银杏二萜内酯葡胺注射液通过抑制p38/p53通路保护氧糖剥夺SY5Y细胞的机制
2017-01-06李雪珂许治良王振中伟1
李雪珂,刘 秋,许治良,周 军,曹 亮,丁 岗,王振中,萧 伟1,
(1.南京中医药大学,江苏 南京 210000;2. 江苏康缘药业股份有限公司;3.中药制药过程新技术国家重点实验室,江苏 连云港 222001)
银杏二萜内酯葡胺注射液通过抑制p38/p53通路保护氧糖剥夺SY5Y细胞的机制
李雪珂1,3,刘 秋2,3,许治良2,3,周 军2,3,曹 亮2,3,丁 岗2,3,王振中2,3,萧 伟1,2,3
(1.南京中医药大学,江苏 南京 210000;2. 江苏康缘药业股份有限公司;3.中药制药过程新技术国家重点实验室,江苏 连云港 222001)
目的 探讨银杏二萜内酯葡胺注射液(diterpene ginkgolides meglumine injection, DGMI)对缺糖/缺氧损伤(oxygen-glucose deprivation,OGD)的人神经母细胞瘤细胞(SY5Y)保护的作用及可能的机制。方法 SY5Y细胞OGD损伤4 h后,予药物复氧1 h,然后测定细胞存活率(CCK-8法)、凋亡坏死比率、线粒体膜电位(ΔΨm);蛋白质免疫印迹检测细胞中p-p38、p-p53、Bcl-2、Bax和cleaved caspase-9、cleaved caspase-3蛋白量的变化。结果 DGMI能明显提高OGD损伤的SY5Y细胞的存活率,降低细胞凋亡比率,挽救下降的线粒体膜电位(ΔΨm)。下调p-p38、p-p53、Bax/Bcl-2、cleaved caspase-9、cleaved caspase-3等蛋白量,抑制p38和p53活性,保护神经细胞。结论 DGMI对OGD损伤的SY5Y细胞具有明显的保护作用,其保护机制可能与细胞内p38/p53/Bcl-2/caspase-9/caspase-3 信号通路的抑制有关。
银杏二萜内酯葡胺注射液;氧糖剥夺;细胞凋亡;p38; p53;Bax/Bcl-2
脑卒中俗称中风,其中缺血性卒中约占85%,是危害人类生存健康的高危疾病。缺血性卒中系由局部脑组织区域血液供应障碍,导致神经细胞缺血/缺氧性病变凋亡和坏死,进而产生临床上对应的神经功能缺失表现,因此保护脑缺血半暗带区神经细胞是其治疗的关键因素之一。银杏二萜内酯葡胺注射液(diterpene ginkgolides meglumine injection, DGMI)是含银杏内酯B(GB,60%)、银杏内酯A(GA,35%)、银杏内酯K(GK,2%)、银杏内酯C(GC,2%)的5类新药,用于脑卒中的治疗。研究表明,各单体除作为血小板活化因子(PAF)受体拮抗剂[1]外,还可通过抑制炎症反应、线粒体凋亡和氧化应激等[2-5]途径保护神经细胞。本实验室已有研究表明,DGMI可通过激活PI3K/Akt和PKA通路,促进Bad(Ser 136)和Bad(Ser 112)磷酸化,抑制氧糖剥夺SY5Y细胞线粒体途径凋亡[6]。p38 MAPK是有丝分裂原激活蛋白激酶 (mitogen-activated protein kinase, MAPK)的重要成员,激活后发生核转位,对许多蛋白激酶和转录因子具有磷酸化激活的作用,参与体内多种过程,不仅在炎症反应中,也在细胞凋亡中发挥重要的作用[7-8]。p53是体内的一种检测基因组完整性,调控凋亡的蛋白,在脑缺血神经元凋亡中发挥重要作用,其活性受磷酸化、乙酰化、甲基化、泛素化等翻译修饰后调控,特别是Ser15位点磷酸化能抑制泛素化降解,增强其稳定性活性。本研究选用人神经母细胞瘤细胞(SY5Y)缺糖/缺氧(oxygen-glucose deprivation,OGD)模型模拟缺血性脑卒中,研究DGMI对OGD损伤细胞凋亡的抑制作用及抑制p38/p53信号通路治疗脑卒中的机制。
1 材料与方法
1.1 材料与仪器 SY5Y购自中科院上海细胞库;RPMI 1640培养基、胎牛血清购自美国Gibco公司;青链霉素混合液购自 Biotopped公司;胰蛋白酶购自美国Amresco公司;CCK-8试剂盒购自上海贝博公司;Annexin V-FITC细胞凋亡检测试剂盒购自上海碧云天公司;罗丹明123购自美国Sigma公司;p-p38(Thr 180/Tyr 182) antibody、p-p53(Ser 15) antibody、Bcl-2 antibody、cleaved caspase-3 antibody、GAPDH antibody、tublin antibody、HRP标记二抗购自美国CST公司;Bax antibody、caspase-3 antibody购自美国Santa Cruz公司;caspase-9 antibody购自英国Abcam公司;CO2细胞培养箱购自美国Thermo公司;三气培养箱购自德国BINDER公司;微板检测系统Flex Station 3购自美国MD公司;ChemiDoc XRS系统购自美国Bio Rad公司;银杏二萜内酯葡胺注射液(DGMI)为江苏康缘药业生产,产品批号为141201;金纳多注射液(JND)产品批号为H4040。
1.2 方法
1.2.1 细胞培养、OGD模型和给药 SY5Y细胞培养于RPMI 1640培养基(含10%胎牛血清,100 kU·L-1青霉素,100 mg·L-1链霉素)中,置于37 ℃,5% CO2的培养箱中培养,选取对数生长期细胞进行实验。将SY5Y细胞接种至细胞板中培养24 h,以无糖RPMI 1640完全置换RPMI 1640培养基,置于三气培养箱中,控制95% N2和5% CO2混合气体条件,待氧气浓度降为0.2%时计时,培养4 h后,取出细胞板置于含氧细胞培养箱中,给一定浓度DGMI和JND药物,复氧1 h。然后进行细胞活力、凋亡和相关激酶蛋白的测定。
1.2.2 细胞活力的测定 将SY5Y细胞以每孔2×104的密度接种至96孔板,OGD 4 h后加入终浓度为6.25、12.5、25、50 mg·L-1的DGMI,一起复氧1 h,采用CCK-8试剂盒,按说明书每孔加入底物10 μL,细胞培养箱中孵育2.5 h后,于450 nm处测定各组吸光度值OD450 nm,计算细胞相对活力。
1.2.3 Annexin V-FITC检测细胞凋亡 将SY5Y细胞以每孔5×105的密度接种至6孔板,OGD 4 h后加入终浓度为25 mg·L-1的药物,一起复氧1 h,应用Annexin V-FITC细胞凋亡检测试剂盒,参考说明书,测定细胞凋亡比率。将细胞培养液吸出至一灭菌离心管内,胰酶消化收集,合并液体,1 000×g离心5 min,弃上清,PBS重悬计数,取10万重悬细胞至1.5 mL离心管内。1 000×g离心5 min,弃上清,加入195 μL Annexin V-FITC结合液,轻轻重悬,加入5 μL Annexin V-FITC,再加入10 μL碘化丙啶染色液,轻轻混匀,室温避光孵育15 min,随即进行流式细胞仪检测。获取散点图后,调节四分象限,计算各组细胞凋亡比率。
1.2.4 罗丹明123染色 将SY5Y细胞以每孔2×104的密度接种至96孔板,OGD 4 h后加入终浓度为25 mg·L-1的药物,一起复氧1 h,吸除细胞培养液,加PBS洗涤1次,加入100 μL的Rhodamine123染色液,轻轻混匀,细胞培养箱中避光孵育15 min,PBS洗涤2次,于荧光倒置显微镜下拍照观察。1.2.5 Western blot检测蛋白质水平 将SY5Y细胞以每孔5×106的密度接种于100 mm培养皿中,收集各组细胞,加入蛋白裂解液提取总蛋白,应用BCA法,测定蛋白浓度。经SDS-PAGE电泳后,电转蛋白至PVDF膜,封闭,加入1 ∶1 000稀释的兔抗人的一抗,4 ℃孵育过夜,回温2 h,TBST洗涤3次,加入HRP标记的羊抗兔二抗(1 ∶1 500)溶液室温 孵育2 h后洗涤膜,ECL法显影,应用ChemiDoc XRS系统拍照,采用Quantity One图像分析软件进行显影条带灰度值分析。
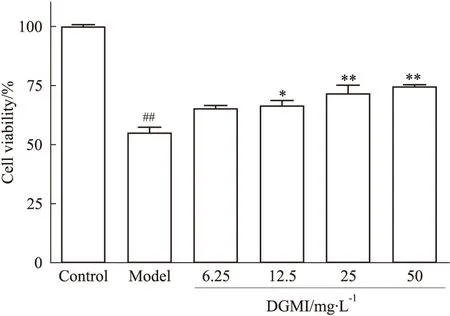
Fig 1 DGMI improves viabilities of
##P<0.01vscontrol group;*P<0.05,**P<0.01vsmodel group
A
B
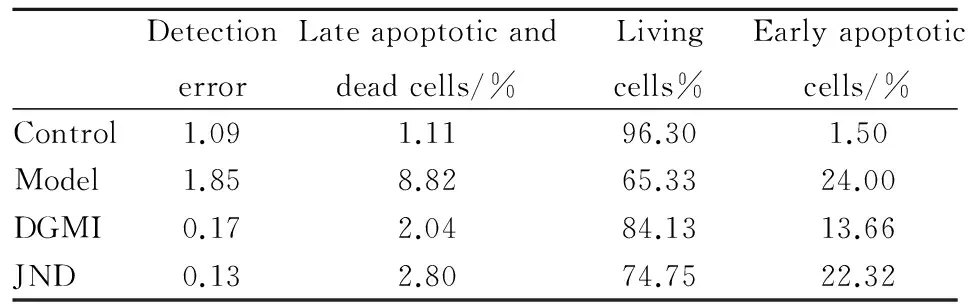
DetectionerrorLateapoptoticanddeadcells/%Livingcells%Earlyapoptoticcells/%Control1.091.1196.301.50Model1.858.8265.3324.00DGMI0.172.0484.1313.66JND0.132.8074.7522.32
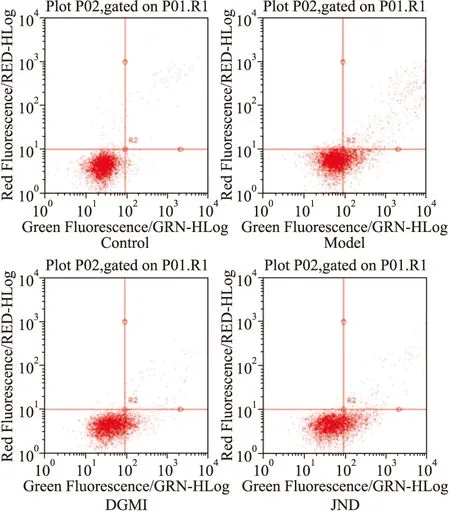
Fig 2 Effect of DGMI on OGD-induced apoptosis of SY5Y cells
A:Preventive effects of DGMI and JND on OGD-induced apoptosis. The cells were stained by Annexin V-FITC/PI and detected by flow cytometry.B:The diagram describes the percentage of cells in each quadrant.
2 结果
2.1 DGMI明显提高OGD的SY5Y细胞的存活率 应用CCK-8试剂盒测定不同给药浓度的SY5Y细胞活力,结果显示(Fig 1),与Control组相比,OGD 4 h组细胞存活率降低至54.80%,DGMI终浓度为6.25、12.5、25、50 mg·L-1时,细胞活力分别升至65.18%、66.26%、71.57%和74.45%,且呈浓度依赖性,选取25 mg·L-1作为后续试验药物浓度。
2.2 DGMI抑制OGD诱导的SY5Y细胞的凋亡 为了明确DGMI对细胞凋亡的抑制作用,本研究检测了细胞凋亡比率和线粒体膜电位(ΔΨm)。Annexin V-FITC实验结果显示(Fig 2),与对照组相比,SY5Y细胞OGD损伤后,细胞凋亡比率明显升高至34.24%,复氧给药DGMI后降至15.68%, JND组下降至24.26%。线粒体膜电位实验荧光结果显示(Fig 3),氧糖剥夺后细胞绿色荧光减弱,膜电位(ΔΨm)明显下降,孵育药物后荧光增强,膜电位(ΔΨm)升高。说明DGMI能保护线粒体,抑制细胞凋亡。
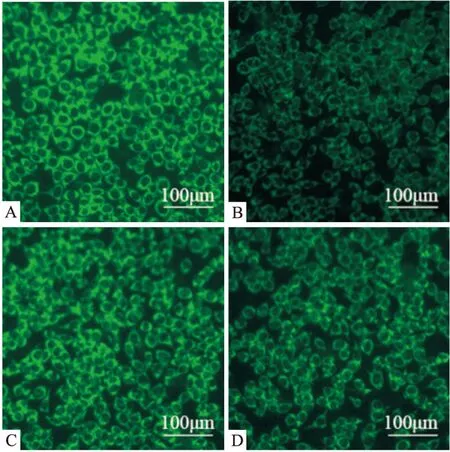
Fig 3 Effect of DGMI on loss of MMP in SY5Y cells
A:Control;B:Model;C:DGMI;D:JND.The cells were stained by Rhodamine123 and photographed at 100×
2.3 DGMI抑制SY5Y细胞OGD激活的p38/p53活性 本研究检测了DGMI对p38/p53通路信号通路的调节作用。蛋白质分析结果显示(Fig 4),缺糖/缺氧损伤后,与OGD组相比,DGMI组p38 (Thr 180/Tyr 182)和p53(Ser 15)磷酸化明显下降(P<0.01),抑制了SY5Y细胞中OGD诱导的p38/p53活性。
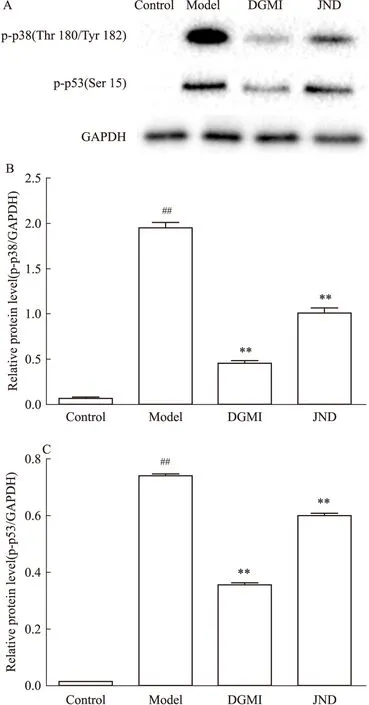
Fig 4 p-p38(Thr 180/Tyr 182) and p-p53(Ser 15) levels of OGD-induced SY5Y cells treated with ±s,n=3)
A:Protein levels of p-p38(Thr180/Tyr182) and p-p53(Ser15);B~C: The protein levels quantified by band gray value ration to GAPDH;B:p-p38, C:p-p53.##P<0.01vscontrol group.**P<0.01vsmodel group
2.4 DGMI降低OGD诱导的SY5Y细胞中的Bax/Bcl-2比率、caspase-9和caspase-3的活性 本研究检测了DGMI对Bcl-2家族蛋白表达的影响。蛋白分析结果显示(Fig 5, 6),缺糖/缺氧损伤后,与OGD组相比,DGMI组Bax含量减少,Bcl-2含量增加,Bax/Bcl-2表达明显下降(P<0.01),procaspase- 3含量明显下降(P<0.05),活化caspase-9和caspase-3水平明显下降(P<0.01),可见其抑制了SY5Y细胞中OGD诱导的p53调控线粒体途径的细胞凋亡。
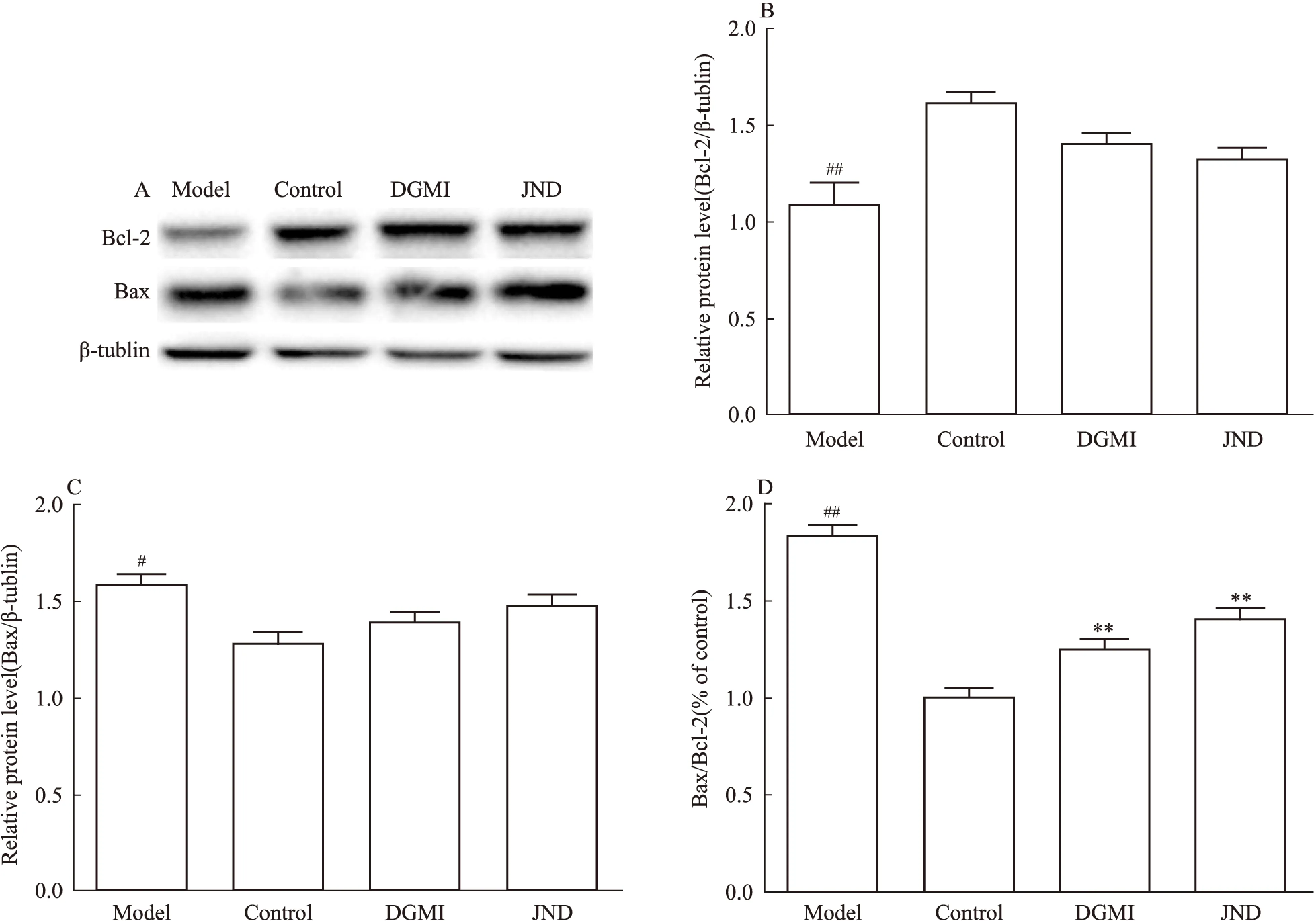
Fig 5 Bax/Bcl-2 ratio of OGD-induced SY5Y cells treated with ±s,n=3)
A:Protein levels of Bcl-2 and Bax; B-D: The protein levels quantified by band gray value ration to β-tublin; B: Bcl-2;C:Bax; D:Bax/Bcl-2 ratio.#P<0.05,##P<0.01vscontrol group;**P<0.01vsmodel group
3 讨论
缺血性脑卒中是常见多发的神经系统性疾病,约占脑卒中的85%[9],是世界范围内危害人类健康的重要疾病之一,体外氧糖剥夺SY5Y细胞模拟这一病征,是研究缺血性脑卒中损伤机制及相关药效机制的重要手段。本研究表明,缺血性卒中发生后,DGMI可浓度依赖性地提高OGD损伤SY5Y细胞活性,减少凋亡比率,且在抑制p38和p53磷酸活化方面,优于含银杏黄酮类的JND。
大量研究结果表明,线粒体在脑缺血神经元凋亡中起着十分重要的作用,被认为是中枢调控者。当神经细胞发生缺血损伤时,至少有3种因素诱导线粒体膜通透性转运孔开放:线粒体内钙离子超载、自由基对线粒体膜的氧化性损伤和产生的能量水平下降[10]。引起细胞色素C进入细胞质,与Adaf-1羧基端的WD-40重复序列结合,激活后者氨基末端的caspase募集区,剪切活化酶原caspase-9,再激活caspase-3引发凋亡[11]。除剪切活化外,脑缺血也可引起caspase-3 mRNA升高,增加表达[12]。在线粒体膜通透性转运孔结构改变中,Bcl-2、Bax等Bcl-2家族成员起着至关重要的作用[13-15],两者比例影响细胞受刺激后是否凋亡。当Bax 高表达时,可形成Bax-Bax 同源二聚体,促进细胞凋亡;当Bcl-2 高表达时,可竞争形成更稳定的Bax-Bcl-2 异源二聚体,封闭Bax 形成孔道的活性,抑制细胞凋亡[16]。
p38/p53通路是机体内调控凋亡的重要信号通路。研究报道活化的p38能促进p53在Ser 15位磷酸化[17.18],从而抑制p53泛素化降解,促进其激活和积累。活化的p53可直接作用于Bax启动子,也可减少Bcl-2的mRNA含量,上调Bax/Bcl-2比率,促进细胞凋亡[19-20]。由本研究结果看出,DGMI可抑制OGD损伤SY5Y细胞p38激酶磷酸化,降低p53的Ser 15位点活化,下调氧糖剥夺引起的Bax/Bcl-2升高,调控线粒体凋亡途径促进在氧糖剥 夺SY5Y细胞的存活。可见,抑制p38/p53通路是DGMI抑制氧糖剥夺SY5Y细胞凋亡的重要机制之一。
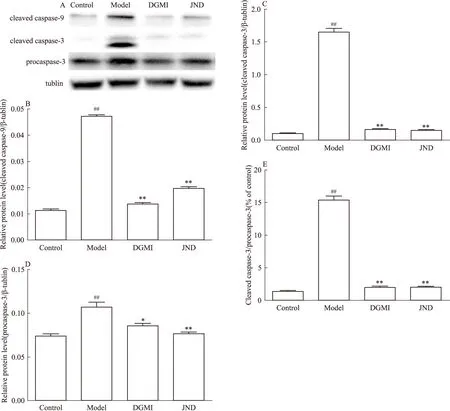
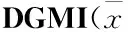
A:Protein levels of cleaved caspase-9 and cleaved caspase-3;B~E:The protein levels quantified by band gray value ration to β-tublin;B:cleaved caspase-9; C:eleaved caspase-3;D:procaspase-3;E:cleaved caspase-3/procaspase-3.##P<0.01vscontrol group;*P<0.05,**P<0.01vsmodel group
(致谢: 本实验全部在江苏康缘药业药理毒理所许治良老师课题组独立完成,感谢我的导师萧伟老师、许治良老师及刘秋研究员在实验思路设计上提出的建议和意见及实验技术上给予的指导!)
[1] Kondratskaya E L, Pankratov Y V, Lalo U V, et al. Inhibition of hippocampal LTP by ginkgolide B is mediated by its blocking action on PAF rather than glycine receptors[J].NeurochemInt,2004,44: 171-7.
[2] Wang X, Qin Z H, Shi H, et al. Protective effect of Ginkgolids (A + B) is associated with inhibition of NIK/IKK/IκB/NF-κB signaling pathway in a rat model of permanent focal cerebral ischemia[J].BrainRes, 2008, 1234: 8-15.
[3] Wang X, Jiang C M, Wan H Y, et al. Neuroprotection against permanent focal cerebral ischemia by ginkgolides A and B is associated with obstruction of the mitochondrial apoptotic pathway via inhibition of c-Jun N-terminal kinase in rats[J].JNeurosciRes, 2014,92(2): 232-42.
[4] Ma W, Hu J, Cheng J, et al. Ginkgolide B protects against cisplatin-induced ototoxicity: enhancement of Akt-Nrf2-HO-1 signaling and reduction of NADPH oxidase[J].CancerChemothPharm,2015, 75(5): 949-59.
[5] Ma S, Liu X, Xun Q, et al. Neuroprotective effect of ginkgolide K against acute ischemic stroke on middle cerebral ischemia occlusion in rats[J].JNatMed,2012, 66(1): 25-31.
[6] 刘 秋, 许治良, 周 军, 等. 银杏二萜内酯葡胺注射液对缺糖/缺氧损伤的SH-SY5Y细胞保护作用[J]. 中国药理学通报,2015, 31(7): 994-9.
[6] Liu Q, Xu Z L, Zhou J, et al. Neuroprotective effects of YXETNZ injection on SH-SY5Y cells against injury induced by oxygen-glucose deprivation[J].ChinPharmacolBull,2015, 31(7): 994-9.
[7] Johnson G L, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases[J].Science, 2002, 298:1911-2.
[8] Kim M J,Lee K H,Lee S J.Ionizing radiation utilizes c-Jun N-terminal kinase for amplification of mitochondrial apoptotic cell death in human cervical cancer cells[J].FEBSJ, 2008, 275:2096-108.
[9] Agudo-Lopez A, Miguel B G, Femandez I, et al. Involvement of mitochondria on neuroprotective effect of sphigosine-1-phosphate in cell death in aninvitromodel of brain ischemia[J].NeurosciLett, 2010, 470(2):130-3.
[10]Venkata P, Anchal G, Suresh L, et al. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunitues[J].MolNeurobiol,2008, 37: 7-38.
[11]Twiddy D, Brown D G, Adrain C, et al.Pro-apoptotic proteins released from the mitochondrial regulate the protein composition and caspase-processing activity of the native Adaf-1/caspase-9 apoptosome complex[J].JBiolChem, 2004, 279(19): 19665-82.
[12]罗 璨, 郭莲军. 牛磺酸对大鼠急性脑缺血神经元凋亡的影响[J]. 中国药理学通报,2005, 9(21): 1057-61.
[12]Luo C, Guo L J. Effect of taurine on acute ischemia-induced neuron apoptosis in rats[J].ChinPharmacolBull,2005, 9(21): 1057-61.
[13]Lan R, Zhang Y, Xiang J, et al. Xiao-Xu-Ming decoction preserves mitochondrial integrity and reduces apoptosis after focal cerebral ischemia and reperfusion via the mitochondrial p53 pathway[J].JEthnopharmacol,2014, 151:307-16.
[14]Cheng C Y, Tang N Y, Kao S T, et al. Ferulic acid administered at various time points against cerebral infaction by activating p38 MAPK/p90RSK/CREB/Bcl-2 anti-apoptotic signaling in the subacute phase of cerebral ischemia-reperfusion injury in rats[J].PLoSOne,2016, 11(5): e0155748.
[15]Anilkumar U, Prehn J H. Anti-apoptotic Bcl-2 family in acute neural injury[J].FrontCellNeurosci,2014, 8: 281.
[16]Hara A, Iwai T. Niwa M, et al. Immunohistochemical detection of Bax and Bcl-2 Protein in gerbil hippocampus following transient forebrain ischemia[J].BrainRes,1996,711(1-2): 249-53.
[17]Gong X W, Liu A H, Ming X Y, et al. UV-induced interaction between p38 MAPK and p53 serves as a molecular switch in determining cell fate[J].FEBSLett,2010, 584: 4711-6.
[18]Lee Y J, Kuo H C, Chu C Y, et al. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells[J].BiochemPharmacol,2003, 66: 2281-9.
[19]Yamaratee J, Anusorn T, Benjawan M, et al. Curcumin I protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis[J].NeurosciLett, 2016, 489(3): 192-6.
[20]He J L, Ji X Y, li Y F, et al. Subchronic exposure of benzo(a)pyrene interferes with the expression of Bcl-2, Ki-67, C-myc and p53, Bax, Caspase-3 in sub-regions of cerebral cortex and hippocampus[J].ExpToxicolPathol, 2016, 68: 149-56.
Involvement of p38-p53 signal pathway in neuroprotective effects of DGMI on SH-SY5Y cells damaged by oxygen-glucose deprivation
LI Xue-ke1,2,LIU Qiu2,3,XU Zhi-liang2,3,ZHOU Jun2,3, CAO Liang2,3, DING Gang2,3,WANG Zhen-zhong2,3, XIAO Wei1,2,3
(1.NanjingUniversityofChineseMedicine,Nanjing210000,China;2.JiangsuKanionPharmaceuticalCo.Ltd,LianyungangJiangsu222001,China;3.StateKeyLaboratoryofNew-techforChineseMedicinePharmaceuticalProcess,LianyungangJiangsu222001,China)
Aim To investigate the protective effects of Diterpene Ginkgolides Meglumine Injection(DGMI) on SY5Y cells damaged by oxygen-glucose deprivation and its functional mechanisms.Methods After 4 h of OGD, the cells were treated with 25 mg·L-1drugs for 1 h. Subsequently, cell viabilities were measured by cell counting kit-8(CCK-8 kit) and cell apoptosis was measured by flow cytometric analysis. Furthermore, the mitochondrial membrane potential was detected by rhodamine123 staining. The levels of phospho-p38, phospho-p53, Bcl-2, Bax and cleaved caspase-9/3 were evaluated by western blot.Results DGMI significantly increased the cell viabilities of SY5Y cells damaged by OGD, and reduced OGD-elicited dissipation of mitochondrial membrane potential and cell apoptosis. Furthermore, DGMI also reduced p-p38,p-p53,Bax/Bcl-2 ratio,cleaved caspase-9 and cleaved caspase-3.Conclusion DGMI shows good neuroprotective effects on SY5Y cells after oxygen-glucose deprivation. The underlying mechanisms may be associated with the suppression of p38/p53/Bcl-2 /caspase-9/caspase-3 signaling pathway.
DGMI; oxygen-glucose deprivation; apoptosis; p38; p53; Bax/Bcl-2
时间:2016-12-5 15:14
http://www.cnki.net/kcms/detail/34.1086.R.20161205.1514.026.html
2016-07-22,
2016-08-29
国家科技部“重大新药创制”科技重大专项资助项目(No 2013ZX09402203)
李雪珂(1993-),女,硕士生,研究方向:药物分子药理学,E-mail: xkl9347@163.com; 萧 伟(1959-),男,博士,教授,博士生导师,研究方向:中药新剂型的研究与开发,通讯作者,E-mail: wzhzh-nj@163.net
10.3969/j.issn.1001-1978.2016.12.013
A
1001-1978(2016)12-1699-06
R282.71;R329.25;R341;R743.31;R845.22