联合使用脱甲基化药物和紫杉醇对肾癌细胞的侵袭能力影响机制研究
2017-01-04杨凯钧朱斌潘卫兵谢礼仁
杨凯钧 朱斌 潘卫兵 谢礼仁
联合使用脱甲基化药物和紫杉醇对肾癌细胞的侵袭能力影响机制研究
杨凯钧 朱斌 潘卫兵 谢礼仁
目的 观察联合使用脱氧杂胞苷(DAC)与紫杉醇(PTX)对肾患细胞是否存在协同作用,并探究其机制。方法离体培养肾透明细胞癌ACHN细胞株,将120培养皿随机分为空白对照组、DAC组、PTX组、联合组,每组30个。DAC组:用DAC:0.5、1、2、4、8 μmol/L处理 3 d;PTX组:用 PTX:1、2、4、8、16 nmol/L处理 3 d;联合组:用DAC+ PTX处理 3 d。使用高速分选流式细胞仪观察T肽协同树突状细胞对肾癌细胞的凋亡率;通过ELISA检测淋巴细胞增强因子(LEF1)和E-cadherin在肾癌细胞中的表达;通过ELISA技术检测凋亡蛋白caspass-3在肾癌细胞中的表达。结果DAC组、PTX组以及联合组对肿瘤细胞的抑制作用明显,其中联合组对肿瘤细胞的抑制作用最强(P<0.05),而且DAC和PTX对肿瘤细胞的抑制作用与药物浓度成正比(P<0.05)。与DAC组、PTX组比较,联合组中LEF1的表达水平最低(P<0.05),E-cadherin、caspass-3的表达水平最高(P<0.05),而且DAC和PTX对LEF1表达的抑制作用、对E-cadherin、caspass-3的表达的促进作用与药物浓度成正比(P<0.05)。结论DAC与 PTX联合干预肾癌细胞,具有协同作用,而且 LEF1是新的肾癌治疗靶点,DAC与 PTX合用可以提高肾癌细胞的凋亡率,并且降低肾癌细胞的侵袭转移能力。
脱甲基化药物;紫杉醇;肾癌;侵袭能力;分子机制
肾细胞癌(renal cell carcinoma,RCC)是最常见的泌尿系统恶性肿瘤[1-3],其发病率仅略低于膀胱肿瘤。基因甲基化的功用是稳定基因结构的碱基序列,使其能够编码正常的基因。如果基因组的基因甲基化和去甲基化的平衡发生异常变化,造成染色质复制不稳定,可引起细胞突变,造成生长失控,从而诱发肿瘤。脱氧杂氮胞苷亦称地西他滨(5-aza-2 deoxycytidine,DAC)是经典的去甲基化药物,其可有效抑制胞嘧啶甲基化的过程,随着细胞分裂逐渐减低DNA甲基化的程度[4-6]。紫杉醇(paclitaxel,PTX)属于抗微管药,其能下调细胞浆内的IkB-α水平,使得可以诱导和促进微管蛋白聚合,抑制微管解聚,使得NF-κB被激活,其转移至细胞核内参与调节多种参与凋亡蛋白,如半胱天冬酶(caspase)家族,使癌细胞凋亡[7-9]。若联合使用DAC 和PTX ,除了两者皆具有的细胞毒效应影响癌细胞的凋亡外,很可能通过干扰癌细胞的细胞黏附分子从而对肿瘤细胞的侵袭能力产生间接影响。本研究即观察联合使用DAC 与PTX对肾患细胞是否存在协同作用,并探究其分子机制。
1 材料与方法
1.1 主要试剂及仪器 地西他滨(DAC;美国 Sigma公司); Paclitaxel (PTX;美国 Sigma公司);人肾癌细胞株(美国 ATCC公司)FACSArill型高速分选流式细胞仪(美国 BD公司);Freedom EVO 75 全自动酶联免疫吸附分析仪(瑞士 Tecan公司)。
1.2 研究方案
1.2.1 细胞复苏:肾透明细胞癌ACHN细胞株均置于-80℃保存。复苏时置37℃水浴解冻,加RPMI-1640 培养液,1 500 r/min,5 min离心,去上清,加Rpmi-1640 培养基液,置5%CO2、37℃的培养箱中培养。
1.2.2 细胞培养:细胞培养基选用10%胎牛血清、100 U/ml青霉素、RPMI-1640、100 mg/ml链霉素,5%CO2、37℃的培养箱中孵育,ACHN肾癌细胞株为单层粘壁生长类型,将细胞培养浓度1×107/ml。
1.2.3 试验设计和分组:通过随机数字发生器将120培养皿随机分为4组(n=30):空白对照组;DAC组,脱甲基化药物 DAC干预;PTX组,化疗药物PTX干预;联合组,DAC联合PTX干预。
1.2.4 试验干预措施:PTX溶于DMSO之中,浓度10 mg/ml,使用无血清 RPMI-1640 配制样品(1 mg/ml)浓度为 1 000、100、10、1、0.1 μg/ml,500 nmol/L PBS 稀释,0.22 μm滤膜过滤,置-80℃。DAC 500 μmol/L PBS 稀释,0.22 μm滤膜过滤,置-80℃。①DAC干预方案:用DAC 0.5、1、2、4、8 μmol/L处理3 d。 ②PTX干预方案:用 PTX 1、 2、4、8、16 nmol/L处理 3 d。③DAC联合PTX治疗方案:用DAC+ PTX处理 3 d。
1.3 观察指标 (1)使用高速分选流式细胞仪观察T肽协同树突状细胞对肾癌细胞的凋亡率;(2)通过酶联免疫吸附测定(enzyme linked immunosorbent assay,ELISA)检测淋巴细胞增强因子(lymphoid enhance facter 1,LEF1)和E-cadherin在肾癌细胞中的表达;(3)通过ELISA技术检测凋亡蛋白caspass-3在肾癌细胞中的表达。
2 结果
2.1 肾癌细胞凋亡率比较 DAC组、PTX组以及联合组对肿瘤细胞的抑制作用明显,其中联合组对肿瘤细胞的抑制作用最强,而且DAC和PTX对肿瘤细胞的抑制作用与药物浓度呈正比,差异有统计学意义(P<0.05)。见表1。


组别0.5μmol/L1μmol/L2μmol/L4μmol/L8μmol/LP值空白对照组23.89±2.7524.10±2.0124.27±2.2125.09±3.9525.55±3.69>0.05DAC组25.78±4.4935.57±3.0946.87±3.2758.15±4.5561.82±.88<0.05PTX组25.78±4.4935.57±3.0946.87±3.2758.15±4.5575.90±2.88<0.05联合组26.02±1.5442.28±2.0258.68±2.2579.30±3.5092.08±2.50<0.05 P值<0.05<0.05<0.05<0.05<0.05-
2.2 LEF1在肾癌细胞中的表达比较 联合组中LEF1的表达水平最低,而且DAC和PTX对LEF1表达的抑制作用与药物浓度呈正比,差异有统计学意义(P<0.05)。见表2。
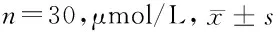
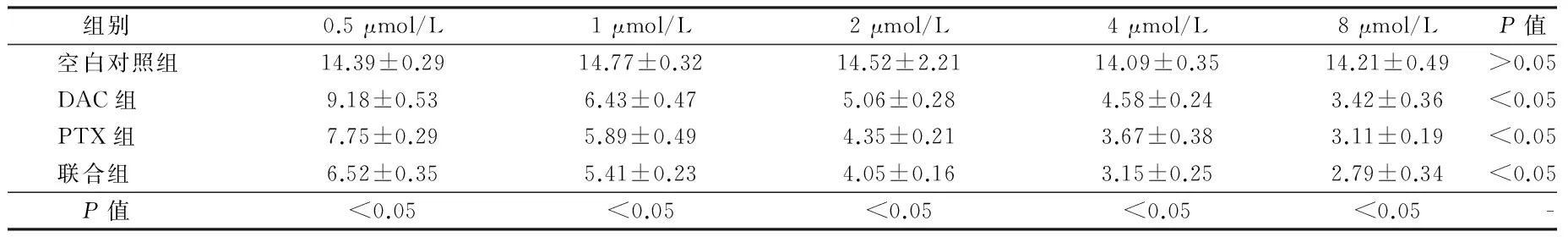
组别0.5μmol/L1μmol/L2μmol/L4μmol/L8μmol/LP值空白对照组14.39±0.2914.77±0.3214.52±2.2114.09±0.3514.21±0.49>0.05DAC组9.18±0.536.43±0.475.06±0.284.58±0.243.42±0.36<0.05PTX组7.75±0.295.89±0.494.35±0.213.67±0.383.11±0.19<0.05联合组6.52±0.355.41±0.234.05±0.163.15±0.252.79±0.34<0.05 P值<0.05<0.05<0.05<0.05<0.05-
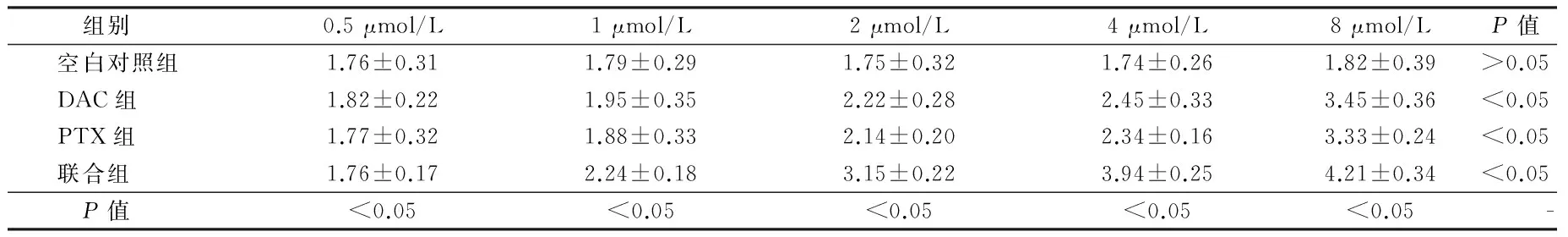
2.3 E-cadherin在肾癌细胞中的表达比较 联合组中E-cadherin的表达水平最高,而且DAC和PTX对E-cadherin表达的促进作用与药物浓度呈正比,差异有统计学意义(P<0.05)。见表3。
2.4 caspass-3在肾癌细胞中的表达的比较:比较DAC组、PTX组以及联合组中caspass-3在肾癌细胞中的表达,其中联合组中caspass-3的表达水平最高,而且DAC和PTX对LEF1表达的促进作用与药物浓度呈正比,差异有统计学意义(P<0.05)。见表4。
3 讨论
肿瘤发生的特征是生长失控,癌细胞基因组中的一种肿瘤发生诱因是基因组超甲基化,即抑癌基因(tumor suppressor gene)的超甲基化(hyper methylation),使该基因失活,不能转录复制,诱发肿瘤发生[10-12]。目前在肾癌中,已发现多个基因存在超甲基化的变化,例如肿瘤抑制蛋白 RDA32、凋亡相关蛋白激酶 1、蛋白分化酶 3的抑制因子等。近年,DNA甲基化的作用受到关注,因为甲基化水平同时影响肿瘤细胞的凋亡和转移能力,而肿瘤细胞的绝对数目以及是否容易从瘤体脱落是决定肿瘤细胞侵袭能力的关键,尤其是甲基化(methylation)在肿瘤的发展的影响成为肿瘤研究的热点[13-15]。
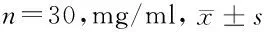
组别0.5μmol/L1μmol/L2μmol/L4μmol/L8μmol/LP值空白对照组1.76±0.311.79±0.291.75±0.321.74±0.261.82±0.39>0.05DAC组1.82±0.221.95±0.352.22±0.282.45±0.333.45±0.36<0.05PTX组1.77±0.321.88±0.332.14±0.202.34±0.163.33±0.24<0.05联合组1.76±0.172.24±0.183.15±0.223.94±0.254.21±0.34<0.05 P值<0.05<0.05<0.05<0.05<0.05-
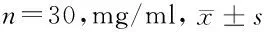

组别0.5μmol/L1μmol/L2μmol/L4μmol/L8μmol/LP值空白对照组14.39±0.2914.77±0.3214.52±2.2114.09±0.3514.21±0.49>0.05DAC组9.18±0.536.43±0.475.06±0.284.58±0.243.42±0.36<0.05PTX组7.75±0.295.89±0.494.35±0.213.67±0.383.11±0.19<0.05联合组6.52±0.355.41±0.234.05±0.163.15±0.252.79±0.34<0.05 P值<0.05<0.05<0.05<0.05<0.05-
淋巴细胞增强因子(lymphoid enhance facter 1,LEF1)在胚胎组织细胞中多为高表达,其在出生后迅速下降[16-18]。LEF1通过和β-catenin结合激活靶基因调控细胞增殖和凋亡,继而干预肿瘤的发展过程。LEF-1在肾肿瘤中的表达多增高,其通过β-catenin信号通路抑制 E-cadherin在肿瘤细胞内的表达水平,继而促进肿瘤细胞迁移。E-cadherin属于钙依赖性细胞间黏附分子,其可以维持组织细胞的完整性。β-catenin的一端与钙依赖性黏附分子(calcium-dependent cell adhesion molecule,E-cadherin)末端进行耦联,继而形成 E-cadherin/β-catenin复合物,β-catenin的另一端通过与胞内肌动蛋白连接,稳定细胞间的连接。E-cadherin蛋白表达下调可以会诱发该复合体功能障碍,使细胞间形成稳定连接失去功能,最终诱发肿瘤细胞发生浸润和转移。
脱甲基化药物 DAC可以和 DNA结合,继而抑制DNA甲基化转移酶的活性,使甲基化胞嘧啶去甲基,使DNA低甲基化。目前,DAC 已用于临床造血系统肿瘤、转移性肺癌和转移性前列腺癌的临床治疗。
单独使用DAC 或者PTX治疗肾患细胞的临床效果并不佳,其总缓解率仅达到 5%,其原因与肾癌细胞的增殖和侵袭能力没有得到有效遏制有关[19]。本研究证实, DAC和 PTX单独使用可以抑制肾癌细胞的生长,即诱发caspass-3在肾癌细胞中的高表达,使得肾癌细胞的凋亡率上调。其具有剂量依赖性。尤为重要的是,实验发现联合应用 DAC与 PTX,其对肿瘤细胞的生长抑制显著高于单独使用的效果,也即联合治疗能够比较低的浓度达到较好的疗效。
本研究发现,LEF1的表达水平在DAC和PTX干预肾癌症细胞之后明显下调,而且联合作用后 LEF1表达水平的下降幅度更大,即两种药物呈现协同作用。本实验结果还发现,DAC和PTX联合处理肾癌细胞之后 LEF1的表达显著低于单一药物的作用。本研究结果表明,LEF1是在DAC和PTX联合作用的靶点,即LEF1所在的Wnt信号通路密切参与肾癌的发展过程,并且其是调控肾癌细胞生长的重要通路。
综上所述,本研究证实了 DAC与 PTX联合干预肾癌细胞,具有协同作用,而且 LEF1是新的肾癌治疗靶点,DAC与 PTX合用可以提高肾癌细胞的凋亡率,并且降低肾癌细胞的侵袭转移能力。
1 Aguiar P Jr,Padua TC,Guimaraes DP.Brazilian data of renal cell carcinoma in a public university hospital. Int Braz J Urol,2016,42:29-36.
2 Zhang S, Hong Z, Li Q, et al. Effect of MicroRNA-218 on the viability, apoptosis and invasion of renal cell carcinoma cells under hypoxia by targeted downregulation of CXCR7 expression. Biomed Pharmacother,2016,80:213-219.
3 Mitash N, Agnihotri S, Mittal B, et al. Molecular cystoscopy: Micro-RNAs could be a marker for identifying genotypic changes for transitional cell carcinoma of the urinary bladder. Indian J Urol,2015,32:149-153.
4 Jiang J, Yi BO, Ding S, et al. Demethylation drug 5-Aza-2'-deoxycytidine-induced upregulation of miR-200c inhibits the migration, invasion and epithelial-mesenchymal transition of clear cell renal cell carcinoma in vitro.Oncol Lett,2016,11:3167-3172.
5 Anders NM,Liu J,Wanjiku T,et al.Simultaneous quantitative determination of 5-aza-2'-deoxycytidine genomic incorporation and DNA demethylation by liquid chromatography tandem mass spectrometry as exposure-response measures of nucleoside analog DNA methyltransferase inhibitors.J Chromatogr B Analyt Technol Biomed Life Sci,2016,1022:38-45.
6 Zhu X, Yi F, Chen P, et al. 5-Aza-2'-Deoxycytidine and CDDP Synergistically Induce Apoptosis in Renal Carcinoma Cells via Enhancing theAPAF-1 Activity. Clin Lab,2015,61:1821-1830.
7 Luan X, Guan YY, Lovell JF, et al.Tumor priming using metronomic chemotherapy with neovasculature-targeted, nanoparticulate paclitaxel. Biomaterials,2016,95:60-73.
8 Wang W, Shi Y, Li J, et al.Up-regulation of KIF14 is a predictor of poor survival and a novel prognostic biomarker of chemoresistance to paclitaxel treatment in cervical cancer. Biosci Rep,2016,36:315.
9 Najlah M, Kadam A, Wan KW, et al. Novel paclitaxel formulations solubilized by parenteral nutrition nanoemulsions for application against glioma cell lines. Int J Pharm,2016,506:102-109.
10 Cheedipudi S, Puri D, Saleh A,et al. A fine balance: epigenetic control of cellular quiescence by the tumor suppressor PRDM2/RIZ at a bivalent domain in the cyclin a gene.Nucleic Acids Res,2015,43:6236-6256.
11 Li Z, Ding Y, Zhu Y, et al. Both gene deletion and promoter hyper-methylation contribute to the down-regulation of ZAC/PLAGL1 gene in gastric adenocarcinomas: a case control study.Clin Res Hepatol Gastroenterol,2015,38: 744-750.
12 Tahara T, Shibata T, Yamashita H, et al. Chronic nonsteroidal anti-inflammatory drug (NSAID) use suppresses multiple CpG islands hyper methylation (CIHM) of tumor suppressor genes in the human gastric mucosa.Cancer Sci,2009,100:1192-1197.
13 Chun HJ, Lim EL, Heravi-Moussavi A, et al. Genome-Wide Profiles of Extra-cranial Malignant Rhabdoid Tumors Reveal Heterogeneity and Dysregulated Developmental Pathways.Cancer Cell,2016,29:394-406.
14 Richter AM, Walesch SK, Dammann RH. Aberrant Promoter Methylation of the Tumour Suppressor RASSF10 and Its Growth Inhibitory Function in Breast Cancer. Cancers (Basel),2016,25:26.
15 Cohen DO, Duchin S, Feldman M, et al. Engineering of Methylation State Specific 3xMBT Domain Using ELISA Screening. PLoS One, 2016, 11:154-207.
16 Kennedy MW, Chalamalasetty RB, Thomas S, et al. Sp5 and Sp8 recruit β-catenin and Tcf1-Lef1 to select enhancers to activate Wnt target gene transcription.Proc Natl Acad Sci U S A, 2016, 113:3545-3350.
18 Jia M, Zhao HZ, Shen HP, et al. Overexpression of lymphoid enhancer-binding factor-1 (LEF1) is a novel favorable prognostic factor in childhood acute lymphoblastic leukemia.Int J Lab Hematol,2015,37:631-640.
19 Chandel N, Ayasolla KS, Lan X, et al. Epigenetic Modulation of Human Podocyte Vitamin D Receptor in HIV Milieu.J Mol Biol,2015,427:3201-3215.
Effects of demethylation drugs combined with paclitaxel on invasion ability of renal carcinoma cells and their action mechanism
YANGKaijun,ZHUBin,PANWeibing,etal.
DepartmentofUrinarySurgery,People’sHospitalofPingshanDistrict,Guangdong,Shenzhen518118,China
Objective To observe whether combination application of deoxidation cytidine (DAC) with paclitaxel (PTX) has a synergistic effect on renal carcinoma cells, and to explore their action mechanism. Methods The renal clear cell carcinoma-ACHN cell line was cultured in vitro. The 120 culture dishes were randomly divided into 4 groups:blank control group,DAC group,PTX group,combination group (DAC+PTX),with 30 dishes in each group. The cells in DAC group were treated by DAC 0.5, 1, 2, 4, 8 μmol/L, respectively for 3 days;the celles in PTX group were treated by PTX 1, 2, 4, 8, 16 nmol/L, respectively for 3 days; the cells in combination group were treated by DAC+PTX for 3 days. The apoptosis rates of renal carcinoma cells induced by T peptide combined with dendritic cells were detected by flow cytometry,and the expression levels of lymphocyte enhancement factor (LEF1),E-cadherin and Caspass-3 in renal carcinoma cells were detected by ELISA. Results The inhibitory effects on tumor cells in DAC group,PTX group and combination group were obvious,in which the inhibitory effects in combination group were the most obvious (P<0.05). Moreover the inhibitory effects on tumor cells in DAC group and PTX group were in direct proportion to the drug concentrations (P<0.05). As compared with those in DAC group and in PTX group,the expression levels of LEF1 in combination group were the lowest (P<0.05), however, the expression levels of E-cadherin and Caspass-3 were the highest. Moreover the inhibitory effects of DAC and PTX on the expressions of LEF1 as well as the promotive effects on the expressions of E-cadherin,Caspass-3 were in opposite proportion to the drug concentrations (P<0.05).Conclusion The combination intervention of DAC and PTX on renal carcinoma cells has a synergistic effect.Moreover LEF1 is an new therapeutic target for renal carcinoma.The combination application of DAC with PTX can increase the apoptosis rate of renal carcinoma cells, and can decrease the abilities of invasion and metastasis of renal carcinoma cells.
demethylation drugs; paclitaxel; renal carcinoma; invasive ability; molecular mechanism
10.3969/j.issn.1002-7386.2016.24.009
518118 广东省深圳市坪山新区人民医院泌尿外科
R 737.11
A
1002-7386(2016)24-3717-04
2016-06-08)
