Amberlyst-15催化C(sp3)—H对靛红类化合物的加成反应
2016-12-27董道青胡东岳寻之玉李宗慧史大鹏杨家岐王祖利
董道青,胡东岳,寻之玉,李宗慧,史大鹏,杨家岐,王祖利
·研究论文·
Amberlyst-15催化C(sp3)—H对靛红类化合物的加成反应
董道青,胡东岳,寻之玉,李宗慧,史大鹏,杨家岐,王祖利*
(青岛农业大学 化学与药学院,山东 青岛 266109)
以Amberlyst-15为催化剂,甲基吡啶类化合物中的C(sp3)—H键对靛红类化合物经加成反应合成了24个3-羟基-2-吲哚酮类化合物(3a~3x),其中3u~3x为新化合物,其结构经1H NMR,13C NMR,IR和HR-MS(ESI)表征。催化剂Amberlyst-15循环使用8次,不影响反应收率。
C(sp3)—H键;Amberlyst-15;绿色化学;靛红;合成;吲哚酮
Amberlyst-15 是一种磺化的聚苯乙烯树脂,具有无毒,化学物理性质稳定,容易从反应体系中分离等优点,在有机合成中得到了广泛应用。 其催化的各种有机反应已被报道[1],如酯化反应[2-4],Michael加成反应[5-6],Prins反应[7],傅克反应[8-9],卤化反应[10],环氧化合物开环反应[11-13],缩合反应[14-16]及多组分反应[17-18]等。
C(sp3)—H键能高,反应相对惰性。C(sp3)—H键的功能化反应是有机化学研究中的重要和热点领域[19-23]。2014年,Yang等[24]研究了醋酸银催化的C(sp3)—H键活化形成C—N键的反应;Qian等[25]实现了三氟甲磺酸钪催化的C(sp3)—H键对亚胺的加成反应;Wang等[26]研究了醋酸促进的C(sp3)—H键对醛的加成反应合成醇类化合物的反应。
3-羟基-2-吲哚酮类化合物是许多天然产物及药物活性分子的结构单元,对其合成的研究具有重要意义。本文研究了Amberlyst-15催化甲基吡啶类化合物(1)中的C(sp3)—H键对靛红类化合物(2)的加成反应(Scheme 1),高效合成了24个3-羟基-2-吲哚酮类化合物(3a~3x),其中3u~3x为新化合物,其结构经1H NMR,13C NMR,IR和HR-MS(ESI)表征。催化剂Amberlyst-15循环使用8次,不影响反应收率。

Scheme 1
1 实验部分
1.1 仪器与试剂
Bruker AV300型核磁共振仪(DMSO-d6为溶剂,TMS为内标);MATRIX-1型红外光谱仪;APEX II型质谱仪。
所用试剂均为分析纯。
1.2 合成
(1) 3a~3x的合成通法
在25 mL的真空管中加入 1 0.75 mmol,2 0.25 mmol,Amberlyst-15催化剂30 mg和DMSO 0.6 mL,升温至100 ℃反应24 h。反应液用乙醚(3×10 mL)萃取,合并有机相,用无水硫化钠干燥,减压旋蒸除溶,残余物经硅胶柱层析[洗脱剂:A=V(石油醚):V(乙酸乙酯)=1:3]纯化得3a~3x。
3-羟基-1-甲基-3-[(6-甲基吡啶基-2-)甲基]-吲哚-2-酮(3a):黄色固体,收率88%;1H NMRδ:7.42(t,J=7.65 Hz,1H),7.16(t,J=7.2 Hz,1H),6.97~6.79(m,5H),6.35(s,1H),3.29(d,J=10.8 Hz,1H),3.25(d,J=10.8 Hz,1H),3.03(s,3H),2.22(s,3H);13C NMRδ:177.4,156.6,155.8,143.9,136.6,131.0,129.2,124.3,122.0,121.3,121.1,108.3,76.7,45.2,26.1,24.1。
3-羟基-3-[(6-甲基吡啶基-2-)甲基]-1-苯基吲哚-2-酮(3b):白色固体,收率75%;1H NMRδ:8.01(s,1H),7.57~7.34(m,6H),7.25~7.11(m,2H),7.00~6.88(m,3H),6.82(d,J=7.8 Hz,1H),3.38(d,J=14.7 Hz,1H),3.20(d,J=14.7 Hz,1H),2.60(s,3H);13C NMRδ:176.1,157.2,156.7,142.8,137.4,134.2,131.0,129.5,129.1,127.9,126.4,124.2,123.1,122.0,121.6,109.5,76.2,42.8,24.2。
5-溴-3-羟基-3-[(6-甲基吡啶-2-)甲基]-吲哚-2-酮(3c):白色固体,收率90%;1H NMRδ:10.28(s,1H),7.50(t,J=7.65 Hz,1H),7.27(dd,J=2.1 Hz,8.4 Hz,1H),7.01~6.96(m,3H),6.63(d,J=8.4 Hz,1H),6.39(s,1H),3.27(d,J=13.2 Hz,1H),3.10(d,J=13.2 Hz,1H),2.27(s,3H);13C NMRδ:178.7,156.8,155.5,141.5,136.7,134.0,131.7,128.2,121.7,121.3,113.0,111.5,76.1,49.9,24.1。
3-羟基-5-甲基-3-[( 6-甲基吡啶-2-)甲基]吲哚-2-酮(3d):白色固体,收率73%;1H NMRδ:10.09(s,1H),7.55(t,J=7.65 Hz,1H),7.08~6.95(m,4H),6.73~6.61(m,3H),3.28(d,J=13.2 Hz,1H),3.13(d,J=13.2 Hz,1H),2.36(s,3H),2.21(s,3H);13C NMRδ:179.1,156.7,155.9,139.6,136.6,131.6,130.0,129.3,125.8,121.7,121.2,109.3,76.0,45.1,24.2,21.1。
3-羟基-3-[(6-甲基吡啶-2-)甲基]吲哚-2-酮(3e):白色固体,收率80%;1H NMRδ:10.13(s,1H),7.48(t,J=7.5 Hz,1H),7.09(dt,J=1.2 Hz,6.9 Hz,1H),7.01~6.94(m,2H),6.89~6.79(m,2H),6.67(d,J=7.8 Hz,1H),6.39(s,1H),3.24(d,J=13.5 Hz,1H),3.11(d,J=13.5 Hz,1H),2.28(s,3H);13C NMRδ:179.0,156.8,155.9,142.2,136.6,131.6,129.1,125.0,121.6,121.4,121.3,109.6,76.0,45.1,24.2。
7-溴-3-羟基-3-[(6-甲基吡啶-2-)甲基)]吲哚-2-酮(3f):淡黄色固体,收率92%;1H NMRδ:10.43(s,1H),7.48(t,J=7.65 Hz,1H),7.28(d,J=8.1 Hz,1H),6.99~6.93(m,3H),6.78(t,J=7.65 Hz,1H),6.44(s,1H),3.27(d,J=13.8 Hz,1H),3.18(d,J=13.8 Hz,1H),2.25(s,3H);13C NMRδ:178.9,156.8,155.5,141.8,136.7,133.8,131.9,123.9,123.1,121.4,121.3,102.1,76.7,44.9,24.0。
3-羟基-3-[(6-甲基吡啶-2-)甲基]-5-硝基吲哚-2-酮(3g):淡黄色固体,收率95%;1H NMRδ:10.08(s,1H),8.10(dd,J=2.4 Hz,8.7 Hz,1H),7.82(d,J=2.1 Hz,1H),7.50(t,J=7.65 Hz,1H),7.01~6.97(m,2H),6.88(d,J=8.4 Hz,1H),6.53(s,1H),3.41(d,J=13.5 Hz,1H),3.22(d,J=13.5 Hz,1H),2.21(s,3H);13C NMRδ:179.5,156.9,155.1,149.1,142.0,136.8,132.7,126.5,121.7,121.3,120.8,109.7,75.5,44.6,23.9。
3-羟基-5-甲氧基-3-[(6-甲基吡啶-2-)甲基]吲哚-2-酮(3h):白色固体,收率84%;1H NMRδ:9.97(s,1H),7.50(t,J=7.5 Hz,1H),7.03~6.97(d,J=8.1 Hz,2H),6.45(s,1H),6.36(s,1H),3.60(s,3H),3.24(d,J=13.5 Hz,1H),3.06(d,J=13.5 Hz,1H),2.31(s,3H);13C NMRδ:179.0,156.8,155.9,154.8,136.6,135.3,132.8,121.8,121.3,114.0,112.0,110.0,76.4,55.8,45.0,24.2。
3-羟基-5-硝基-3-(吡啶基-2-甲基)吲哚-2-酮(3i):淡黄色固体,收率85%;1H NMRδ:10.86(s,1H),8.25(d,J=3.9 Hz,2H),8.08(dd,J=2.4 Hz,8.7 Hz,1H),7.83(d,J=2.1 Hz,H),7.60(dt,J=2.0 Hz,8.5 Hz,1H),7.20~7.11(m,2H),6.84(d,J=8.7 Hz,1H),6.56(s,1H),3.45(d,J=13.2 Hz,1H),3.28(d,J=13.2 Hz,1H);13C NMRδ:179.3,155.8,148.9,142.1,136.5,132.5,126.6,124.8,122.3,120.7,109.9,75.7,45.0。
5-溴-3-羟基-3-(吡啶基-2-甲基)吲哚-2-酮(3j):白色固体,收率83%;1H NMRδ:10.30(s,1H),8.33(d,J=6.0 Hz,2H),7.33(d,J=2.1 Hz,1H),7.31(s,1H),6.94(d,J=6.0 Hz,2H),6.61(d,J=8.1 Hz,1H),6.40(s,1H),3.21(d,J=12.6 Hz,1H),3.00(d,J=12.6 Hz,1H);13C NMRδ:178.3,149.3,144.2,141.2,133.4,132.2,127.9,125.9,113.5,111.9,76.5,42.7。
3-羟基-5-甲氧基-3-(吡啶基-2-甲基)吲哚-2-酮(3k):白色固体,收率81%;1H NMRδ:9.95(s,1H),8.31(d,J=4.2 Hz,1H),7.60(dt,J=1.5 Hz,7.65 Hz,1H),7.18~7.12(m,2H),6.65(dd,J=2.4 Hz,5.4 Hz,1H),6.56(d,J=8.4 Hz,1H),6.46(d,J=2.4 Hz,1H),6.28(s,1H),3.60(s,3H),3.30(d,J=12.9 Hz,1H),3.12(d,J=12.9 Hz,1H);13C NMRδ:178.9,156.6,154.8,148.7,136.2,135.2,132.6,124.9,122.1,113.9,112.0,110.0,76.4,55.7,45.5。
3-羟基-1-苯基-3-(吡啶基-2-甲基)吲哚-2-酮(3l):白色固体,收率78%;1H NMRδ:8.58(d,J=4.2 Hz,1H),7.65(dt,J=7.8 Hz,1.8 Hz,1H),7.47(t,J=12.0 Hz,2H),7.41~7.35(m,3H),7.28(t,J=5.5 Hz,1H),7.22~7.16(m,1H),7.10(d,J=7.8 Hz,1H),6.98(d,J=4.2 Hz,2H),6.80(d,J=8.1 Hz,1H),3.42(d,J=14.7 Hz,1H),3.30(d,J=14.7 Hz,1H);13C NMRδ:176.1,157.3,148.1,142.9,137.2,134.2,130.6,129.5,129.3,127.9,126.4,124.8,124.3,123.2,122.3,109.5,76.3,43.2。
3-羟基-3-(吡啶基-2-甲基)吲哚-2-酮(3m):白色固体,收率86%;1H NMRδ:10.12(s,1H),8.29(d,J=4.5 Hz,1H),7.57(dt,J=1.8 Hz,7.8 Hz,1H),7.14~7.05(m,3H),6.91(d,J=6.9 Hz,1H),6.82(t,J=7.5 Hz,1H),6.65(d,J=7.8 Hz,1H),6.28(s,1H),3.33(d,J=12.9 Hz,1H),3.17(d,J=12.9 Hz,1H);13C NMRδ:179.0,156.5,148.7,142.0,136.2,131.4,129.2,125.0,124.7,122.1,121.5,109.6,76.1,45.5。
3-羟基-5-甲基-3-(吡啶基-2-甲基)吲哚-2-酮(3n):白色固体,收率77%;1H NMRδ:10.09(s,1H),8.30(t,J=2.7 Hz,1H),7.61~7.55(m,1H),7.15~7.12(m,2H),6.88(d,J=7.5 Hz,1H),6.71(s,1H),6.53(d,J=7.8 Hz,1H),6.23(s,1H),3.26(d,J=12.9 Hz,1H),3.14(d,J=12.9 Hz,1H),2.15(s,3H);13C NMRδ:179.0,156.6,148.7,139.6,136.2,131.5,130.1,129.4,125.7,124.7,122.0,109.3,76.1,45.6,21.1。
3-羟基-1-甲基-3-(吡啶基-4-甲基)吲哚-2-酮(3o):白色固体,收率82%;1H NMRδ:8.24(d,J=6.0 Hz,2H),7.28~7.23(m,1H),7.17(d,J=6.9 Hz,1H),7.05(t,J=7.5 Hz,1H),6.88(d,J=6.0 Hz,2H),6.63(d,J=7.8 Hz,1H),3.29(d,J=12.6 Hz,1H),3.14(d,J=12.6 Hz,1H),2.97(s,3H);13C NMRδ:177.4,148.6,143.9,142.9,129.9,128.9,125.5,124.2,123.0,108.4,76.7,43.9,25.9。
3-羟基-3-(吡啶基-4-甲基)吲哚-2-酮(3p):红色固体,收率70%,m.p.209~210 ℃;1H NMRδ:10.14(s,1H),8.29(d,J=4.8 Hz,2H),6.94~6.89(m,3H),6.63(d,J=7.8 Hz,1H),6.24(s,1H),3.17(d,J=12.6 Hz,1H),2.98(d,J=12.6 Hz,1H);13C NMRδ:178.8,149.2,144.5,141.9,130.9,129.6,125.9,124.9,121.8,109.9,76.4,43.0;IRν:1 722,1 621,1 604,1 551,750 cm-1。
3-[(4,6-二甲基吡啶基-2-)甲基]-3羟基吲哚-2-酮(3q):淡黄色固体,收率76%,m.p.217~218 ℃;1H NMRδ:10.13(s,1H),7.09(t,J=7.05 Hz,1H),6.84~6.69(m,5H),6.47(s,1H),3.18(d,J=13.5 Hz,1H),3.03(d,J=13.5 Hz,1H),2.24(s,3H),2.15(s,3H);13C NMRδ:179.0,156.5,155.9,147.0,142.1,131.8,129.1,124.9,122.5,122.2,121.4,109.6,76.0,44.7,24.0,20.8;IRν:1 712,1 620,1 560,1 485,768 cm-1。
3-羟基-1-甲基-3-(喹啉基-2-甲基)吲哚-2-酮(3r):收率86%,白色固体,m.p.217~218 ℃;1H NMRδ:8.11(t,J=9.3 Hz,2H),7.85~7.31(m,3H),7.56(t,J=7.5 Hz,1H),7.28~7.16(m,2H),6.90~6.80(m,3H),3.56(d,J=15.0 Hz,1H),3.23(d,J=15.0 Hz,1H),3.20(s,3H);13C NMRδ:176.6,158.6,146.6,143.0,137.1,131.2,130.1,129.3,128.7,127.7,127.0,126.6,124.0,122.7,122.7,108.2,43.1,26.2;IRν:1 712,1 620,1 560,1 485,768 cm-1。
3-羟基-3-[(6-甲基喹啉基-2-)甲基]吲哚-2-酮(3s):黄色固体,收率82%,m.p.197~199 ℃;1H NMRδ:10.17(s,1H),8.04(d,J=8.4 Hz,1H),7.69~7.62(m,2H),7.50~7.47(dd,J=1.8 Hz,8.7 Hz,1H),7.30(d,J=8.4 Hz,1H),7.06(dt,J=1.2 Hz,7.5 Hz,1H),6.88(d,J=6.9 Hz,1H),6.77(t,J=7.3 Hz,1H),6.65(d,J=7.5 Hz,1H),6.3(s,1H),3.48(d,J=13.5 Hz,1H),3.31(d,J=13.5 Hz,1H),2.45(s,3H);13C NMRδ:179.1,156.6,145.8,142.2,135.8,135.2,131.8,131.6,129.2,128.6,126.8,126.8,123.1,109.7,76.0,45.9,21.5;IRν:1 724,1 619,1 595,1 500,746 cm-1。
3-[(6-溴喹啉-2-)甲基]-3-羟基吲哚-2-酮(3t):黄色固体,收率74%,m.p.198~200 ℃;1H NMRδ:10.18(s,1H),8.17~7.13(m,2H),7.78(dd,J=2.1 Hz,9.0 Hz,1H),7.69(d,J=9.0 Hz,1H),7.40(d,J=8.4 Hz,1H),7.07(t,J=7.5 Hz,1H),6.90(d,J=6.9 Hz,1H),6.78(t,J=7.3 Hz,1H),6.65(d,J=7.8 Hz,1H),6.3(s,1H),3.50(d,J=13.5 Hz,1H),3.34(d,J=13.5 Hz,1H);13C NMRδ:179.0,158.3,145.8,142.2,135.0,132.7,131.5,131.0,130.1,129.3,128.2,124.9,124.1,121.5,119.2,109.7,75.9,46.1;IRν:1 735,1 618,1 594,1 560,756 cm-1。
3-(苯并噻唑-2-甲基)-3-羟基-1-甲基喹啉-2-酮(3u):白色固体,收率88%,m.p.130~131 ℃;1H NMRδ:7.99(d,J=4.7 Hz,1H),7.84(d,J=4.8 Hz,1H),7.45~7.36(m,2H),7.26(t,J=4.6 Hz,1H),7.11(d,J=4.3 Hz,1H),6.97~6.93(m,2H),6.62(s,1H),3.69(d,J=8.6 Hz,1H),3.57(d,J=8.6 Hz,1H);13C NMRδ:176.6,165.5,152.3,143.8,135.7,130.0,126.3,125.3,124.4,122.7,122.3,109.0,74.7,42.3,26.4;IRν:1 706,1 615,1 555,1 513 cm-1;HR-MS(ESI)m/z:Calcd for C17H14N2O2SNa {[M+Na]+}333.066 44,found 333.066 82。
3-(苯并噻唑-2-甲基)-3-羟基-5-硝基吲哚-2-酮(3v):黄色固体,收率96%,m.p.150~152 ℃;1H NMRδ:11.0(s,1H),8.17(d,J=5.1 Hz,1H),8.10(s,1H),8.01(d,J=4.7 Hz,1H),7.83(d,J=4.8 Hz,1H),7.44(t,J=4.5 Hz,1H),7.38(t,J=4.4 Hz,1H),6.94(d,J=5.1 Hz,1H),6.86(s,1H),3.85(d,J=8.5 Hz,1H),3.65(d,J=8.6 Hz,1H);13C NMRδ:178.5,164.9,152.5,149.0,142.5,135.6,131.9,127.2,126.4,125.5,122.7,122.4,120.8,110.4,74.8,41.4;IRν:1 737,1 625,1 559,1 524 cm-1;HR-MS(ESI)m/z:Calcd for C16H12N3O4S {[M+H]+}342.053 95,found 342.054 30。
3-(苯并噻唑-2-甲基)-3-羟基-1-苯基吲哚-2-酮(3w):白色固体,收率83%,m.p.186~187 ℃;1H NMRδ:7.99(d,J=4.7 Hz,1H),7.84(d,J=4.8 Hz,1H),7.57(t,J=4.6 Hz,2H),7.44~7.47(m,2H),7.38~7.33(m,3H),7.18(t,J=4.5 Hz,1H),7.03(t,J=8.9 Hz,1H),6.80(s,1H),6.60(d,J=4.7 Hz,1H),3.84(d,J=8.5 Hz,1H),2.98(d,J=8.6 Hz,1H);13C NMRδ:176.1,165.3,152.6,143.7,135.5,134.6,130.0,128.4,126.8,126.4,125.0,123.2,122.6,122.4,109.2,75.0,42.3;IRν:1 717,1 615,1 502,769,702 cm-1;HR-MS(ESI)m/z:Calcd for C22H16N2O2SNa {[M+H]+}395.082 47,found 395.082 18。
3-(苯并噻唑-2-甲基)-7-溴-3-羟基吲哚-2-酮(3x):白色固体,收率93%,m.p 228~229 ℃;1H NMRδ:10.67(s,1H),8.02(d,J=4.6 Hz,1H),7.86(d,J=4.8 Hz,1H),7.47~7.43(m,1H),7.40~7.38(m,2H),7.11(d,J=4.3 Hz,1H),6.87(t,J=4.6 Hz,1H),6.71(s,1H),3.67(d,J=8.5 Hz,1H),3.57(d,J=8.6 Hz,1H);13C NMRδ:178.0,165.3,152.4,141.8,135.8,132.8,132.8,126.3,125.4,124.0,123.8,122.7,122.3,102.6,75.7,42.2;IRν:1 705,1 623,1 559,1 509,1 474 cm-1;HR-MS(ESI)m/z:Calcd for C16H11N2O2SBrNa {[M+Na]+}396.961 68,found 396.961 27。
2 结果与讨论
2.1 反应条件优化
以2,6-二甲基吡啶(1a)与1-甲基靛红(2a)的反应为模板,Amberlyst-15为催化剂,考察了溶剂、催化剂用量和反应温度等因素对反应的影响,结果见表1。由表1可以看出,当DMSO为溶剂时,收率最高(74%,No.5);当反应在Dioxane,THF,EtOH或DMF中进行时,反应也可以发生,但是收率有所降低(No.1~4);以Toluene或H2O作溶剂,反应几乎不发生(No.6~7)。当催化剂Amberlyst-15用量减半,收率降至60%(No.8);当Amberlyst-15用量增加一倍时,收率几乎不变(No.9)。不同反应温度影响不同,反应温度从80 ℃升至100 ℃时,收率提高至88%(No.10),如反应温度进一步升至120 ℃,收率没有变化(No.11)。
综上所述,最佳反应条件为:溶剂为DMSO,Amberlyst-15 30 mg,反应温度100 ℃。
表1 不同反应条件对收率的影响a
Table 1 Effects of different conditions on the yield

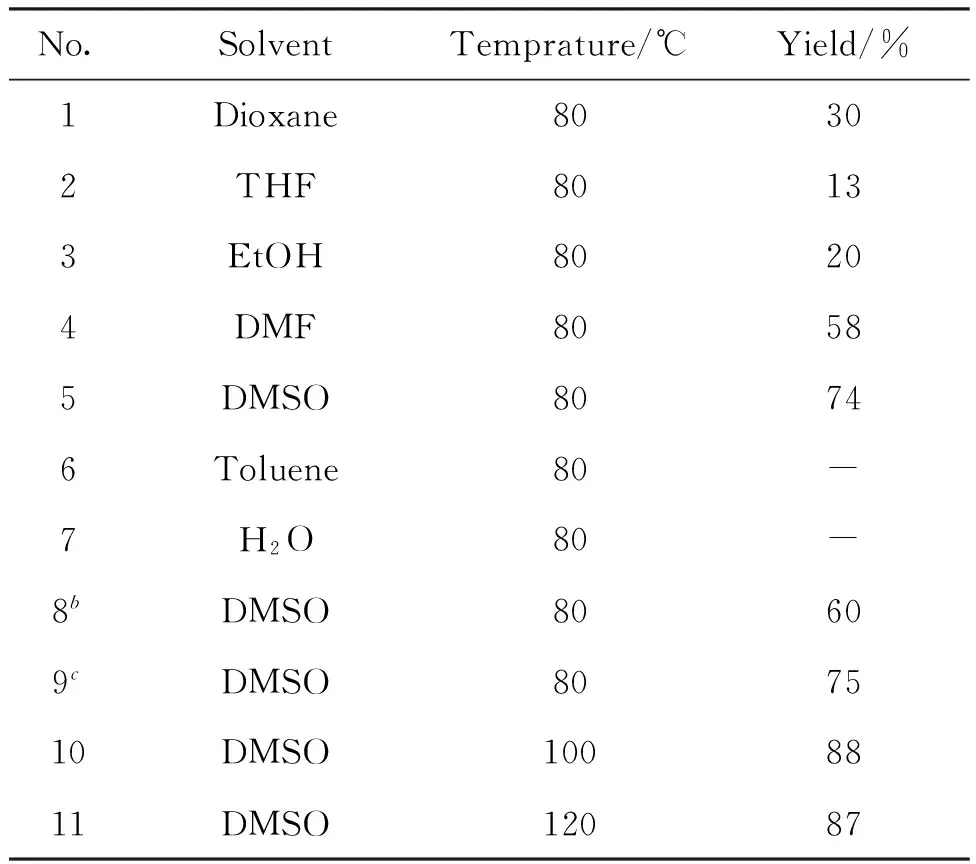
No.SolventTemprature/℃Yield/%1Dioxane80302THF80133EtOH80204DMF80585DMSO80746Toluene80-7H2O80-8bDMSO80609cDMSO807510DMSO1008811DMSO12087
a2a 0.25 mmol,1a 0.75 mmol,Amberlyst-15 30 mg,DMSO 0.8 mL,reaction for 24 h;bAmberlyst-15 15 mg;cAmberlyst-15 60 mg。
2.2 反应普适性
在最佳反应条件下,对反应的普适性进行了研究,结果见Chart 1。由Chart 1可见,靛红类化合物苯环上无论是吸电子基团还是给电子基团都可取得较高的收率。另外,靛红类化合物的氮原子是否带有取代基都可与2,6-二甲基吡啶反应,收率较高(3a~3h)。除 2,6-二甲基吡啶以外,2-甲基吡啶和2,4,6-二甲基吡啶也可以很好地参与反应(3i~3n,3q)。值得注意的是,4-甲基吡啶也可以与靛红类化合物发生反应(3o~3p)。以较高的收率得到了甲基喹啉类化合物和2-甲基苯并噻唑与2的反应产物(3u~3x)。表明该催化剂对不同的底物均有较好的催化性能。
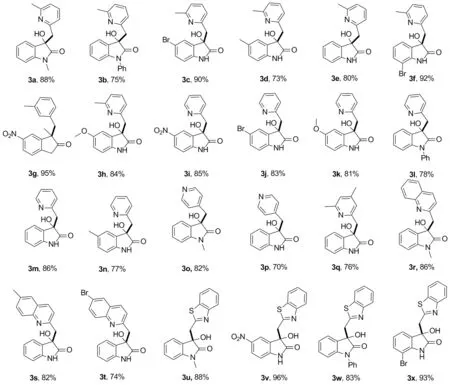
a反应条件 :1 0.75mmol,2 0.25mmol和 Amberlyst-15 30 mg,于100 ℃,在DMSO(0.8mL)为溶剂中反应24 h。
Chart 1
2.3 催化剂的重复利用
以1a与2a的反应为模板,研究了催化剂的回收利用情况。反应结束后,催化剂经过滤后分别用丙酮和乙醚淋洗。于40 ℃干燥3 h,可直接投入使用无需纯化。催化剂可重复使用8次,催化活性无明显降低。

表2 Amberlyst-15的回收利用
a反应条件:2a 0.25 mmol,1a 0.75 mmol,Amberlyst-15 30 mg,DMSO 0.8 mL,反应 24 h。
报道了一种Amberlyst-15催化的C(sp3)—H键对靛红类化合物的加成反应合成3-羟基-2-吲哚酮类化合物的新方法。此反应所使用的催化剂稳定,经过滤后可回收和重复使用八次且产率没有明显的降低。该方法操作简单、底物适用范围广且产物收率高,符合“绿色化学” 的基本要求,具有较好的应用前景和科研价值。
[1] Pal R,Sarkar T,Khasnobis S.Amberlyst-15 in organic synthesis[J].Arkivoc,2012:570-609.
[2] Petrini M,Ballini R,Marcantoni E.Amberlyst 15:A practical,mild and selective catalyst for methyl esterification of carboxylic acids.[J].Synth Commun,1988,18:847-853.
[3] Chavan S P,Subbarao T,Dantale S W,etal.Transesterification of ketoesters using amberlyst-15[J].Synth Commun,2001,31:289-294.
[4] Pappu V K S,Yanez A J,Peereboom L,etal.A kinetic model of the Amberlyst-15 catalyzed transesterification of methyl stearate withn-butanol[J].Bioresource Technology,2011,102:4270-4272.
[5] Das B,Damodar K,Chowdhury N.Amberlyst-15:A mild,efficient and reusable heterogeneous catalyst for Michael addition of pyrroles toα,β-unsaturated ketones[J].J Mol Cata A Chem,2007,269:81-84.
[6] Bandini M,Fagioli M,Umani-Ronchi A.Solid acid-catalysed michael-yype conjugate addition of indoles to electron-poor C=C bonds:Towards high atom economical semicontinuous processes[J].Adv Synth Catal,2004,346:545-548.
[7] Yadav J S,Reddy B V S,Sekhar K C,etal.Amberlyst-15-catalyzed novel synthesis of tetrahydropyranols[J].Synthesis,2001,6:885-888.
[8] Kadam S T,Thirupathi P,Kim S S.Amberlyst-15:An efficient and reusable catalyst for the Friedel-Crafts reactions of activated arenes and heteroarenes withα-amido sulfones[J].Tetrahedron,2009,65:10383-10389.
[9] Wu L,Yang C,Zhang C,Yang L L.H3PW12O40-SiO2and amberlyst 15:Two efficient heterogeneous catalysts for synthesis ofN-acylsulfonamides under solvent-free conditions[J].Bull Korean Chem Soc,2009,30:1665-1666.
[10] Meshram H M,Reddy P N,Sadashiv K,Yadav J S.Amberlyst-15-promoted efficient 2-halogenation of 1,3-keto-esters and cyclic ketones usingN-halosuccinimides[J].Tetrahedron Lett,2005,46:623-626.
[11] Vijender M,Kishore P,Narender P.Amberlist-15 as heterogeneous reusable catalyst for regioselective ring opening of epoxides with amines under mild conditions[J].J Mol Catal A Chem,2007,266(12):290-293.
[12] Liu Y,Liu Q,Zhang Z.Amberlyst-15 as a new and reusable catalyst for regioselective ring-opening reactions of epoxides toβ-alkoxy alcohols[J].J Mol Catal A Chem,2008,296:42-46.
[13] Solladie-Cavallo A,Lupattelli P,Bonini G.Regio- and stereoselective ring opening of 2,3-diaryl oxiranes by LiBr/Amberlyst 15:A new stereocontrolled access to 1,2-diaryl-2-bromo alcohols[J].J Org Chem,2005,70:1605-1611.
[14] Ramesh C,Banerjee J,Pal R,etal.Silica supported sodium hydrogen sulfate and amberlyst-15:Two efficient heterogeneous catalysts for facile synthesis of bis- and tris(1H-indol-3-yl)methanes from indoles and carbonyl compounds[J].Adv Synth Catal,2003,345:557-559.
[15] Lu J,Bai Y.Inhibition of Kupffer cell activity induces hepatic triglyceride synthesis in fasted rats,independent of lipopolysaccharide challenge[J].Synthesis,2002,36:466-473.
[16] Sarada T,Kobayashi F,Sakai N,etal.An unprecedented approach to 4,5-disubstituted pyrimidine derivatives by a ZnCl-catalyzed three-component coupling reaction[J].Org Lett,2009,40:2161-2164.
[17] Das B,Reddy K.Facile one-pot multicomponent synthesis ofβ-acetamido ketones with amberlyst-15 as heterogeneous catalyst[J].Helv Chem Acta,2006,89:3109.
[18] Das B,Banerjee J.Silica-supported sodium hydrogen sulfate and amberlyst-15:Two efficient heterogeneous catalysts for single-step synthesis of 4(3H)-quinazolinones from anthranilic acid,ortho esters,and amines under solvent free conditions[J].Chem Lett,2004,3:960-961.
[19] Li B,Shi Z.From C(sp2)—H to C(sp3)—H:Systematic studies on transition metal-catalyzed oxidative C—C formation[J].Chem Soc Rev,2012,41:5588-5598.
[20] Godula K,Sames D.Mechanism of Pd-catalyzed selective C—H activation of aliphatic amines via four-membered-ring cyclometallation pathway[J].Science,2006,312:67-72.
[21] Niu R,Xiao J,Liang T,etal,Facile synthesis of azaarene-substituted 3-hydroxy-2-oxindoles via brønsted acid catalyzedC(sp3)—H functionalization[J].Org Lett,2012,14:676-679.
[22] Raghu M,Rajasekhar M,Reddy B C O,etal.Polyethylene glycol(PEG-400):A mild and efficient reaction medium for one-pot synthesis of 3-hydroxy-3-(pyridin-2-ylmethyl)indolin-2-ones[J].Tetrahedron Lett,2013,54:3503-3506.
[23] Mulla S A R,Pathan M Y,Chavan S S.A novel and effi cient synthesis of azaarene-substituted 3-hydroxy-2-oxindolesviaC(sp3)—H functionalization of 2-methyl azaarenes and(2-azaaryl)methanes over a heterogeneous,reusable silica-supported dodecatungstophosphoric acid catalyst[J].RSC Adv,2013,3:20281-20286.
[24] Yang M,Su B,Wang Y,etal.Silver-catalysed direct amination of unactivated C—H bonds of functionalized molecules[J].Nat Commun,2014,5:4707-4713.
[25] Qian B,Guo S,Xia C,etal.Lewis acid-catalyzed C—H functionalization for synthesis of isoindolinones and isoindolines[J].Adv Synth Catal,2010,352:3195-3200.
[26] Wang F,Luo C,Wang Y,etal.Brønsted acid promoted benzylic C—H bond functionalization of azaarenes:Nucleophilic addition to aldehydes[J].Org Biomol Chem,2012,10:8605-8608.
The Nucleophilic Addition Reactions of C(sp3)—H to Isatins Catalyed by Amberlyst-15
DONG Dao-qing,HU Dong-yue,XUN Zhi-yu,LI Zong-hui, SHI Da-peng,YANG Jia-qi,WANG Zu-li*
(College of Chemistry and Pharmaceutical Sciences,Qingdao Agricultural University,Qingdao 266109,China)
Twenty four 3-hydroxy-2-oxindoles(3a~3x) were synthesized by the nucleophilic addition reactions of C(sp3)—H bond in methyl pyridine compounds to isatins,using Amberlyst-15 as catalyst.Among them,3u~3x were novel compounds and the structures were characterized by1H NMR,13C NMR,IR and HR-MS(ESI).The catalyst can be reused for eight times without significant loss of its catalytic activity.
C(sp3)—H bond;amberlyst-15;green chemistry;isatin;synthesis;oxindole
2016-04-14;
2016-09-23
国家自然科学基金资助项目( 21402103);山东省优秀中青年基金资助项目(BS2013YY024);中国博士后基金资助项目(150030)
董道青(1982-),女,汉族,山东青岛人,硕士,主要从事有机化学的研究。 E-mail:why20062002@163.com
王祖利,副教授,Tel.0532-86080895,E-mail:wangzulichem@163.com
O621.25;O621.3
A
10.15952/j.cnki.cjsc.1005-1511.2016.12.16104
