation in real space led to no analytical theory—only approximate or numerical solutions existed.In investigating the same nucleation process in a phase space of nucleation probability vs separation, we have achieved an analytical theory for the separationLsas a function of surface diffusion rateνand deposition rateF[17]
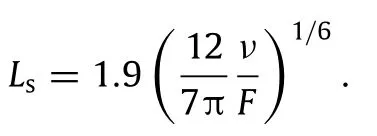
Again,we have verified the validity of this theory of nuclei separation using LKMC simulations.
Next,weputthetwotheoriestovaliditytest.AsshowninFig.3, accordingtothetheories,nanoroddiametershouldbeontheorder of tens of nanometers as experimentally observed.Further,the two theories predict a critical point,which separate the growth of thin films from that of nanorods,and indeed,this is what our experimentsshow.Beforethetheoriescametolight,wehadtocall the experimental result an‘anomaly’.
Now equipped with verified and validated theories of nanorod growth—for both nanorod diameter and nanorod separation, we grow nanorods by design.From Fig.3,we realize that the intrinsic separation of nanorods is only slightly larger than the nanorods even in the domain of nanorod growth.In order to grow well-separated nanorods,the separation must be increased or the diameter must be decreased.This can be accomplished in multiple ways,for example,by decreasing substrate temperature, decreasing the substrate wettability,and by patterning substrates. Making substrates rough and non-wetting can be done simply and introduces a pre-defined separation of nanorods.As shown in Fig.4,small well-separated Cu nanorods are realized for the first time,thanks to the guidance of theories that became reality through the synergy of atomistic simulations,analytical formulations,and experiments.
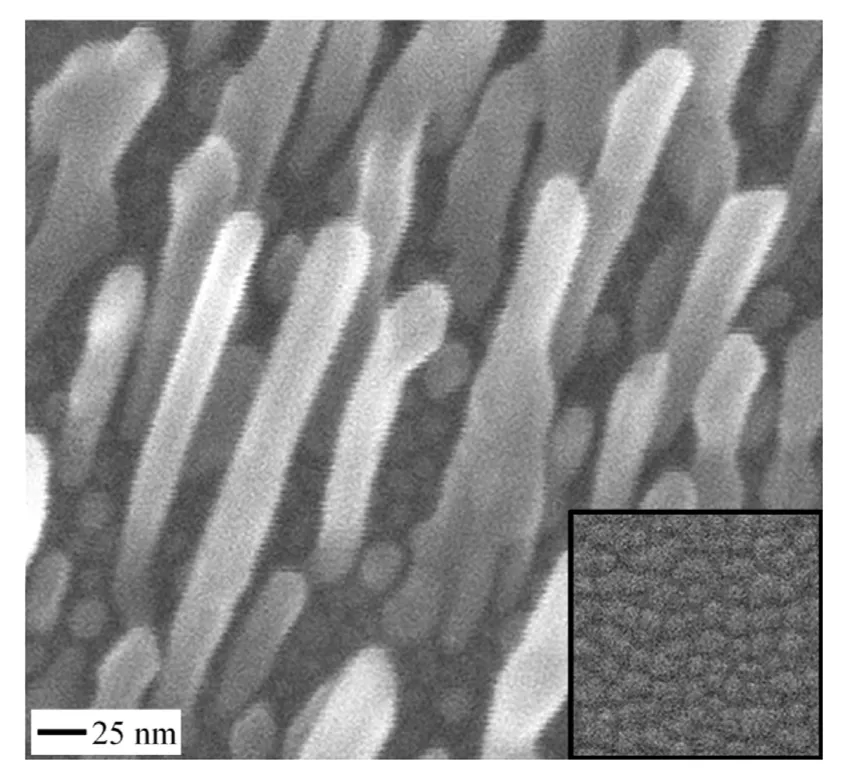
Fig.4.Scanning electron microscopy images of well-separated Cu nanorods at an early stage;the insets with the same scale show the morphologies of substrates. Source:Reprinted with permission from Ref.[15].
As engineers,we enjoy scientific discoveries through synergies, but do not stop at the discoveries.More importantly,we translate scientific discoveries to technological innovations.Using the small well-separated nanorods,we can glue two solids together,as schematically shown in Fig.5.Because of the small dimensions and the large separations,it takes very small pressure of a few megapascals to press the two groups of nanorods into each other. Because of the small dimensions,surface diffusion is fast so sintering takes places at room temperature in a few seconds. The introduction of a eutectic coating such as In and Ga on the nanorods also introduces liquid formation upon contact and then solidification upon interdiffusion.
Our experiments indeed show that this process gives rise to a continuous glue at room temperature, in air, and under a small pressure; Fig. 6(a). Either the increase of temperature to 100°C or the introduction of eutectic alloy coating can lead to even denser glue; Fig. 6(b). This glue technology has the potential of replacing soldering or welding, in ambient environments instead of at high temperature or high pressure or both. The title of Yahoo! News report, ‘Nanoscale metallic glue could replace welding and soldering’, is a reflection of this expectation.
3.Summary
Using nanorod growth as a prototype process,we have demonstrated the synergy of atomistic simulations,analytical formulations,and experiments.Atomistic simulations enabled the discovery of a diffusion mechanism,the identification of a deficiency in the classical theory,and the verification of analytical theories.The analytical formulations result in two closed-form or analytical theories that guide the design of nanorod growth.The experiments,in addition to validating the theories and the simulations,provoke further understanding through the observations of anomalies.This synergy in nanorod growth has led to the scientific discovery of the smallest well-separated nanorods and then to the innovation of metallic glue technology.
Acknowledgments
Author HH gratefully acknowledges the program of Hseu Shen Tsien Engineering Science Lecture,which this paper is based on, and also acknowledges the sponsorship of the US Department of Energy Office of Basic Energy Science(DE-SC0014035)and the US National Science Foundation(NSF 1506966).

Fig.5.Low-temperaturemetallicgluingenabledbywellseparatedmetallicnanorods:(a)Twosetsofwellseparatednanorods,whichhavemetalliccoresandshellelements that form a eutectic alloy,are brought together,(b)they interpenetrate under fingertip pressure,(c)shell elements meet and form a eutectic alloy,which is liquid at room temperature.
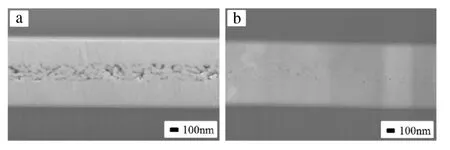
Fig. 6. Metallic glue formed in air and under a small pressure of 9 MPa (a) at room temperature, and (b) at 100°C. Source: Reprinted with permission from Ref. [19].
References
[1]L.S.Wang,X.Z.Zhang,S.Q.Zhao,etal.,Synthesisofwell-alignedZnOnanowires by simple physical vapor deposition on c-oriented ZnO thin films without catalysts or additives,Appl.Phys.Lett.86(2005)024108.
[2]J.J.Wu,S.C.Liu,Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition,Adv.Mater.14(2002)215–218.
[3]Y.G.Sun,B.Gates,B.Mayers,Crystalline silver nanowires by soft solution processing,Nano Lett.2(2002)165–168.
[4]K.Robbie,J.C.Sit,M.J.Brett,Advanced techniques for glancing angle deposition,J.Vac.Sci.Technol.B 16(1998)1115–1122.
[5]T.Karabacak,G.C.Wang,T.M.Lu,Physical self-assembly and the nucleation of three-dimensional nanostructures by oblique angle deposition,J.Vac.Sci. Technol.A 22(2004)1778–1784.
[6]W.W.Mullins,Theory of thermal grooving,J.Appl.Phys.28(1957)333.
[7]W.K.Burton,N.Cabrera,F.C.Frank,The growth of crystals and the equilibrium structure of their surfaces,Philos.Trans.R.Soc.Lond.Ser.A 243(1951)299.
[8]G.Ehrlich,F.G.Hudda,Atomic view of surface self-diffusion-tungsten on tungsten,J.Chem.Phys.44(1966)1039–1049.
[9]R.L.Schwoebel,E.J.Shipsey,Step motion on crystal surfaces,J.Appl.Phys.37 (1966)3682–3686.
[10]J.Tersoff,A.W.Denier van der Gon,R.M.Tromp,Critical island size for layerby-layer growth,Phys.Rev.Lett.72(1994)266–269.
[11]J.Krug,Four lectures on the physics of crystal growth,Phys.A 313(2002) 47–82.
[12]S.J.Liu,H.C.Huang,C.H.Woo,Schwoebel-Ehrlich barrier:from two to three dimensions,Appl.Phys.Lett.80(2002)3295–3297.
[13]S.K.Xiang,H.C.Huang,Ab initio determination of Ehrlich-Schwoebel barriers on Cu{111},Appl.Phys.Lett.92(2008)101923.
[14]R.X.Zhang,H.C.Huang,Another kinetic mechanism of stabilizing multiplelayer surface steps,Appl.Phys.Lett.98(2011)221903.
[15]X.B.Niu,S.P.Stagon,H.C.Huang,Smallest metallic nanorods using physical vapor deposition,Phys.Rev.Lett.110(2013)136102.
[16]S.P.Stagon,H.C.Huang,J.K.Baldwin,Anomaly of film porosity dependence on deposition rate,Appl.Phys.Lett.100(2012)061601.
[17]L.G.Zhou,H.C.Huang,Smallest separation of nanorods from physical vapor deposition,Appl.Phys.Lett.100(2012)141605.
[18]S.P.Stagon,A.Knapp,P.Elliott,et al.,Metallic glue for ambient environments making strides,Adv.Mater.Proc.174(2016)22–25.
[19]S.P.Stagon,H.C.Huang,Airtight metallic sealing at room temperature under small mechanical pressure,Sci.Rep.3(2013)3066.
∗Corresponding author.
E-mail address:h.huang@northeastern.edu(H.Huang).
http://dx.doi.org/10.1016/j.taml.2016.10.002
2095-0349/©2016 The Author(s).Published by Elsevier Ltd on behalf of The Chinese Society of Theoretical and Applied Mechanics.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Nanorod
Physical vapor deposition
*This article belongs to the Biomechanics and Interdiscipline
杂志排行
Theoretical & Applied Mechanics Letters的其它文章
- Buckling and post-buckling analyses of size-dependent piezoelectric nanoplates
- Numerical simulation of domain switching in multilayer ferroelectric actuators
- Coriolis effect on responses of rotating thin piezoelectric hollow cylinder
- On the role of piezoelectricity in phonon properties and thermal conductivity of GaN nanofilms
- On transition of type V interaction in double-wedge flow with non-equilibrium effects
- Numerical simulation of Gurney flap on SFYT15thick airfoil
