New energetic ingredients for solid rocket propulsion①
2017-01-05LuigiDeLucaIlariaPalmucciAndreaFranzinVolkerWeiserVolkerGettwertNiklasWingborgMaritaSjblom
Luigi T DeLuca, Ilaria Palmucci, Andrea Franzin,Volker Weiser, Volker Gettwert, Niklas Wingborg, Marita Sjöblom
(1. SPLab, Department of Aerospace Science and Technology, Politecnico di Milano, Milan, I-20156, Italy;2. Fraunhofer-Institut für Chemische Technologie (ICT), Pfintzal, D-76327, Germany;3. Swedish Defence Research Agency (FOI), Tumba, SE-14725, Sweden)
New energetic ingredients for solid rocket propulsion①
Luigi T DeLuca1, Ilaria Palmucci1, Andrea Franzin1,2Volker Weiser2, Volker Gettwert2, Niklas Wingborg3, Marita Sjöblom3
(1. SPLab, Department of Aerospace Science and Technology, Politecnico di Milano, Milan, I-20156, Italy;2. Fraunhofer-Institut für Chemische Technologie (ICT), Pfintzal, D-76327, Germany;3. Swedish Defence Research Agency (FOI), Tumba, SE-14725, Sweden)
Two metallized solid rocket propellant formulations, based on either the standard AP/HTPB matrix commonly used in space propulsion or the new matrix ADN/GAP, were analyzed. The two formulations in terms of ideal thermochemistry and experimental combustion properties were compared. Thus, a dual-oxidizer system bound by an active binder and loaded with a dual metal fuel was proposed as optimum formulation for space exploration missions. For all configurations, only laboratory level testing was discussed. For motor applications, full-scale testing is needed to ensure a complete mastering of the process.
solid propellant;solid oxidizer;metal fuel;steady burning rate
固体火箭推进新型含能组分
Luigi T DeLuca1, Ilaria Palmucci1, Andrea Franzin1,2
Volker Weiser2, Volker Gettwert2, Niklas Wingborg3, Marita Sjöblom3
0 Introduction
Solid rocket propulsion remains one of the most attractive options for low-cost access to space. This paper describes work under progress for a cooperative European project[1-3]. Only laboratory level results are reported, motor application state is still under development[4]. Three main families of metallized composite solid rocket propellants are discussed: standard AP (Ammonium Perchlorate) or PSAN (Phase Stabilized Ammonium Nitrate) and innovative ADN (Ammonium Dinitramide)-based formulations. Systematic work on ADN propellants, including small-scale motor testing, has been performed for years at the Swedish Defence Research Agency (FOI)[5-6].
A comparative analysis in particular between the AP/HTPB and ADN/GAP solid propellant systems in terms of ideal thermochemistry and experimental combustion properties, with respect to a standard solid propellant certified for space flights, will reveal advantages and disadvantages of both. Within this broad framework, innovative formulations are characterized under a variety of operating conditions.
After recalling properties of the main ingredients under consideration (inorganic oxidizers, metallic fuels, and binders), performances of the composite solid rocket propellants actually tested are discussed in detail. The paper conveniently expands on some recent results by the authors[7-10], including assessments on aggregation/agglomeration phenomena relevant to two-phase (2P) flow losses. Recommendations for future work to overcome some ballistic difficulties connected with the use of ADN-based formulations complete the paper. The long-term objective of the HISP project (High performance solid propellants for In-Space Propulsion) of the European Community's Seventh Framework Program (FP7/2007-2013) is to identify a viable high-performance propellant for space exploration. This international joint research effort is coordinated by the Swedish FOI and experimental activites comprise specialized laboratories from several European countries, including Russia.
1 Main energetic ingredients
Different ammonium-based solid crystalline inorganic oxidizers (see Sec. 1.1), metal fuels (different variants of Al and alane AlH3, see Sec. 1.2), and binders (both inert and active, see Sec. 1.3) are under study.
1.1 Solid oxidizers
Candidate oxidizers for solid rocket propulsion are the ever green AN (or PSAN), the ubiquitous AP, and the promising ADN. Their main features as monopropellants are listed in Table 1;Tfis the self-deflagration adiabatic temperature at 6.8 MPa. With respect to AP, AN costs less while ADN costs much more. While AP suffers a negative impact on the environment and personal health, AN and ADN are green oxidizers. Due to its overall unfavorable ballistic properties (hygroscopic, high Pressure Deflagration Limit (PDL), low burning rate and little affected by particle size but strongly sensitive to pressure and initial temperature), AN is mainly used in gas generators (low flame temperature). Being totally gasifiable and nonpolluting, AN is also valued as a (co-)oxidizer but requires phase stabilization. While AN and AP are both well-known and have been used for several decades for a variety of tasks, ADN is a relatively new product whose ideal gravimetric specific impulse neatly overcomes that of the other two oxidizers. For example, see Fig.1 for the generic formulation Oxidizer/GAP expanding from 7 MPa to vacuum with nozzle area rationε=40. A literature survey of ADN important facts was recently offered[10]. As a monopropellant ADN features high burning rates and fair pressure sensitivities, low signature, and full environmental respect. The high burning rate well evidenced in Fig.2 is a drawback for space exploration applications; the shadowed area is where data scattering is concentrated. Note also the much lower burning rates and much larger PDL of AN as a monopropellant. Intermediate values of burning rates and PDL are observed for AP as a monopropellant.
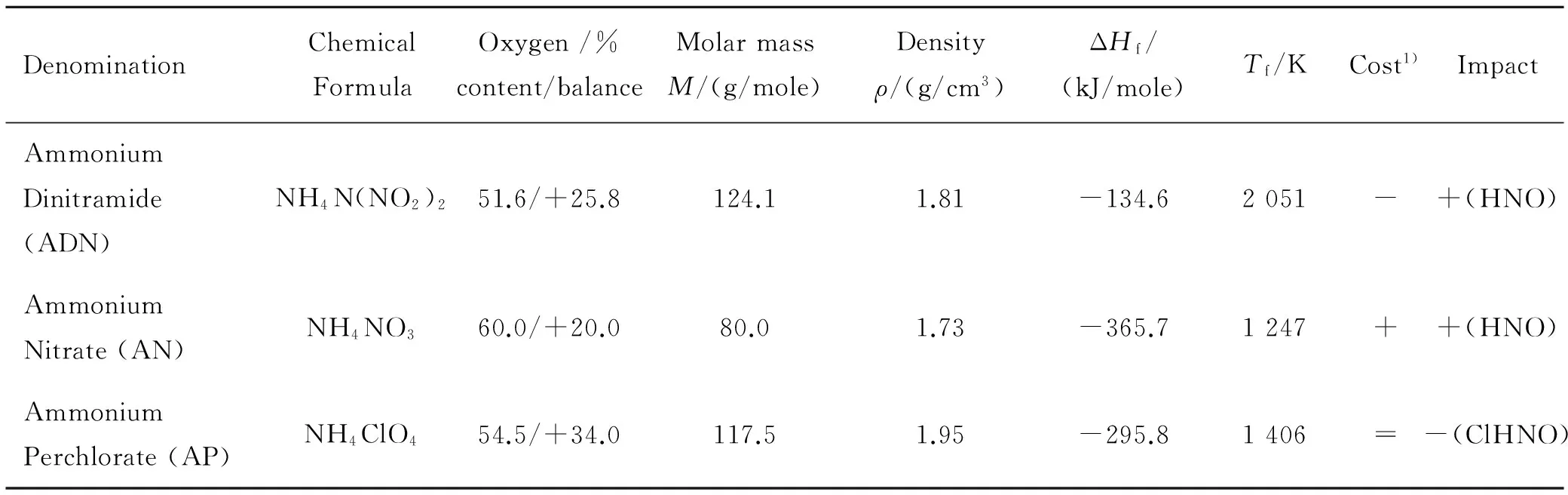
Table 1 Solid oxidizers tested in this investigation[9]
1)=,is the reference value;-,less good than reference;+,better than reference (qualitative scale).

Fig.1 Comparing vacuum Is of indicated solid oxidizers under standard operating conditions

Fig.2 Comparing steady rb of several solid rocket ingredients or propellants
1.2 High-energy metal fuels
The volumetric heat release is maximum for B, followed by Al and Zr; while the gravimetric heat release is maximum for B, followed by Al and Mg. The metal melting temperature is much less than that of the corresponding oxide for Al and Mg, while the opposite is true for B. The peculiar properties of B hamper its efficient combustion and nozzle expansion. Thus, since a longtime, micron-sized propulsion grade Al (μAl) de facto plays the role of reference metal in motor applications[2,9]. Tested variants include μAl, chemically activated μAl (C-ActAl), mechanically activated μAl (M-ActAl), micron-sized aluminum hydride (AlH3), and nano-sized Al (nAl).The density of AlH3(1.477 g/cm3) is much less than that of Al (2.70 g/cm3). The α crystal phase of AlH3is compatible with most ingredients of common use, conducive to superior ballistic properties, prone to excellent performance of solid rocket motor at upper stages, and can be very stable in time. However, stabilization ofα-AlH3is an essential pre-requisite to practical applications. Today, the proper stabilization technique is known only by Russia and not shared.
To achieve the best results, systematic experimental analyses[11-12]suggest a dual-mode Al mixture, (μAl+nAl) or (μAl+AlH3), synergistically exploiting each component. At any rate, the aggregation/ agglomeration phenomena associated with metallic fuel combustion at or near the burning surface are an important factor in evaluating the actually delivered specific impulse and thus deciding the exact composition of the metal fuel.
1.3 Binders
Binders are an essential ingredient for castable composite propellants. They are needed to bind the propellant solid particles (oxidizers and metal, if present) and attain the wanted mechanical properties. Starting in the late 1970's, HTPB became the material of choice for a variety of commercial applications; a “propulsion grade” HTPB is tested in the USA as of this writing. A standard HTPB R-45M polybutadiene prepolymer cured with IPDI is the implemented inert binder[9]. Different curing techniques are implemented for GAP. At ICT a dual curing is applied consisting in a pre-cure with HDI or Desmodur E305 and then in a crosslink reaction with bispropargyl succinate (BPS). At FOI a more conventional isocyanate curing system is used.
2 Tested solid rocket propellant formulation
The metallized composite solid rocket propellant taken as baseline is a standard AP/HTPB/Al formulation 68/14/18 (mass fraction). The laboratory reproduction of a flight certified formulation, containing bimodal AP (200 μm coarse particles and 10 μm fine particles) and a propulsion grade μAl (μAl-30, having 30 μm nominal average grain size), is taken as a benchmark.
2.1 HTPB-based composite propellants tested
Many formulations are tested but keeping in general fixed the reference composition oxidizer/HTPB/metal = 68/14/18 (mass fraction).
2.1.1 Steady burning rate AP/HTPB/metal
A comparison of the main AP/HTPB/Al formulations tested in this investigation was recently discussed and the corresponding Vieille ballistics parameters were summarized[9]. Ideal thermochemistry and a variety of practical considerations point out Al as the most suitable metal fuel. However, the μAl powder commonly used in propulsion applications exhibits drawbacks, such as difficulties in ignition, slow burning, formation of agglomerates, and therefore loss of actually delivered performance. Enhancement of fuel powder reactivity is a possible way to mitigate performance losses.
In this respect, interesting results are in general obtained using nano-sized Al (nAl) instead of μAl; but initial results with ADN/GAP formulations are below expectations. Being characterized by a higher specific surface area, nAl is capable of increasing the propellant burning rate and contemporarily reducing the CCP size. The burning rate increase is essentially controlled by the specific surface area (BET) of the Al particles. Under common operating conditions, the normalized burning rate value with respect to μAl rapidly approaches 100% for increasing BET area and slightly augments for increasing pressure[12]. At any rate, only moderate agglomeration effects are observed for nAl. Unfortunately, the lower metal content and particle clustering typical of nAl also cause a loss of the ideal specific impulse, that could further be influenced by the presence of coatings. Moreover, the high costs and health hazards connected with this ingredient prevent its wide use in space propulsion. A remedy to these difficulties is offered by optimized dual metal formulations (such as μAl+nAl), under real operating conditions, which can lead to improving ballistic properties and mitigating all together the vexing effects on active metal content, propellant viscosity, CCP formation, and final cost.
A further alternative is offered by activated aluminum[11]. The activation process can be executed mechanically or chemically leading to a fair increment of particle reactivity. Since the powder size is kept micrometric, the metal content after activation remains relatively high at a level depending on the specific treatment. While the general target of activation processes is to increase the final product reactivity, the specific approach ranges from chemical to mechanical or mechanochemical procedures. In chemical treatments, the method consists in weakening the external oxide shell contemporarily depositing substances like metals or chemicals based on fluorine. In this case, the original particle shape is preserved and the reactivity increment is mainly obtained by additive-particle proximity. In mechanical activation by ball-milling, reactivity enhancement is obtained by deforming the original particles and embedding a specific additive like PTFE, metal oxides, and so on. In this case the particle shape is strongly modified passing from spherical to flake. The amount of additive depends on the specific application; however, it is generally higher than 10% by mass.
Activation techniques allow to obtain an increment of powder reactivity leading to a reduction of agglomerate size, the main responsible of specific impulse loss. A simultaneous increment of propellant burning rate is observed as well, but the global effect is strongly dependent on both the reference Al and the specific activation treatment; see Fig.3. An experimental campaign, recently carried out at SPLab, demonstrate that the effects of activated Al on uncured solid propellant viscosity is minimal and generally comparable to those obtained using dual metal fuels (μAl+nAl). Finally, the lower volatility of this kind of ingredients reduces handling and health hazards and therefore the costs of risk prevention with respect to nAl. Yet a systematic campaign to evaluate health and explosion risks of activated Al powders is still missing.
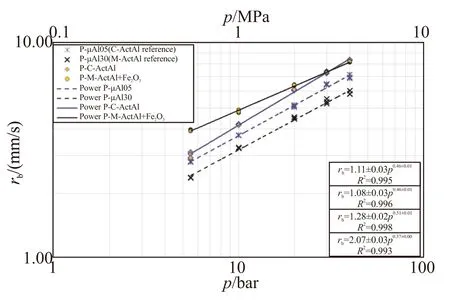
Fig.3 Steady burning rate vs pressure of samples
The samples tested in Fig.3 were loaded with spherical aluminum,μAl-05 (5 μm) and μAl-30 (30 μm), chemically activated aluminum, C-ActAl, and mechanically activated aluminum by ball milling with iron oxide, M-ActAl + Fe2O3.
In spite of its low density, another valuable high-energy metallic fuel is the stabilized AlH3which allows at the same time a sensible increment of ideal specific impulse and reduction of the burning rate pressure sensitivity.
Overall, for all tested AP/HTPB/Al formulations, burning rates at 7 MPa fall in the range 8 to 14 mm/s, except for the stabilized alane formulation close to 24 mm/s. Pressure sensitivity is only slightly affected with respect to baseline (n=0.46), while pressure sensitivity is appreciably mitigated by the alane formulation (n=0.33)[2,9,11].
2.1.2 Steady burning rate (AP+PSAN)/HTPB/metal
AN combustion is characterized[13]by a low stability level: the values of PDL and critical diameter are in general much higher than the corresponding values for double base and composite propellants. This behavior results from a liquid layer which comprises a mixture of melted AN, water, nitric acid, ammonia, nitrogen oxides, and some other less pronounced constituents. Reactions in this layer go on slowly and release the correspondingly low quantities of heat at relatively low temperature.
Thus, double-oxidizers such as (AP+PSAN)/HTPB/Al (40+28)/14/18, with PSAN in turn including 5% phase stabilizer, were tested as reported in Table 2[14]. The measured burning rates are shown in Fig.4 and fall in the low range 4 to 8 mm/s at 7 MPa, with pressure sensitivityn=0.58~0.64 for different stabilizers. The pressure dependence of pure AN, doped with small amounts of additive to make it burn, may be appreciably higher[13-15]. Testing conducted with 50 m Al flakes produce very large agglomeration.
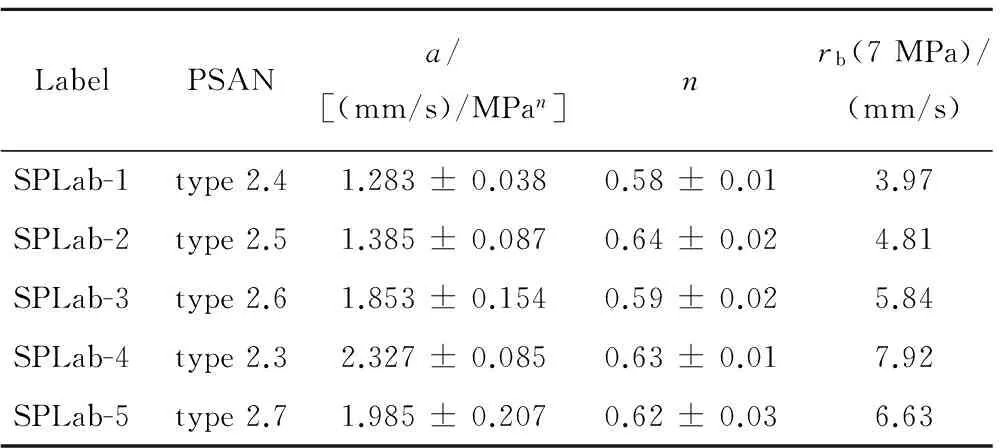
Table 2 Tested AP+PSAN metallized formulations

Fig.4 Comparing steady burning rates of (PSAN+AP)/ HTPB/Al formulations
2.1.3 Steady burning rate ADN/HTPB/metal
Steady ballistics of the formulation ADN/HTPB/μAl were investigated at FOI and TNO, and the results point out moderate burning rates (13.1 to 14.7 mm/s at 7 MPa) with a large pressure sensitivity (n=0.87 to 0.91)[9].
2.2 GAP-based composite propellants tested
2.2.1 Steady burning rate ADN/GAP
ADN/GAP 70/30 propellants, with bimodal oxidizer prills (85% 198 μm + 15% 14 μm) were manufactured and tested at FOI. Runs were recently conducted without or with Al addition, as shown in Table 3 and Fig.5. Confirming previous results[5], a fair pressure sensitivity (n=0.495) and a large burning rate (≈ 24 mm/s at 7 MPa) were found. Further results were obtained at ICT by Gettwert et al[16]with bimodal ADN/GAP 76/24 propellants: a somewhat reduced pressure sensitivity (n=0.370) but larger burning rate (27 mm/s at 7 MPa) were found, as shown in Table 3 and Fig.6. Essentially similar results were found by Menke[17]again at ICT testing ADN/GAP formulations loaded with HMX.
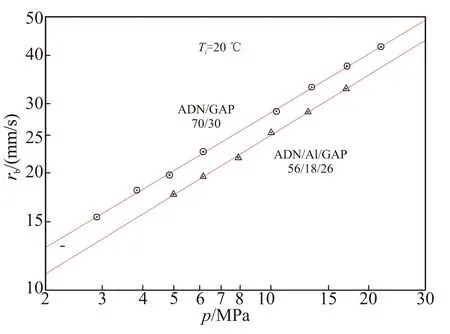
Fig.5 FOI steady burning rate vs pressure of bimodal ADN/GAP propellants with and without μAl

Fig.6 ICT steady burning rate vs pressure of ADN/GAP/ μAl (15c) and ADN/GAP (15h) propellants
In spite of many different details, several variants[5,16-17]of the basic ADN/GAP formulation share similar ballistic properties: burning rates are large but with an acceptable pressure sensitivity in the rangen=0.37~0.52.
Replacing AP with ADN leads to increasing burning rate and pressure sensitivity[16]. While impact and friction sensitivities of ADN/GAP propellants equal those of AP/GAP, sensitivity to detonation is significantly higher (1.1 hazards classification).
2.2.2 ADN grain size effects
Additional tests of ADN/GAP 70/30, with monomodal oxidizer prills or raw crystals, were carried out up to 13 MPa by Franzin[8]in his MSc. thesis for PoliMi conducted at ICT. The several ADN grain sizes tested are listed in decreasing order in Table 4. Only samples H60 contain raw crystals. Sample H58, containing prilled particles of 272 μm, suffered manufacturing problems. Other samples include ADN prill sizes ranging from 212 μm down to 40 μm.
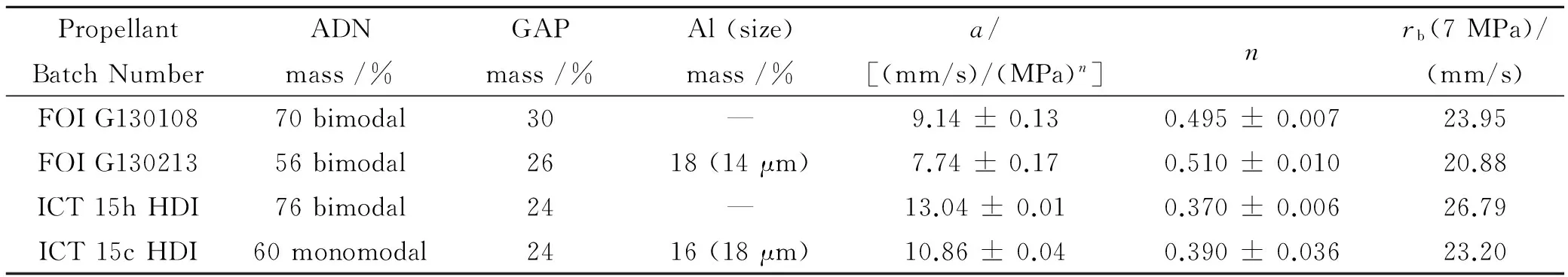
Table 3 Composition, properties, and Vieille parameters ADN/GAP with or without Al
The obtained steady burning rates are displayed in Fig.7. At 1 MPa all values are around 9~10 mm/s. But the ballistic response afterward changes with increasing pressure: samples containing raw ADN show the highest pressure sensitivity (n=0.79) likely for a shape effect; the two samples containing large ADN prills show less pressure sensitivity (n=0.61 and 0.66); and the two samples containing fine ADN prills exhibit the lowest pressure sensitivity (n=0.47 and 0.52). Samples with prill sizes of 212 and 153 μm virtually feature the same burning response to pressure; likewise, the two samples with prill sizes of 55 and 40 μm. Except raw crystals, crossover of all burning rate curves occurs around 2~3 MPa.
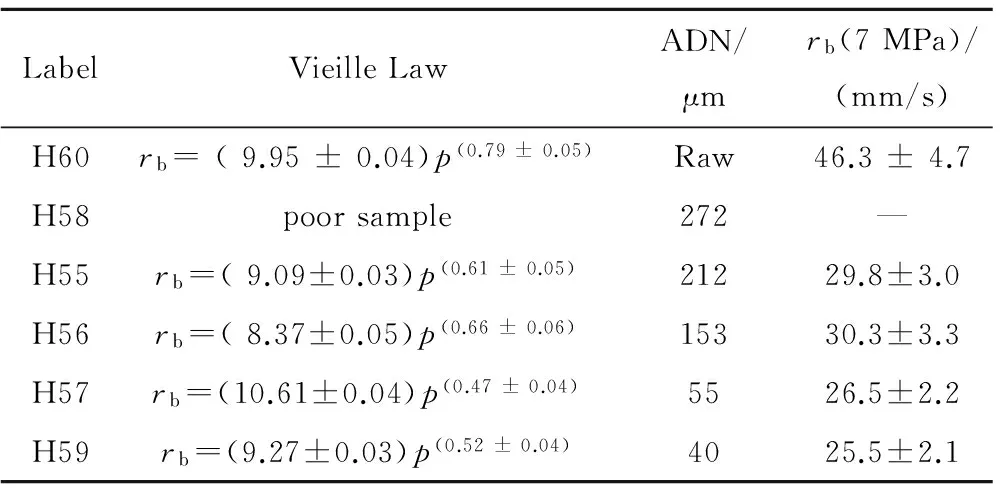
Table 4 Vieille ballistics of monomodal ADN/GAP 70/30 propellants tested at ICT
The obtained Vieille parameters are summarized in Table 4 and point out a strong effect of the particle shape factor: while for prilled ADNnis in the range 0.47~0.66 andrb=26~30 mm/s at 7 MPa, for raw crystalsn=0.79 andrb=46 mm/s. A more important conclusion, within the measurement uncertainties and taking into account the corresponding Vieille multiplicative factors, is that increasing ADN prill size implies a direct increase of the pressure sensitivity and therefore, indirectly, of the burning rate as well.

Fig.7 ICT steady burning rate vs pressure of monomodal ADN/GAP solid propellants
This interpretation is confirmed by the experimental results provided by Shang and Huang[18], showing a dramatic increase of the pressure sensitivity from 0.49 to 1.05 for PGN (polyglycidyl nitrate)/ADN if ADN size increases from <450 μm to 450~900 μm. A further evidence was recently provided by Pang[19]for NEPE (nitrate ester plasticized polyether)/ADN: pressure sensitivity increases from 0.74 to 0.88 for ADN size increasing from 167 μm to 382 μm, while an intermediate value of 0.80 is found for a bimodal (50%~50%) ADN distribution. Therefore, the well-known prediction by Pak[20]could be considered as validated in this broader sense.
2.2.3 Steady burning rate ADN/GAP/metal
With respect to ADN/GAP, results at FOI[5]testing 14 μm Al and over a 3~24 MPa pressure range indicate a slight decrease of burning rate and a slight increase of pressure sensitivity, as shown in Fig.5 and Table 3. Similar results were obtained at ICT[16], with 18 μm Al and over a 2~23 MPa pressure range, but loading slightly more monomodal 228 μm ADN by FOI,as shown in Fig.6 and Table 3.
A systematic analysis over the pressure range 1~15 MPa was carried by Franzin[8]by keeping the formulation 60/24/16 and testing 18 μm Al in the following configurations:
H27, a sample using monomodal 228 μm ADN by FOI;
H31, two monomodal 208 μm ADN samples to study the effects of Al powder sizes (5 μm for H31-D against the standard 18 μm for H31-G);
H32, the target formulation using a bimodal ADN prill (70% 208 μm + 30% 55 μm).
Measured burning rates are shown in Fig.8 and ballistic results summarized in Table 5. Samples H31-D and H31-G show a similar burning response: excluding results at 0.1 MPa, pressure exponents fall in the upper band withn=0.52 for H31-D andn=0.64 for H31-G, with a crossover at 3MPa. An intermediate value of 0.57 and 0.58 was respectively obtained for the slightly larger ADN monomodal H27 and the ADN bimodal H32. Thus, for the explored experimental conditions, increasing the size of Al (18 μm instead of 5 μm) leads to larger pressure sensitivity.
建设全市空地一体化业务管理体系,就是从体制机制、业务管理上着手,建成以自治区级为依托、市级为核心、旗县为支撑、作业点为基础的四级业务管理体系,飞机、火箭、烟炉、高炮为主的空地联合交叉式作业模式,既避免了资源的浪费,又提升了作业科技水平和作业效益,到2020年,争取实现年度增雨量达到3亿吨,防雹保护面积覆盖全市耕地总面积的30%左右。
However, results in Fig.8 do not fully match the findings in Fig.6. Indeed, for the same nominal formulation 60/24/16 (with monomodal 228 μm ADN prills by FOI and 18 μm Al) burning rates at 7 MPa, are 25.3 mm/s for H27 and 23.2 mm/s for 15c HDI, while pressure sensitivities aren=0.57 for H27 and 0.39 for 15c HDI. For H31-G, very similar to H27 (208 μm ADN ICT is used instead of 228 μm ADN FOI), burning rate further increases to 33.0 mm/s and pressure sensitivity increases ton=0.64. This may be due to a combination of several minor changes in propellant manufacturing and, if so, it testifies a pronounced sensitivity of the ballistic response to manufacturing details. The different error bounds reflect ascertained handling differences including:
(1) amount of isocyanate curing agent;
(2) use of glycidyl propagyl ether (1% in formulation 15h and 0.5 % in 15c);
(3) presence of HDI in both formulations 15h and 15c;
(4) amount of GAP-diol before reaction.
2.3 AlH3effects on tested composite propellants
In addition to several Al variants, stabilized alane AlH3was also tested, in view of its highly attractive[21-22]propulsive features (used indeed in the SS-24 third stage). Under all explored circumstances, AlH3appreciably mitigates the burning rate pressure dependence. Alane fast dehydrogenation at the burning surface leaves behind porous Al crystals ready to react, removing heat release from the gas phase to the burning surface and thus decreasing pressure sensitivity. See Fig.3[9]for AP/HTPB formulations and Fig.9 in this paper for ADN/GAP formulations.

Fig.8 Comparing steady burning rates of ADN/GAP/μAl solid propellants
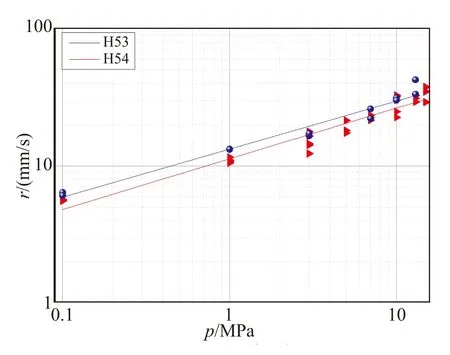
Fig.9 Comparing steady burning rates of bimodal ADN/GAP/AlH3 solid propellants
In the case of AP/HTPB, also an impressive increase of burning rate by a factor of 2 is achieved. In the case of ADN/GAP samples H53 and H54, containing AlH3with a bimodal ADN prill (70% 208 μm + 30% 55 μm), only a minor influence on burning rate is revealed. In spite of the appreciable change in formulation from 16% alane for H53 to 26% for H54, steady burning rates are quite close (24~26 mm/s at 7 MPa), with the 16% sample a bit faster.
2.4 Comparative remarks on steady ballistics
Overall, under the explored operating conditions, no one of the tested formulations looks readily suitable for space propulsion applications: either burning rates are too high because of fast ADN self-deflagration or pressure sensitivity is too large probably due to some effects in the gas-phase flame structure.

Table 5 Vieille ballistics of metallized (Al or Alane) 60/24/16 formulations tested at ICT
1)Bimodal ADN is 70% 208 μm + 30% 55 μm;2)H54 formulation is 52/22/26 (mass fraction).
3 Incipient agglomeration phenomena
Incipient agglomeration phenomena for ADN-based formulations were investigated at ICT in cooperation with PoliMi[7-8,10]. Tests by high-resolution and high-speed video recording were conducted up to 5 MPa for several ADN/GAP/Al propellants. Representative results for the indicated ADN prills and μAl (18 and 5 μm) are shown in Fig.10. Overall, the achieved experimental results essentially point out a phenomenology similar to that already observed for standard AP/HTPB aluminized formulations: a vanishing dependence of the average incipient agglomerate size on μAl particle size (no detectable difference for 18 or 5 μm Al) in contrast with a prominent dependence on ADN particle size (large ADN prills yield large agglomerates) and a distinct decrease for increasing pressure (agglomerate sizes decrease from about 265 μm at ambient pressure to less than 150 μm at 5 MPa). Also, bimodal ADN prill size is shown in Fig.10 to yield larger agglomerates than the monomodal one. In spite of the much faster burning rates and different surface layer structures, the average incipient agglomerate sizes for ADN/GAP/Al are the same order as that of the standard AP/HTPB/Al formulations. However, the surface layer of ADN/GAP/Al formulations appears quite distinct from that of the standard aluminized formulations. Since ADN is a chlorine free oxidizer and GAP generates mainly nitrogen in its exothermic decomposition, Al particles emerging at the burning surface are exposed to drastically different thermal and chemical fields. Moreover, the molten polymer viscosity is different for the two configurations.

Fig.10 Average incipient agglomerate diameters for ADN/GAP/Al propellants showing a sensible decrease for increasing pressure and no dependence on the tested μAl powders
4 Searching for a dual-oxidizer system
Based on the experimental results so far obtained in this investigation and literature findings, the following guidelines are envisaged to achieve ballistic properties suitable to space access/exploration applications.
ADN-based dual oxidizers, such as (ADN+AN) or (ADN+AP), are suggested to slow down large burning rates. Recent results by Pang et al[23]indicate a decrease of burning rate as well as pressure sensitivity for AP replacing ADN: for (AP+ADN)/HTPB/Al 64/13/18 pressure sensitivity over 1~15 MPa decreases from 0.71 for (44% AP+20% ADN) to 0.41 for 64% AP. Some further evidence for AP replacing ADN was offered by Menke[17]testing the mixture AP/HMX. Similar effects for AN replacing ADN were pointed out by Strunin[2,4]long ago. However, it should be borne in mind that while ideal specific impulse is only slightly depressed, see Fig.11 and Fig.12(comma needed instead of dot). AP implies chlorine in the exhausts while AN needs a phase stabilizer. Past investigations on dual-oxidizer propellants show that (AP+PSAN) feature a faster burning and a lower PDL than the PSAN-based compositions[13]. In addition, the size of agglomerates for PSAN sensibly decreases with respect to AN, for which the size could grow up to the order of mm. On the whole, the dual-oxidizer (AP+PSAN) system effectively behaves as a single oxidizer with AP providing high burning rates and low PDL. A similar good synergy is hoped for ADN-based dual oxidizers.
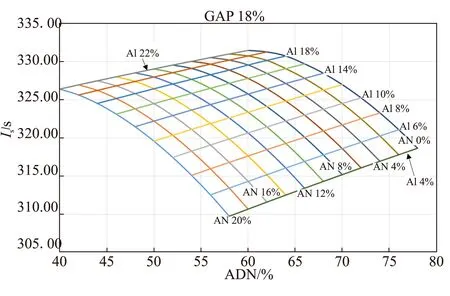
Fig.11 Ideal thermochemistry for dual (ADN+AN) oxidizer with Al as fuel

Fig.12 Ideal thermochemistry for dual (ADN+AP) oxidizer with Al as fuel
Ideal gravimetric specific impulse values computed for the dual-oxidizer formulations (ADN+AN)/GAP/Al and (ADN+AP)/GAP/Al are respectively reported in Fig.11 and Fig.12. For example, for the reference formulation ADN/GAP/Al 60/18/22 a 20% replacement of ADN (1/3 of the oxidizer) implies a decrease ofIsfrom 331.5 s to 326.4 s for AN (-1.50%) and to 327.1 s for AP (-1.35%). Similar values are found for AlH3. However, taking into account AN phase stabilizers might affect this picture.
As for all composite energetic materials, controlling the grain size distribution is a recommended approach for achieving suitable ballistic properties. Small ADN prill size (and large AP crystals, if any) should allow decreasing large pressure sensitivities and slowing down burning rates[8,18-19].
Further support in achieving suitable ballistic properties might be provided by the addition of Oxamide, NH2—CO—CO—NH2, to slow down large burning rates and decrease pressure sensitivity. Recent results by Shang and Huang[18]pointed out for PGN/ADN a dramatic decrease of pressure sensitivity from 0.49 to 0.22 due to a slowdown of burning rates in the pressure range above about 7 MPa (balancing a somewhat increase below 7 MPa). Oxamide is a well-known coolant commonly used to slow down large burning rates, see for example applications to HMX and AP[25-26].
5 Concluding remarks
A dual-oxidizer system, (ADN+AP) or (ADN+AN), bound by a proper binder and loaded with a dual-metal fuel, for example (μAl+nAl) or (μAl+AlH3), is proposed as a promising formulation for space exploration missions. An accurate control of grain size distributions, maybe supported by suitable additives, is recommended to achieve satisfactory ballistic properties. Appropriate combinations of oxidizers and fuels will help in fine tuning the selected formulations in terms of ballistic, mechanical, and rheological properties. A great care is anyway needed to assure at the same time reasonable costs, processability, and handling. Testing is in progress along the above guidelines.
Acknowledgements: The authors gratefully acknowledge the crucial contribution of ENI Donegani Institute, Novara, Italy in performing advanced powder analyses. The authors also wish to thank AVIO, Italy for providing μAl samples; FOI, Sweden for actAl samples; and STK, Russia for nAl samples. This work was partially supported by the HISP project (High performance solid propellants for In-Space Propulsion) of the European Community's Seventh Framework Programme (FP7/2007-2013), under Grant Agreement No. 262099, coordinated by FOI.
[1] Wingborg N. 5th EuCASS propulsion physics conference[C]//Munich Germany,01-05 Jul 13,Paper PP.10.04.
[2] Maggi F,Dossi S,Reina A,et al. 5th EuCASS propulsion physics conference[C]//Munich Germany,01-05 Jul 13,Paper PP.10.06.
[3] Weiser V,Franzin A,et al. 5th EuCASS propulsion physics conference[C]//Munich Germany,01-05 Jul 13,Paper PP.10.08.
[4] Calabro M. 5th EuCASS propulsion physics conference[C]//Munich Germany,01-05 Jul 13,Paper PP.10.05.
[5] Wingborg N,Andreasson S,et al. Development of ADN-based minimum smoke propellants[R]. AIAA 2010-6586.
[6] de Flon J,Andreasson S,Liljedahl M,et al. Solid Propellants based on ADN and HTPB[R]. AIAA 2011-6136.
[7] Weiser V,DeLuca L T,Franzin,A,et al. Combustion behavior of aluminum particles in ADN/GAP composite propellants[R]. HEM Workshop,Tokyo,Japan,07-09 Oct 13.
[8] Franzin A MSc. Thesis Politecnico di Milano[R]. Milan,Italy,20 Dec 13.
[9] DeLuca L T,Maggi F,Dossi S,et al. High-energy metal fuels for rocket propulsion: characterization and performance[J].Chinese Journal of Explosives & Propellants,2013,36(6):1-14.
[10] DeLuca L T,Maggi F,Dossi S,et al. Survey of aggregation and agglomeration phenomena in solid rocket propulsion [R]. HEM Workshop,Tokyo,Japan,07-09 Oct 13.
[11] Dossi S,Reina A,Maggi F,et al. Innovative metal fuels for solid rocket propulsion[J]. Int. J. Energetic Materials. Chem. Prop.,2012,11(4):299-322.
[12] De Luca L T,Galfetti L,Colombo G,et al. Microstructure effects in solid rocket propellants[J]. Journal of Propulsion and Power,2010,26: 724-733.
[13] DeLuca L T,Galfetti L,Severini F,et al. 2nd international conference on green propellants for space propulsion (ESA SP-557)[C]//Chia Laguna,(CA),Italy,2004:23.
[14] Babuk V A,Glebov A,Arkhipov V A,et al. Dual-oxidizer solid rocket propellants for low-cost access to space[R].10-IWCP,Lerici,Italy,2005:15.
[15] Sinditskii V P,Egorshev V Yu,Levshenkov A I,et al. Ammonium nitrate: combustion mechanism and the role of additives [J]. Propellants,Explosives,Pyrotechnics,2005,30(4):269-280.
[16] Gettwert V,Fischer S,Menke K. 44th international annual conference of ICT[C]//Karlsruhe,Germany,2013:57.
[17] Menke K,Heintz T,Schweikert W,et al. Formulation and properties of ADN/GAP propellants[J]. 2009,34:218-230.
[18] Shang Dong-qin,Huang Hong-yong. Combustion properties of PGN/ADN propellants [J].Chinese Journal of Energetic Materials,2010,18(4):372-376.
[19] Pang W Q,DeLuca L T,Fan X Z,et al. Effects of ADN on the properties of NEPE solid propellant[C]//International Workshop on New Energetic Materials and Propulsion Techniques for Space Exploration. Proceedings of 12-IWCP,Paper 07-03,Politecnico di Milan,Milan,Italy,2014,9-10.
[20] Pak Z P. Some ways to higher environmental safety of solid rocket propellant application[R].AIAA 93-1755.
[21] DeLuca L T,Galfetti L,Severini F,et al. Physical and ballistic characterization of AlH3-based space propellants [J].Aerospace Science and Technology,2007,11(1):18-25.
[22] DeLuca L T,Rossettini L,Kappenstein C,et al. Ballistic characterization of AlH3-based propellants for solid and hybrid rocket propulsion [R]. AIAA 2009-4874.
[23] Pang W Q,et al. Effect of ammonium dinitramide (ADN) on the characteristics of hydroxyl terminated polybutadiene (HTPB) based composite solid propellant [J]. Journal of Chemical Science and Technology,2013,2(2):53-60.
[24] Strunin V A,D'Yakov A P,Manelis G B. Combustion of ammonium dinitramide [J]. Combustion and Flame,1999,117:429-434.
[25] DeLuca L T,Caveny L H,Ohlemiller T J,et al. Radiative ignition of double-base propellants I. Some formulation effects [J].AIAA Journal,1976,14(7): 940-946.
[26] Klager K,Zimmerman G A. Steady burning rate and affecting factors: experimental results [J]. AIAA PAAS,1992,143:59-109.
(编辑:薛永利)
(1. 米兰理工大学 航天科技系,米兰 I-20156,意大利;2. 弗劳恩霍夫化学技术研究所,D-76327,德国;
3.瑞典国防研究机构,通巴,SE-14725,瑞典)
针对2种含金属粉的固体推进剂——用于空间推进的标准AP/HTPB推进剂及ADN/GAP新体系的推进剂,分析了其配方,比较了这2种配方的理论热化学和实验燃烧性能的优缺点。分析结果表明,含双金属燃料和双氧化剂及活性粘合剂的体系被认为是空间探测任务的优化配方。仅讨论了实验室水平的测试,对于发动机的应用,需要进行全尺寸测试,以确保全面掌握此过程。
固体推进剂;固体氧化剂;金属燃料;稳态燃速
V512 Document Code:A Article ID:1006-2793(2016)06-0765-10
10.7673/j.issn.1006-2793.2016.06.006
①Receivied date:2016-09-28。
Biography:Luigi T DeLuca(1944—),male, PhD in aerospace and mechanical sciences from Princeton university, Princeton, USA; metalized solid propellants and novel propellant formulations for rocket propulsion.E-mail:luigi.deluca@polimi.it
